Heart Valve Involvement in Patients with Antiphospholipid Syndrome: A Long-Term Follow-Up Study of a Single Centre
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Silbiger, J.J. The cardiac manifestations of antiphospholipid syndrome and their echocardiographic recognition. J. Am. Soc. Echocardiogr. 2009, 22, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Cervera, R.; Piette, J.-C.; Font, J.; Khamashta, M.A.; Shoenfeld, Y.; Camps, M.T.; Jacobsen, S.; Lakos, G.; Tincani, A.; Kontopoulou-Griva, I.; et al. Antiphospholipid syndrome: Clinical and immunologic manifestations and patterns of disease expression in a cohort of 1000 patients. Arthritis Rheum. 2002, 46, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Cervera, R.; Serrano, R.; Pons-Estel, G.J.; Ceberio-Hualde, L.; Shoenfeld, Y.; De Ramón, E.; Buonaiuto, V.; Jacobsen, S.; Zeher, M.M.; Tarr, T.; et al. Morbidity and mortality in the antiphospholipid syndrome during a 10-year period: A multicentre prospective study of 1000 patients. Ann. Rheum. Dis. 2015, 74, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Pardos-Gea, J.; Ordi-Ros, J.; Avegliano, G.; Cortés-Hernández, J.; Balada, E.; Evangelista, A.; Vilardell, M. Echocardiography at diagnosis of antiphospholipid syndrome provides prognostic information on valvular disease evolution and identifies two subtypes of patients. Lupus 2010, 19, 575–582. [Google Scholar] [CrossRef]
- Krause, I.; Lev, S.; Fraser, A.; Blank, M.; Lorber, M.; Stojanovich, L.; Rovensky, J.; Chapman, J.; Shoenfeld, Y. Close association between valvar heart disease and central nervous system manifestations in the antiphospholipid syndrome. Ann. Rheum. Dis. 2005, 64, 1490–1493. [Google Scholar] [CrossRef]
- Turiel, M.; Muzzupappa, S.; Gottardi, B.; Crema, C.; Sarzi-Puttini, P.; Rossi, E. Evaluation of cardiac abnormalities and embolic sources in primary antiphospholipid syndrome by transesophageal echocardiography. Lupus 2000, 9, 406–412. [Google Scholar] [CrossRef]
- Perez-Villa, F.; Font, J.; Azqueta, M.; Espinosa, G.; Pare, C.; Cervera, R.; Reverter, J.C.; Ingelmo, M.; Sanz, G. Severe valvular regurgitation and antiphospholipid antibodies in systemic lupus erythematosus: A prospective, long-term, followup study. Arthritis Rheum. 2005, 53, 460–467. [Google Scholar] [CrossRef]
- Cieśla, M.; Wypasek, E.; Undas, A. IgA Antiphospholipid Antibodies and Anti-Domain 1 of Beta 2 Glycoprotein 1 Antibodies are Associated with Livedo Reticularis and Heart Valve Disease in Antiphospholipid Syndrome. Adv. Clin. Exp. Med. 2014, 23, 729–733. [Google Scholar] [CrossRef]
- Djokovic, A.; Stojanovich, L.; Kontic, M.; Stanisavljevic, N.; Radovanovic, S.; Marisavljevic, D. Association between cardiac manifestations and antiphospholipid antibody type and level in a cohort of Serbian patients with primary and secondary antiphospholipid syndrome. Isr. Med. Assoc. J. 2014, 16, 162–167. [Google Scholar]
- Vivero, F.; Gonzalez-Echavarri, C.; Ruiz-Estevez, B.; Maderuelo, I.; Ruiz-Irastorza, G. Prevalence and predictors of valvular heart disease in patients with systemic lupus erythematosus. Autoimmun. Rev. 2016, 15, 1134–1140. [Google Scholar] [CrossRef]
