Impact of the Family and Household Environment on Pediatric Atopic Dermatitis in Japan
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Questionnaire and Outcomes
2.4. Statistical Analysis
3. Results
3.1. Study Population Diagnosed with AD
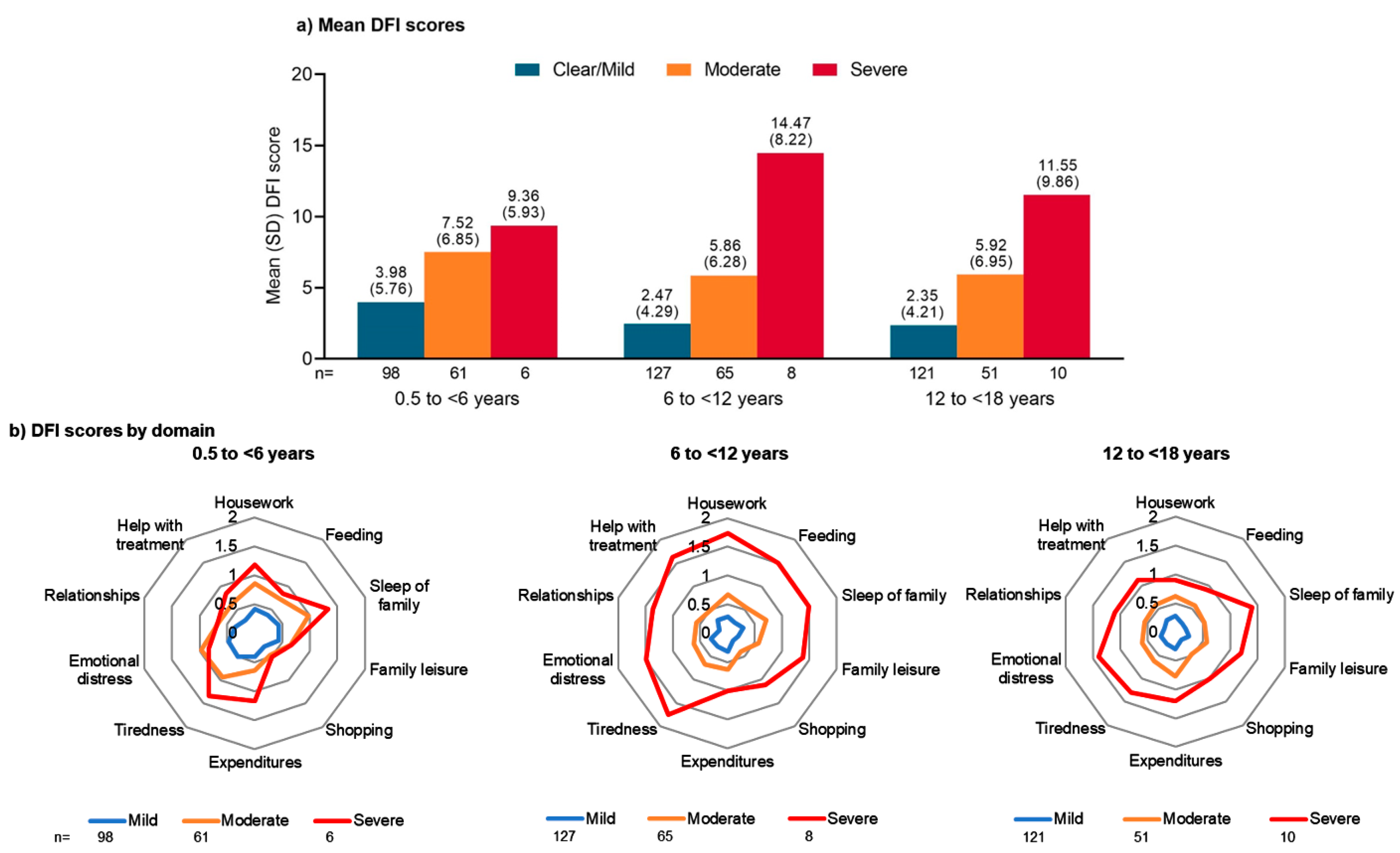
3.2. Impact on Family QoL
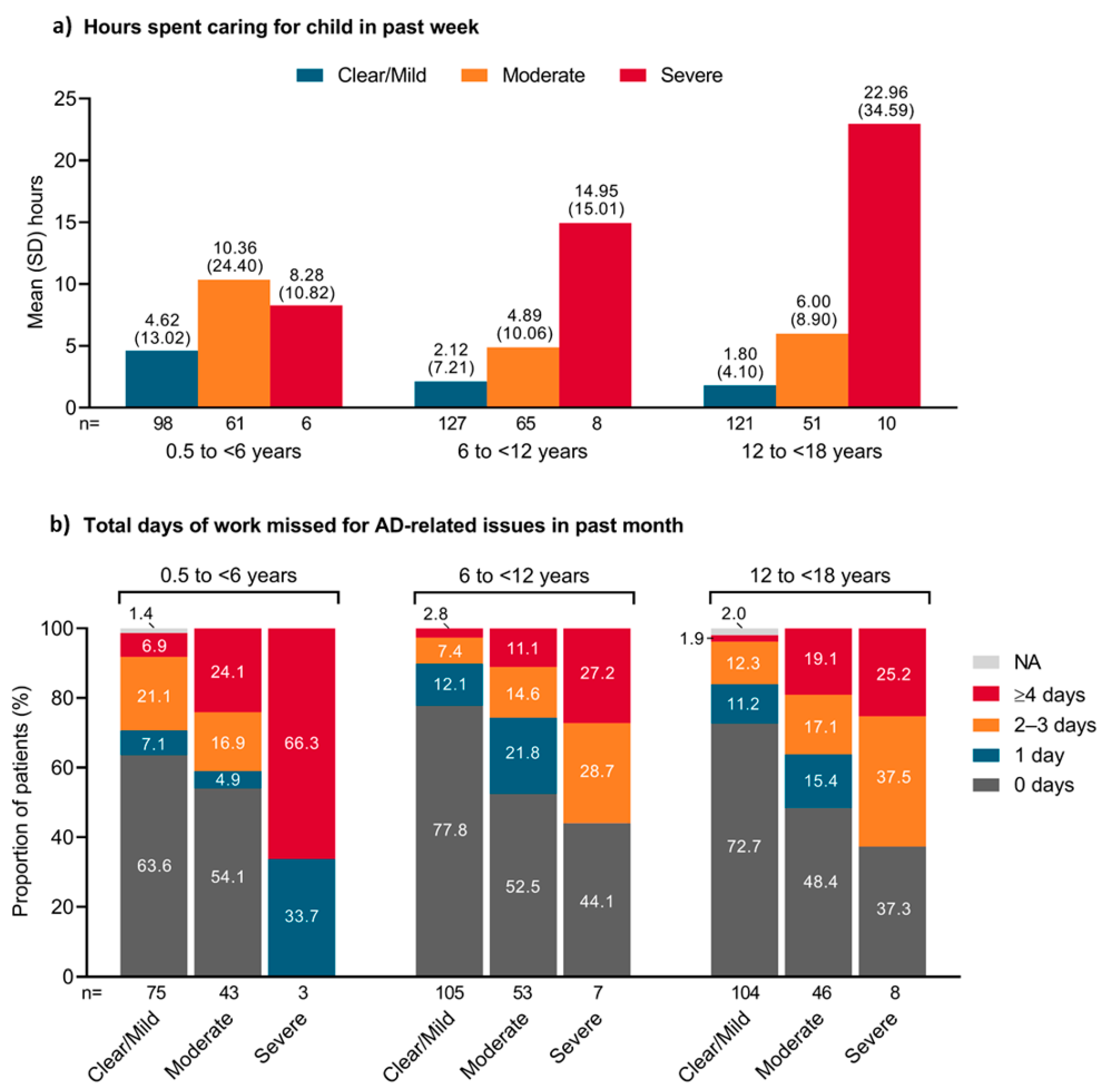
3.3. Impact on Parents’ Time
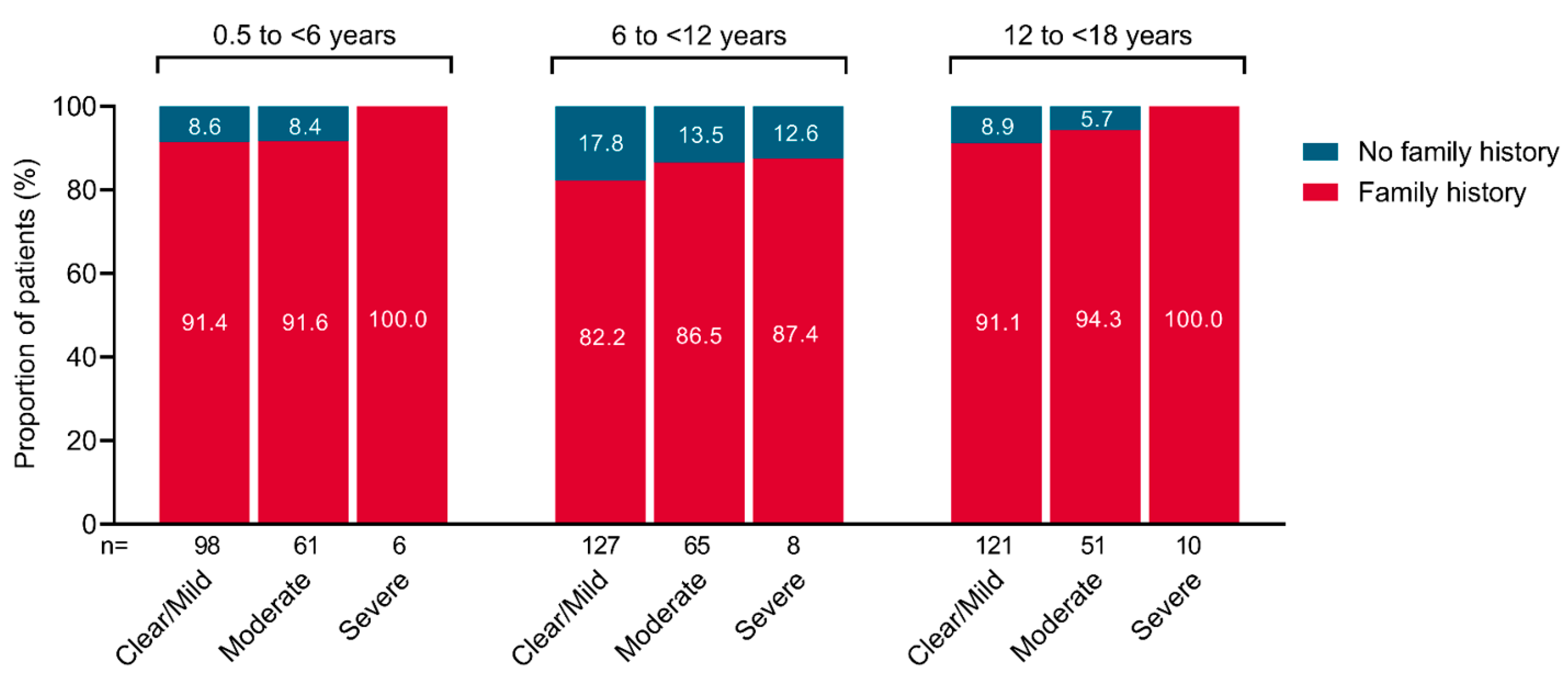
3.4. Family History of Allergic Conditions
3.5. Current Residency
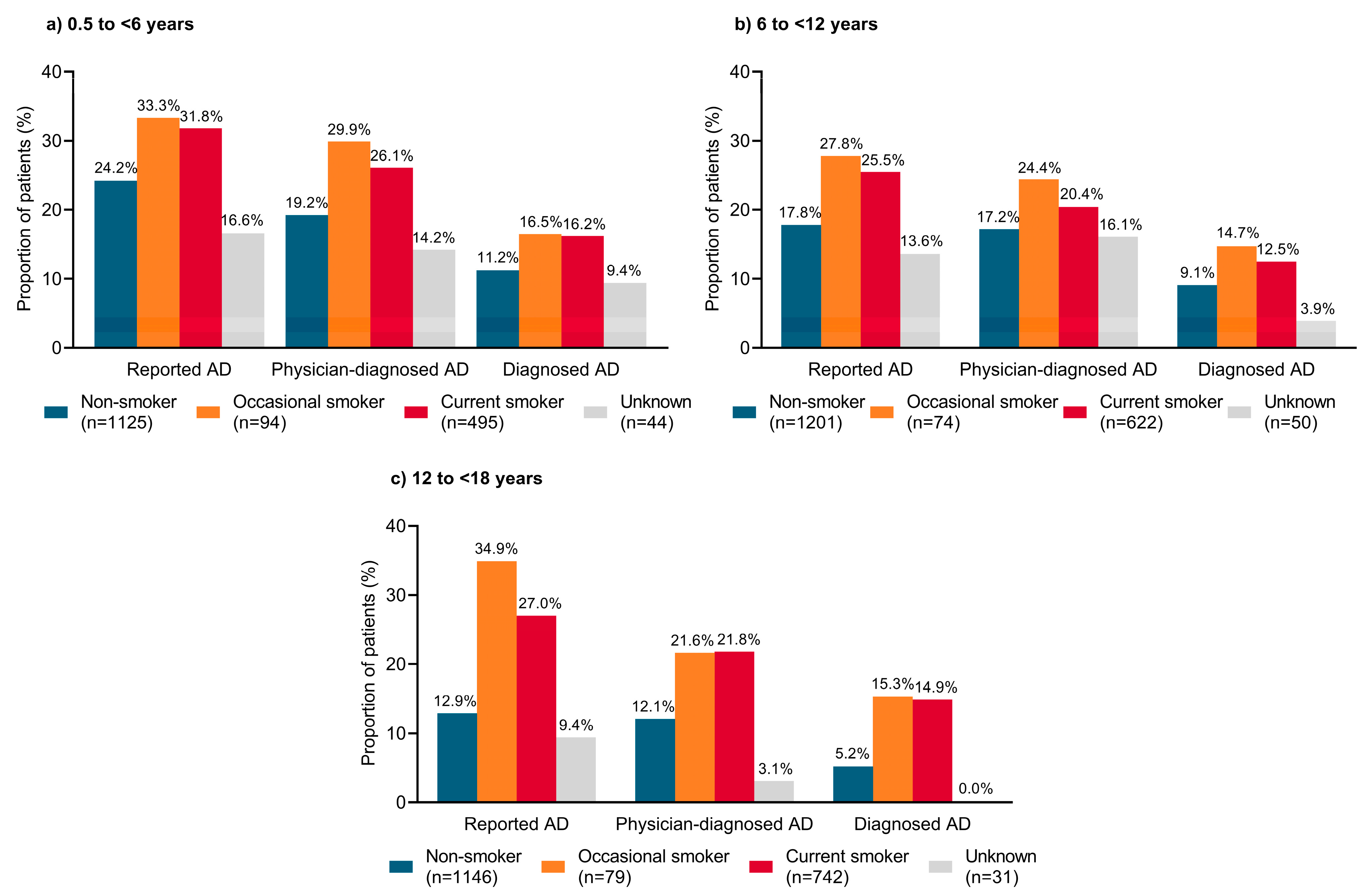
3.6. Second-Hand Smoke Exposure
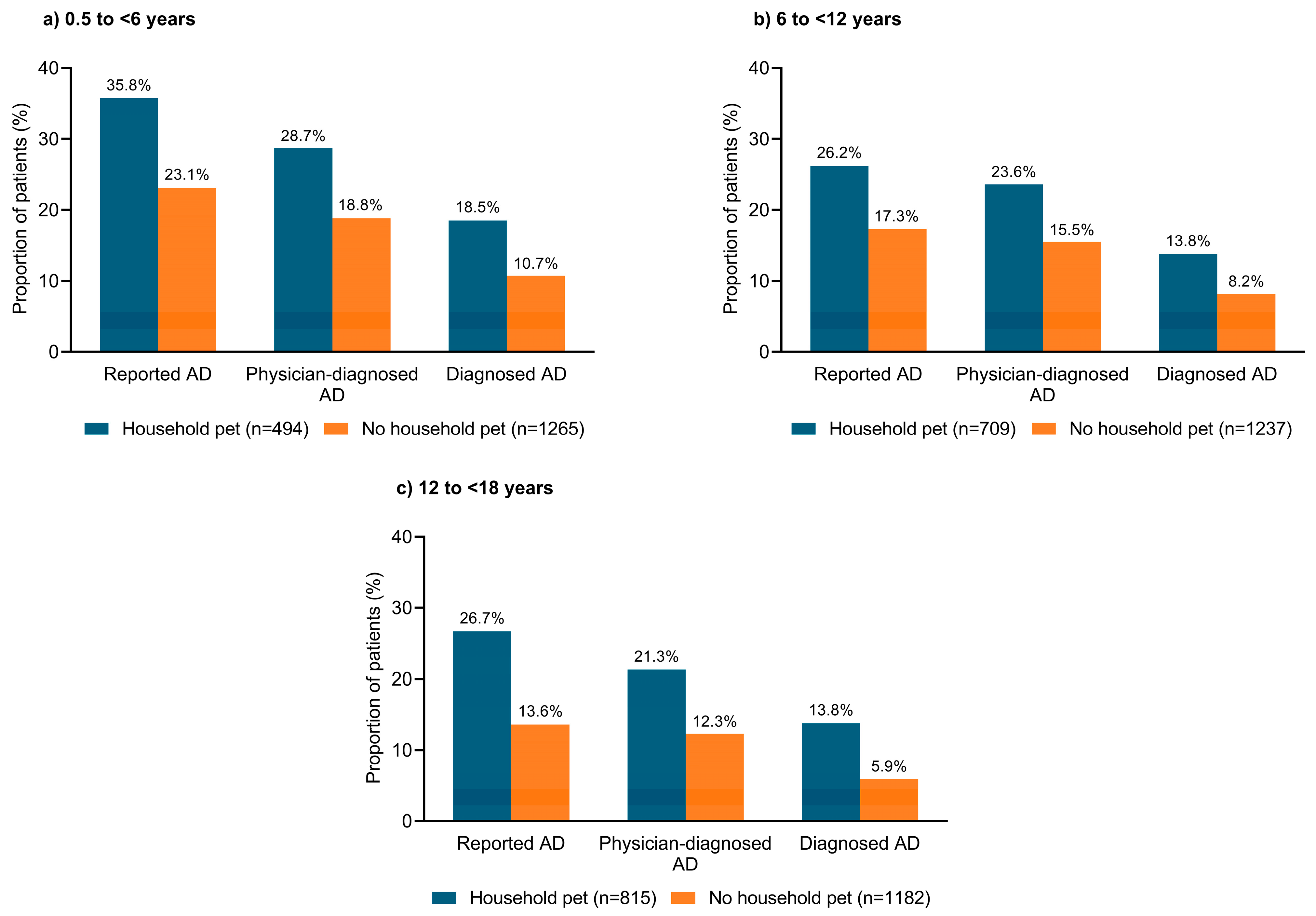
3.7. Household Pets
3.8. Parent Education and Employment Status
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Irvine, A.D.; Mina-Osorio, P. Disease trajectories in childhood atopic dermatitis: An update and practitioner’s guide. Br. J. Dermatol. 2019, 181, 895–906. [Google Scholar] [CrossRef]
- Lyons, J.J.; Milner, J.D.; Stone, K.D. Atopic dermatitis in children: Clinical features, pathophysiology, and treatment. Immunol. Allergy Clin. N. Am. 2015, 35, 161–183. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, M.; Fabbrocini, G.; Martora, F.; Genco, L.; Noto, M.; Patruno, C. Children atopic dermatitis: Diagnosis, mimics, overlaps, and therapeutic implication. Dermatol. Ther. 2022, 35, e15901. [Google Scholar] [CrossRef]
- Novak, N.; Bieber, T. Allergic and nonallergic forms of atopic diseases. J. Allergy Clin. Immunol. 2003, 112, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Celebi Sozener, Z.; Özbey Yücel, Ü.; Altiner, S.; Ozdel Oztürk, B.; Cerci, P.; Türk, M.; Gorgülü Akin, B.; Akdis, M.; Yilmaz, I.; Ozdemir, C.; et al. The external exposome and allergies: From the perspective of the epithelial barrier hypothesis. Front. Allergy 2022, 3, 887672. [Google Scholar] [CrossRef]
- Molina-García, M.; Granger, C.; Trullàs, C.; Puig, S. Exposome and skin: Part 1. Bibliometric analysis and review of the impact of exposome approaches on dermatology. Dermatol. Ther. 2022, 12, 345–359. [Google Scholar] [CrossRef]