- Ruiz, D.; Oates, J.C.; Kamen, D.L. Antiphospholipid Antibodies and Heart Valve Disease in Systemic Lupus Erythematosus. Am. J. Med. Sci. 2018, 355, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Zuily, S.; Regnault, V.; Selton-Suty, C.; Eschwège, V.; Bruntz, J.F.; Bode-Dotto, E.; De Maistre, E.; Dotto, P.; Perret-Guillaume, C.; Lecompte, T.; et al. Increased risk for heart valve disease associated with antiphospholipid antibodies in patients with systemic lupus erythematosus: Meta-analysis of echocardiographic studies. Circulation 2011, 124, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Espínola-Zavaleta, N.; Vargas-Barrón, J.; Colmenares-Galvis, T.; Cruz-Cruz, F.; Romero-Cárdenas, A.; Keirns, C.; Amigo, M.-C. Echocardiographic evaluation of patients with primary antiphospholipid syndrome. Am. Heart J. 1999, 137, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Zavaleta, N.E.; Montes, R.M.; Soto, M.E.; Vanzzini, N.A.; Amigo, M.C. Primary antiphospholipid syndrome: A 5-year transesophageal echocardiographic followup study. J. Rheumatol. 2004, 31, 2402–2407. [Google Scholar]
- Turiel, M.; Sarzi-Puttini, P.; Peretti, R.; Bonizzato, S.; Muzzupappa, S.; Atzeni, F.; Rossi, E.; Doria, A. Five-year follow-up by transesophageal echocardiographic studies in primary antiphospholipid syndrome. Am. J. Cardiol. 2005, 96, 574–579. [Google Scholar] [CrossRef]
- Kampolis, C.; Tektonidou, M.; Moyssakis, I.; Tzelepis, G.E.; Moutsopoulos, H.; Vlachoyiannopoulos, P.G. Evolution of cardiac dysfunction in patients with antiphospholipid antibodies and/or antiphospholipid syndrome: A 10-year follow-up study. Semin. Arthritis Rheum. 2014, 43, 558–565. [Google Scholar] [CrossRef]
- Espinosa, G.; Cervera, R. Current treatment of antiphospholipid syndrome: Lights and shadows. Nat. Rev. Rheumatol. 2015, 11, 586–596. [Google Scholar] [CrossRef]
- Xourgia, E.; Tektonidou, M.G. Management of Non-criteria Manifestations in Antiphospholipid Syndrome. Curr. Rheumatol. Rep. 2020, 22, 51. [Google Scholar] [CrossRef]
- Andrade, D.; Tektonidou, M. Emerging Therapies in Antiphospholipid Syndrome. Curr. Rheumatol. Rep. 2016, 18, 22. [Google Scholar] [CrossRef]
- Sevim, E.; Willis, R.; Erkan, D. Is there a role for immunosuppression in antiphospholipid syndrome? Hematol. Am. Soc. Hematol. Educ. Program 2019, 2019, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Miyakis, S.; Lockshin, M.D.; Atsumi, T.; Branch, D.W.; Brey, R.L.; Cervera, R.; Derksen, R.H.; DE Groot, P.G.; Koike, T.; Meroni, P.L.; et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J. Thromb. Haemost. 2006, 4, 295–306. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino Sr, R.B.; Vasan, R.S.; Pencina, M.J.; Wolf, P.A.; Cobain, M.; Massaro, J.M.; Kannel, W.B. General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation 2008, 117, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Devreese, K.M.J.; de Groot, P.G.; de Laat, B.; Erkan, D.; Favaloro, E.J.; Mackie, I.; Martinuzzo, M.; Ortel, T.L.; Pengo, V.; Rand, J.H.; et al. Guidance from the Scientific and Standardization Committee for lupus anticoagulant/antiphospholipid antibodies of the International Society on Thrombosis and Haemostasis: Update of the guidelines for lupus anticoagulant detection and interpretation. J. Thromb. Haemost. 2020, 18, 2828–2839. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.; Mackie, I.J.; Devreese, K.M.J.; International Society for Thrombosis and Haemostasis Scientific and Standardization Committee for Lupus Anticoagulant/Antiphospholipid Antibodies. Clinical and laboratory practice for lupus anticoagulant testing: An International Society of Thrombosis and Haemostasis Scientific and Standardization Committee survey. J. Thromb. Haemost. 2019, 17, 1715–1732. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, W.A.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Kraft, C.D.; Levine, R.A.; Nihoyannopoulos, P.; Otto, C.M.; Quinones, M.A.; Rakowski, H.; et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J. Am. Soc. Echocardiogr. 2003, 16, 777–802. [Google Scholar] [CrossRef]
- Falk, V.; Baumgartner, H.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Muñoz, D.R.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. J. Cardiothorac. Surg. 2017, 52, 616–664. [Google Scholar] [CrossRef]
- Vianna, J.L.; Khamashta, M.A.; Ordi-Ros, J.; Font, J.; Cervera, R.; Lopez-Soto, A.; Tolosa, C.; Franz, J.; Selva-O’Callaghan, A.; Ingelmo, M.; et al. Comparison of the primary and secondary antiphospholipid syndrome: A European Multicenter Study of 114 patients. Am. J. Med. 1994, 96, 3–9. [Google Scholar] [CrossRef]
- Barbut, D.; Borer, J.S.; Wallerson, D.; Ameisen, O.; Lockshin, M. Anticardiolipin antibody and stroke: Possible relation of valvular heart disease and embolic events. Cardiology 1991, 79, 99–109. [Google Scholar] [CrossRef]
- Kalashnikova, L.A.; Nasonov, E.L.; Borisenko, V.V.; Usman, V.B.; Prudnikova, L.Z.; Kovaljov, V.U.; Kushekbaeva, A.F. Sneddon’s syndrome: Cardiac pathology and antiphospholipid antibodies. Clin. Exp. Rheumatol. 1991, 9, 357–361. [Google Scholar]
- Font, J.; Carvera, R.; Parè, C.; Lòpez-Soto, A.; Pallarès, L.; Azqueta, M.; Khamashta, M.A. Non-infective verrucous endocarditis in a patient with ‘primary’ antiphospholipid syndrome. Br. J. Rheumatol. 1991, 30, 305–307. [Google Scholar] [CrossRef] [PubMed]
- Brenner, B.; Blumenfeld, Z.; Markiewicz, W.; Reisner, S.A. Cardiac involvement in patients with primary antiphospholipid syndrome. J. Am. Coll. Cardiol. 1991, 18, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Cervera, R.; Khamashta, M.A.; Font, J.; Reyes, P.A.; Vianna, J.L.; López-Soto, A.; Amigo, M.-C.; Asherson, R.A.; Azqueta, M.; Paré, C.; et al. High prevalence of significant heart valve lesions in patients with the ‘primary’ antiphospholipid syndrome. Lupus 1991, 1, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Galve, E.; Ordi, J.; Barquinero, J.; Evangelista, A.; Vilardell, M.; Soler-Soler, J. Valvular heart disease in the primary antiphospholipid syndrome. Ann. Intern. Med. 1992, 116, 293–298. [Google Scholar] [CrossRef]
- Khamashta, M.A.; Cervera, R.; Asherson, R.A.; Font, J.; Gil, A.; Coltart, D.J.; Vázquez, J.J.; Paré, C.; Ingelmo, M.; Oliver, J.; et al. Association of antibodies against phospholipids with heart valve disease in systemic lupus erythematosus. Lancet 1990, 335, 1541–1544. [Google Scholar] [CrossRef] [PubMed]
- Nihoyannopoulos, P.; Gomez, P.M.; Joshi, J.; Loizou, S.; Walport, M.J.; Oakley, C.M. Cardiac abnormalities in systemic lupus erythematosus. Association with raised anticardiolipin antibodies. Circulation 1990, 82, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Cervera, R.; Font, J.; Paré, C.; Azqueta, M.; Pérez-Villa, F.; López-Soto, A.; Ingelmo, M. Cardiac disease in systemic lupus erythematosus: Prospective study of 70 patients. Ann. Rheum. Dis. 1992, 51, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Jouhikainen, T.; Pohjola-Sintonen, S.; Stephansson, E. Lupus anticoagulant and cardiac manifestations in systemic lupus erythematosus. Lupus 1994, 3, 167–172. [Google Scholar] [CrossRef]
- Leung, W.H.; Wong, K.L.; Lau, C.P.; Wong, C.K.; Liu, H.W. Association between antiphospholipid antibodies and cardiac abnormalities in patients with systemic lupus erythematosus. Am. J. Med. 1990, 89, 411–419. [Google Scholar] [CrossRef]
- Gleason, C.B.; Stoddard, M.F.; Wagner, S.G.; A Longaker, R.; Pierangeli, S.; Harris, E. A comparison of cardiac valvular involvement in the primary antiphospholipid syndrome versus anticardiolipin-negative systemic lupus erythematosus. Am. Heart J. 1993, 125, 1123–1129. [Google Scholar] [CrossRef]
- Cervera, R.; Tektonidou, M.; Espinosa, G.; Cabral, A.R.; González, E.B.; Erkan, D.; Vadya, S.; E Adrogué, H.; Solomon, M.; Zandman-Goddard, G.; et al. Task Force on Catastrophic Antiphospholipid Syndrome (APS) and Non-criteria APS Manifestations (I): Catastrophic APS, APS nephropathy and heart valve lesions. Lupus 2011, 20, 165–173. [Google Scholar] [CrossRef]
- Granowicz, E.; Chung, K. Improvement of Cardiac Vegetations in Antiphospholipid Syndrome with Enoxaparin and Corticosteroids after Rivaroxaban Failure. Case Rep. Hematol. 2018, 2018, 8097539. [Google Scholar] [CrossRef] [PubMed]
- Yuriditsky, E.; Torres, J.; Izmirly, P.M.; Belmont, H.M. Resolution of large aortic valve vegetations in antiphospholipid syndrome treated with therapeutic anticoagulation: A report of two cases and literature review. Lupus 2018, 27, 2269–2273. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.M.; Shaikh, N.A.; Pradeep, R. An extraordinary case of recurrent stroke, disseminated thrombosis and endocarditis. BMJ Case Rep. 2018, 2018, bcr2018224172. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, K.; Negi, P.; Merwaha, R.; Sharma, M. Isolated tricuspid valve Libman-Sacks endocarditis in a patient with antiphospholipid antibody syndrome. BMJ Case Rep. 2017, 2017, bcr2017219217. [Google Scholar] [CrossRef] [PubMed]
- Arif, R.; Farag, M.; Seppelt, P.; Beller, C.J.; Ruhparwar, A.; Karck, M.; Kallenbach, K. Patients with systemic lupus erythematosus and antiphospholipid syndrome undergoing cardiac valve surgery. J. Heart Valve Dis. 2015, 24, 228–235. [Google Scholar]
- Bai, Z.; Hou, J.; Ren, W.; Guo, Y. Diagnosis and surgical treatment for isolated tricuspid Libman-Sacks endocarditis: A rare case report and literatures review. J. Cardiothorac. Surg. 2015, 10, 93. [Google Scholar] [CrossRef]
- Hachiya, K.; Wakami, K.; Tani, T.; Yoshida, A.; Suzuki, S.; Suda, H.; Ohte, N. Double-valve replacement for mitral and aortic regurgitation in a Patient with Libman-Sacks endocarditis. Intern. Med. 2014, 53, 1769–1773. [Google Scholar] [CrossRef]
- Correia, M.; Silva, A.; Mota-Garcia, R.; Mendes, S.; Martins, L.; Santos, M. An unusual case of pacemaker endocarditis in a patient with antiphospholipid syndrome. Rev. Port. Cardiol. 2012, 31, 309–312. [Google Scholar] [CrossRef]
- Teunisse, C.C.; Kalsbeek, A.J.; De Vries, S.T.; Huisman, S.J.; E Boers, J.; Breeman, A.; Beukhof, J.R. Reversible cardiac valvular disease in catastrophic antiphospholipid syndrome. Neth. J. Med. 2010, 68, 215–220. [Google Scholar]
- Bhimani, A.A.; Hoit, B.D. Extensive nonbacterial thrombotic endocarditis isolated to the tricuspid valve in primary antiphospholipid syndrome. J. Am. Soc. Echocardiogr. 2010, 23, 107.e5–107.e6. [Google Scholar] [CrossRef]
- Salzberg, S.P.; Nemirovsky, D.; Goldman, M.E.; Adams, D.H. Aortic valve vegetation without endocarditis. Ann. Thorac. Surg. 2009, 88, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Bridges, J.S.; Kumar, K.; Raphael, J.A.; Acharjee, S.; Welty, F.K. Complete resolution of a mitral valve vegetation with anticoagulation in seronegative antiphospholipid syndrome. Clin. Rheumatol. 2008, 27, 1577–1579. [Google Scholar] [CrossRef] [PubMed]
- Lønnebakken, M.T.; Gerdts, E. Libman-Sacks endocarditis and cerebral embolization in antiphospholipid syndrome. Eur. J. Echocardiogr. 2008, 9, 192–193. [Google Scholar] [CrossRef]
- Sasahashi, N.; Harada, H.; Saji, Y.; Marui, A.; Nishina, T.; Komeda, M. Aortic valve replacement for aortic regurgitation in a patient with antiphospholipid antibody syndrome. Gen. Thorac. Cardiovasc. Surg. 2007, 55, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Falode, O.; Hunt, I.; Chambers, J.; Blauth, C. Large tricuspid mass in primary antiphospholipid syndrome. Ann. Thorac. Surg. 2006, 82, 1538. [Google Scholar] [CrossRef] [PubMed]
- Letsas, K.P.; Filippatos, G.S.; Kounas, S.P.; Pappas, L.K.; Kardaras, F.; Gavrielatos, G. Primary antiphospholipid syndrome and factor V Leiden mutation in a young patient with non-bacterial thrombotic endocarditis and transient ischemic stroke. Thromb. Haemost. 2005, 94, 1331–1332. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Juanatey, C.; Gonzalez-Gay, M.A. Libman-sacks endocarditis and primary antiphospholipid syndrome. J. Heart Valve Dis. 2005, 14, 700–702. [Google Scholar]
- Kolitz, T.; Shiber, S.; Sharabi, I.; Winder, A.; Zandman-Goddard, G. Cardiac Manifestations of Antiphospholipid Syndrome With Focus on Its Primary Form. Front. Immunol. 2019, 10, 941. [Google Scholar] [CrossRef]
- Ziporen, L.; Goldberg, I.; Arad, M.; Hojnik, M.; Ordi-Ros, J.; Afek, A.; Blank, M.; Sandbank, Y.; Vilardell-Tarres, M.; de Torres, I.; et al. Libman-Sacks endocarditis in the antiphospholipid syndrome: Immunopathologic findings in deformed heart valves. Lupus 1996, 5, 196–205. [Google Scholar] [CrossRef]
- Amigo, M.C.; García-Torres, R. Morphology of vascular, renal, and heart lesions in the antiphospholipid syndrome: Relationship to pathogenesis. Curr. Rheumatol. Rep. 2000, 2, 262–270. [Google Scholar] [CrossRef]
- Gartshteyn, Y.; Bhave, N.; Joseph, M.S.; Askanase, A.; Bernstein, E.J. Inflammatory and thrombotic valvulopathies in autoimmune disease. Heart 2022, 109, 583–588. [Google Scholar] [CrossRef]
- Hussain, K.; Gauto-Mariotti, E.; Cattoni, H.M.; Arif, A.W.; Richardson, C.; Manadan, A.; Yadav, N. A Meta-analysis and Systematic Review of Valvular Heart Disease in Systemic Lupus Erythematosus and Its Association with Antiphospholipid Antibodies. J. Clin. Rheumatol. 2021, 27, e525–e532. [Google Scholar] [CrossRef] [PubMed]
- Aykan, A.Ç.; Gökdeniz, T.; Kalçık, M.; Astarcıoğlu, M.A.; Gündüz, S.; Karakoyun, S.; Gürsoy, M.O.; Oğuz, A.E.; Ertürk, E.; Çakal, B.; et al. Role of anticardiolipin antibodies in the pathogenesis of prosthetic valve thrombosis: An observational study. Herz 2015, 40, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Pardos-Gea, J.; Cortés-Hernández, J.; Castro-Marrero, J.; Balada, E.; Ordi-Ros, J. Autoantibodies to types I and IV collagen and heart valve disease in systemic lupus erythematosus/antiphospholipid syndrome. Clin. Rheumatol. 2017, 36, 1401–1406. [Google Scholar] [CrossRef] [PubMed]
- Tufano, A.; Lembo, M.; Di Minno, M.N.; Nardo, A.; Esposito, R.; Santoro, C.; Buonauro, A.; Cerbone, A.M.; Di Minno, G.; Galderisi, M. Left ventricular diastolic abnormalities other than valvular heart disease in antiphospholipid syndrome: An echocardiographic study. Int. J. Cardiol. 2018, 271, 366–370. [Google Scholar] [CrossRef]
- Erkan, D.; Yazici, Y.; Peterson, M.G.; Sammaritano, L.; Lockshin, M.D. A cross-sectional study of clinical thrombotic risk factors and preventive treatments in antiphospholipid syndrome. Rheumatology 2002, 41, 924–929. [Google Scholar] [CrossRef]
- Belizna, C.C.; Richard, V.; Primard, E.; Kerleau, J.M.; Cailleux, N.; Louvel, J.P.; Marie, I.; Hamidou, M.; Thuillez, C.; Lévesque, H. Early atheroma in primary and secondary antiphospholipid syndrome: An intrinsic finding. Semin. Arthritis Rheum. 2008, 37, 373–380. [Google Scholar] [CrossRef]
- Lewandowska, E.; Wierzba-Bobrowicz, T.; Wagner, T.; Bogusławska, R.; Rudnicka, A.; Leszczyńska, A.; Pasennik, E.; Lechowicz, W.; Stepień, T.; Kuran, W. Sneddon’s syndrome as a disorder of small arteries with endothelial cells proliferation: Ultrastructural and neuroimaging study. Folia Neuropathol. 2005, 43, 345–354. [Google Scholar]
- Christodoulou, C.; Sangle, S.; D’Cruz, D.P. Vasculopathy and arterial stenotic lesions in the antiphospholipid syndrome. Rheumatology 2007, 46, 907–910. [Google Scholar] [CrossRef]
- Der, H.; Kerekes, G.; Veres, K.; Szodoray, P.; Toth, J.; Lakos, G.; Szegedi, G.; Soltesz, P. Impaired endothelial function and increased carotid intima-media thickness in association with elevated von Willebrand antigen level in primary antiphospholipid syndrome. Lupus 2007, 16, 497–503. [Google Scholar] [CrossRef] [PubMed]

| With Valve Involvement (n = 72) N (%) | Without Valve Involvement (n = 72) N (%) | p | |
|---|---|---|---|
| Sex (F/M) | 60/12 (83/17) | 46/26 (64/36) | 0.013 |
| Age at APS diagnosis (years) | 41.2 ± 15.1 | 41 ± 13.9 | 0.699 |
| Follow-up (months) | 115.3 ± 57.6 | 104.6 ± 61.2 | 0.213 |
| TTE made at time of APS diagnosis | 30 (42) | 23 (32) | 0.275 |
| Primary APS | 48 (67) | 57 (80) | 0.133 |
| SLE associated APS | 22 (30) | 14 (19) | 0.177 |
| Cardiovascular risk factors a | |||
| Current smoking | 21/68 (31) | 14/69 (20) | 0.112 |
| Ever smoking | 16/68 (31) | 22/69 (32) | 0.260 |
| Arterial hypertension | 34 (47) | 20 (28) | 0.025 |
| Dyslipidemia | 14 (19) | 17 (24) | 0.686 |
| Diabetes mellitus | 5 (7) | 3 (4) | 0.719 |
| Hormonal contraceptives use | 7/60 (12) | 8/46 (17) | 0.196 |
| APS-related clinical manifestations | |||
| Thrombosis | 63 (88) | 60 (83) | 0.638 |
| Arterial | 38 (53) | 24 (33) | 0.028 |
| Venous | 23 (32) | 36 (50) | 0.042 |
| Both | 3 (4) | 4 (7) | 1.000 |
| Peripheral DVT | 17 (24) | 27 (37) | 1.000 |
| Pulmonary embolism | 6 (8) | 15 (21) | 0.057 |
| Ischemic heart disease | 4 (6) | 3 (4) | 1.000 |
| Stroke | 27 (38) | 15 (21) | 0.043 |
| Transient ischemic attack | 3 (4) | 4 (6) | 1.0000 |
| Cerebral silent ischemia | 4 (6) | 3 (4) | 0.439 |
| Epilepsy | 6 (8) | 3 (4) | 0.494 |
| Migraine | 10 (14) | 8 (11) | 0.802 |
| Multiinfarct dementia | 3 (4) | 2 (3) | 1.000 |
| Obstetric morbidity b | 37/60 (62) | 21/46 (46) | 0.681 |
| Livedo reticularis | 11 (15) | 2 (3) | 0.017 |
| Cutaneous ulcers | 1 (1) | 3 (4) | 0.620 |
| Thrombocytopenia c | 22 (31) | 15 (21) | 0.252 |
| Hemolytic anemia | 3 (4) | 4 (6) | 1.000 |
| Renal thrombotic microangiopathy | 5 (7) | 2 (3) | 0.719 |
| Optic neuritis | 4 (6) | 3 (4) | 1.000 |
| Retinal veno-occlusive disease | 1 (1) | 1 (1) | 1.000 |
| Central retinal artery thrombosis | 2 (3) | 0 (0) | 0.497 |
| Central retinal vein thrombosis | 1 (4) | 1 (1) | 1.000 |
| Thrombotic relapse d | 22/52 (42) | 11/46 (25) | 0.858 |
| Hemorrhagic events d | 11/52 (21) | 9/46 (20) | 0.810 |
| Death | 9 (12) | 1 (1) | 0.017 |
| With Valve Involvement (n = 72) N (%) | Without Valve Involvement (n = 72) N (%) | p | |
|---|---|---|---|
| LAC | 60 (83) | 47 (65) | 0.021 |
| aCL | 56 (78) | 56 (78) | 1.000 |
| IgG aCL | 47 (65) | 44/57 (77) | 1.000 |
| IgM aCL | 26 (36) | 21/58 (36) | 0.348 |
| aβ2GPI | 14/40 (35) | 12/43 (28) | 0.473 |
| IgG aβ2GPI | 10/40 (25) | 5/13 (38) | 1.000 |
| IgM aβ2GPI | 10/40 (25) | 8/13 (61) | 0.255 |
| aPL profile at APS diagnosis | |||
| Isolated LAC | 14 (19) | 13 (18) | 0.414 |
| Isolated aCL | 12 (17) | 26 (36) | 0.013 |
| Isolated aβ2GPI | 1/40 (2) | 1/43 (2) | 1.000 |
| Double positivity | 44 (61) | 28 (39) | 0.045 |
| Triple positivity | 1 (1) | 2 (3) | 1.000 |
| ANA | 43 (60) | 58 (81) | 0.006 |
| Anti-dsDNA abs | 20 (28) | 23 (32) | 0.716 |
| Anti-Ro abs | 5 (7) | 8 (11) | 0.400 |
| Anti-La abs | 3 (4) | 3 (4) | 1.000 |
| Anti-Sm abs | 2 (3) | 1 (1) | 1.000 |
| Anti-U1-RNP abs | 4 (6) | 5 (7) | 0.745 |
| Low levels of C3 | 13 (18) | 15 (21) | 0.834 |
| Low levels of C4 | 10 (14) | 21 (29) | 0.042 |
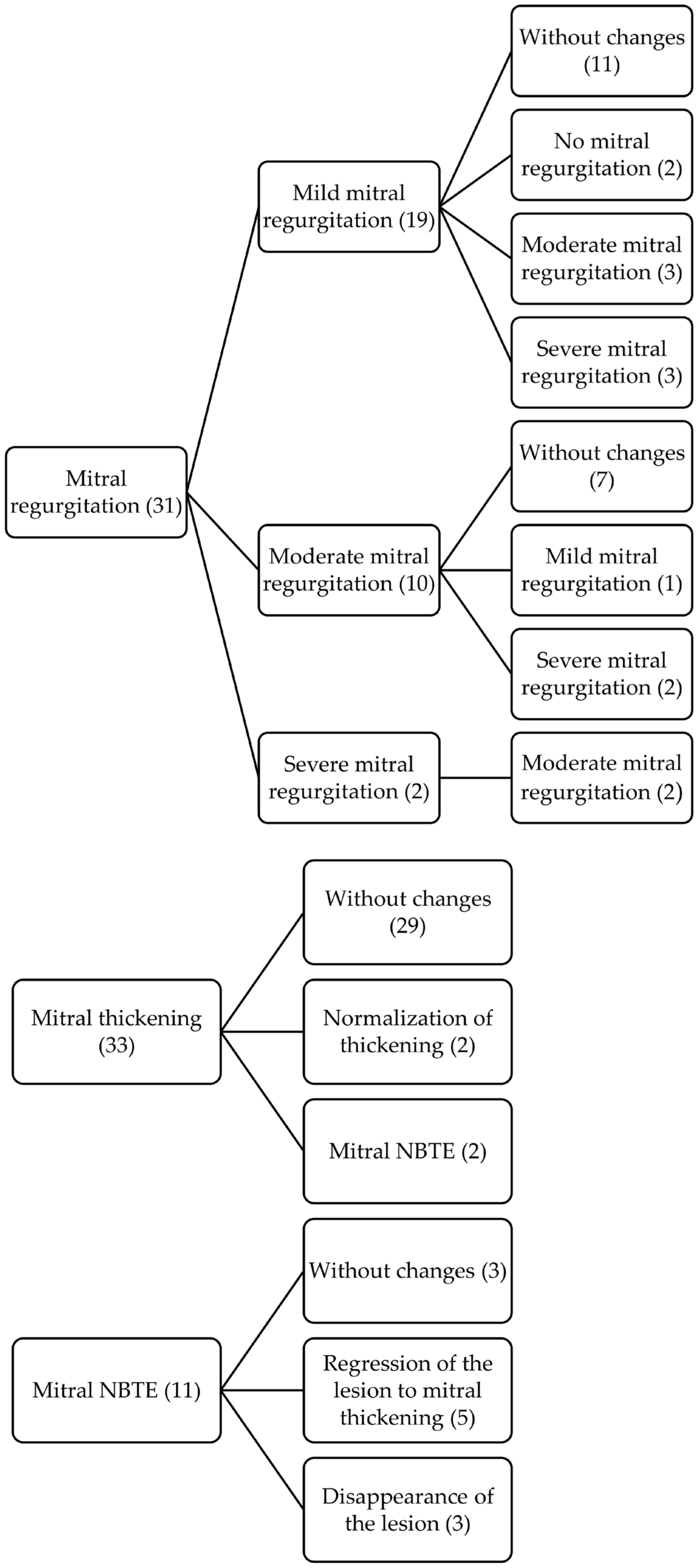
| Heart Valve Involvement | First Echocardiography (n = 72) N (%) |
|---|---|
| Mitral valve | |
| Mitral thickening | 52 (72) |
| Mitral regurgitation | 49 (68) |
| Mild | 33 (67) |
| Moderate | 10 (21) |
| Severe | 6 (12) |
| Mitral stenosis | 1 (1) |
| Non-infectious thrombotic mitral endocarditis * | 21 (29) |
| Aortic valve | |
| Aortic thickening | 18 (25) |
| Aortic regurgitatiom | 16 (22) |
| Mild | 9 (56) |
| Moderate | 6 (38) |
| Severe | 1 (6) |
| Aortic stenosis | 2 (3) |
| Non-infectious thrombotic aortic endocarditis * | 5 (7) |
| Tricuspid valve | |
| Tricuspid thickening | 1 (1) |
| Tricuspid regurgitation | 29 (40) |
| Mild | 29 (100) |
| Moderate | 0 |
| Severe | 0 |
| Significative Valve Involvement N (%) (n = 36) | Without or Mild Valve Involvement N (%) (n = 108) | p | |
|---|---|---|---|
| Cardiovascular risk factors | |||
| Arterial hypertension | 20 (56) | 34 (31) | 0.016 |
| APS-related clinical manifestations | |||
| Thrombosis | 32 (89) | 91 (84) | 0.595 |
| Arterial | 26 (75) | 36 (32) | <0.001 |
| Venous | 4 (11) | 55 (51) | <0.001 |
| Peripheral DVT | 4 (11) | 40 (37) | 0.003 |
| Pulmonary embolism | 1 (3) | 20 (18) | 0.026 |
| Stroke | 21 (61) | 21 (18) | <0.001 |
| Livedo reticularis | 7 (22) | 6 (5) | 0.019 |
| Laboratory features | |||
| LAC | 33 (92) | 74 (68) | 0.007 |
| aPL profile at APS diagnosis | |||
| Isolated LAC | 5 (11) | 22 (20) | 0.137 |
| Isolated aCL | 4 (11) | 34 (31) | 0.346 |
| Isolated aβ2GPI | 0 (0) | 2 (2) | 1.000 |
| Double positivity | 27 (75) | 49 (44) | 0.032 |
| Triple positivity | 1 (3) | 2 (2) | 0.486 |
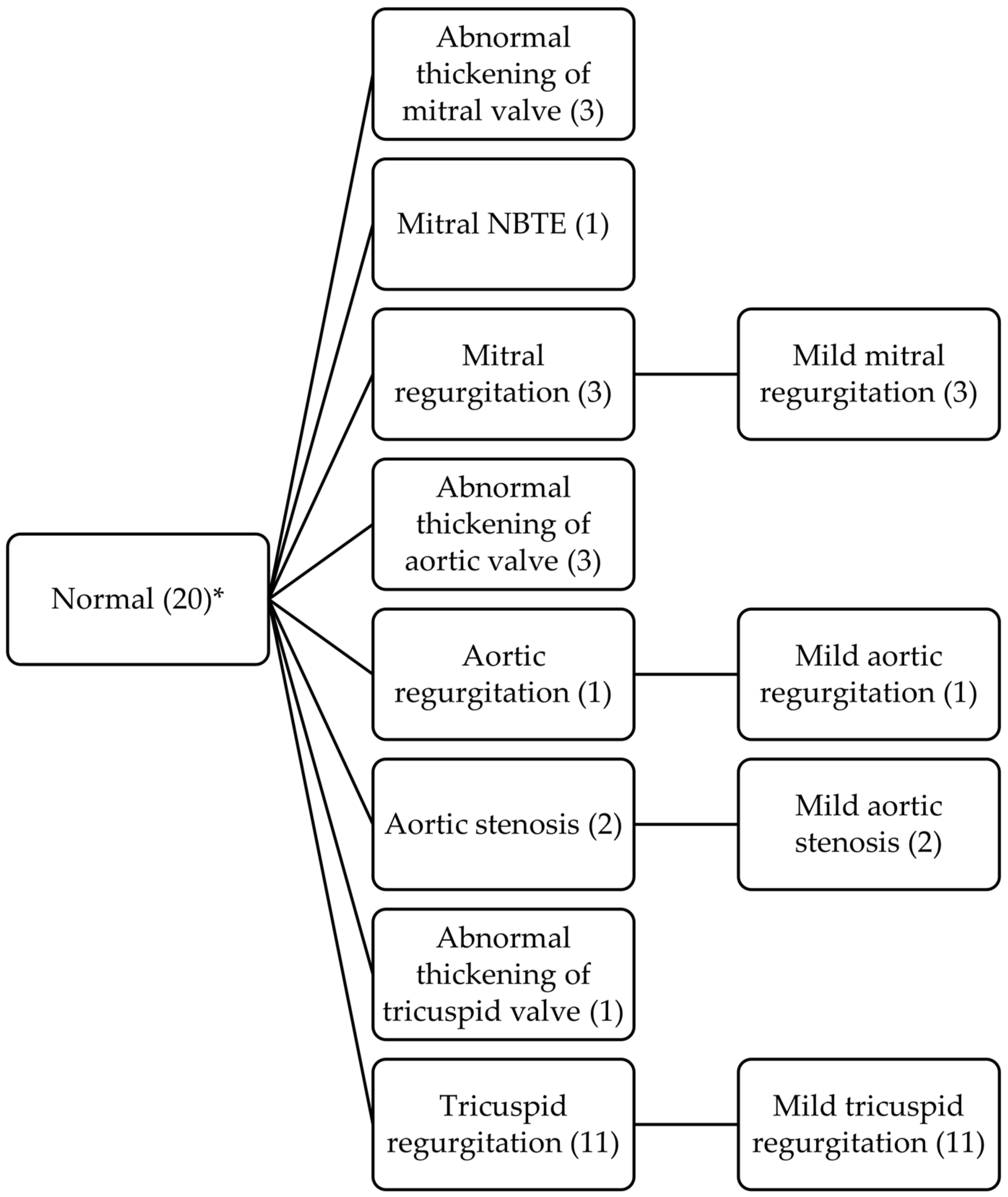
| Initial Echocardiogram | Patients (n = 40) | Follow-Up Echocardiogram | Patients (n = 40) | p |
|---|---|---|---|---|
| Mitral valve | Mitral valve | |||
| Thickening | 33 (82) | Thickening | 32 (80) | 0.711 |
| Regurgitation | 31 (77) | Regurgitation | 32 (80) | 0.628 |
| Mild | 19/31 (61) | Mild | 15/32 (47) | |
| Moderate | 10/31 (32) | Moderate | 12/32 (37) | |
| Severe | 2/31 (6) | Severe | 5/32 (16) | |
| Aortic valve | Aortic valve | |||
| Thickening | 7 (17) | Thickening | 8 (20) | 0.660 |
| Regurgitation | 9 (22) | Regurgitation | 10 (25) | 0.660 |
| Mild | 5/9 (56) | Mild | 9/10 (90) | |
| Moderate | 4/9 (44) | Moderate | 1/10 (10) | |
| Severe | 0/9 (0) | Severe | 0/10 (0) | |
| Stenosis | 1 (2) | Stenosis | 3 (7) | 0.323 |
| Tricuspid valve | Tricuspid valve | |||
| Thickening | 0 (0) | Thickening | 1 (2) | 0.323 |
| Regurgitation | 16 (40) | Regurgitation | 24 (62) | 0.031 |
| Mild | 16/16 (100) | Mild | 24/24 (100) | |
| Moderate | 0/16 (0) | Moderate | 0/24 (0) | |
| Severe | 0/16 (0) | Severe | 0/24 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pons, I.; Louro, J.; Sitges, M.; Vidal, B.; Cervera, R.; Espinosa, G. Heart Valve Involvement in Patients with Antiphospholipid Syndrome: A Long-Term Follow-Up Study of a Single Centre. J. Clin. Med. 2023, 12, 2996. https://doi.org/10.3390/jcm12082996
Pons I, Louro J, Sitges M, Vidal B, Cervera R, Espinosa G. Heart Valve Involvement in Patients with Antiphospholipid Syndrome: A Long-Term Follow-Up Study of a Single Centre. Journal of Clinical Medicine. 2023; 12(8):2996. https://doi.org/10.3390/jcm12082996
Chicago/Turabian StylePons, Isaac, Joana Louro, Marta Sitges, Bàrbara Vidal, Ricard Cervera, and Gerard Espinosa. 2023. "Heart Valve Involvement in Patients with Antiphospholipid Syndrome: A Long-Term Follow-Up Study of a Single Centre" Journal of Clinical Medicine 12, no. 8: 2996. https://doi.org/10.3390/jcm12082996
APA StylePons, I., Louro, J., Sitges, M., Vidal, B., Cervera, R., & Espinosa, G. (2023). Heart Valve Involvement in Patients with Antiphospholipid Syndrome: A Long-Term Follow-Up Study of a Single Centre. Journal of Clinical Medicine, 12(8), 2996. https://doi.org/10.3390/jcm12082996