- Stefanovic, N.; Flohr, C.; Irvine, A.D. The exposome in atopic dermatitis. Allergy 2020, 75, 63–74. [Google Scholar] [CrossRef]
- Stefanovic, N.; Irvine, A.D.; Flohr, C. The role of the environment and exposome in atopic dermatitis. Curr. Treat. Options Allergy 2021, 8, 222–241. [Google Scholar] [CrossRef] [PubMed]
- Celebi Sozener, Z.; Ozdel Ozturk, B.; Cerci, P.; Turk, M.; Gorgulu Akin, B.; Akdis, M.; Altiner, S.; Ozbey, U.; Ogulur, I.; Mitamura, Y.; et al. Epithelial barrier hypothesis: Effect of the external exposome on the microbiome and epithelial barriers in allergic disease. Allergy 2022, 77, 1418–1449. [Google Scholar] [CrossRef]
- Capozza, K.; Gadd, H.; Kelley, K.; Russell, S.; Shi, V.; Schwartz, A. Insights from caregivers on the impact of pediatric atopic dermatitis on families: “I’m tired, overwhelmed, and feel like I’m failing as a mother”. Dermatitis 2020, 31, 223–227. [Google Scholar] [CrossRef]
- Meltzer, L.J.; Flewelling, K.D.; Jump, S.; Gyorkos, E.; White, M.; Hauk, P.J. Impact of atopic dermatitis treatment on child and parent sleep, daytime functioning, and quality of life. Ann. Allergy Asthma Immunol. 2020, 124, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.J.; Beck, K.M.; Sekhon, S.; Bhutani, T.; Koo, J. The impact of pediatric atopic dermatitis on families: A review. Pediatr. Dermatol. 2019, 36, 66–71. [Google Scholar] [CrossRef]
- Barbarot, S.; Silverberg, J.I.; Gadkari, A.; Simpson, E.L.; Weidinger, S.; Mina-Osorio, P.; Rossi, A.B.; Brignoli, L.; Mnif, T.; Guillemin, I.; et al. The family impact of atopic dermatitis in the pediatric population: Results from an international cross-sectional study. J. Pediatr. 2022, 246, 220–226.e5. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.; Ong, P.Y. Severe atopic dermatitis in children. Curr. Allergy Asthma Rep. 2018, 18, 35. [Google Scholar] [CrossRef]
- Capozza, K.; Schwartz, A.; Lang, J.E.; Chalmers, J.; Camilo, J.; Abuabara, K.; Kelley, K.; Harrison, J.; Vastrup, A.; Stancavich, L.; et al. Impact of childhood atopic dermatitis on life decisions for caregivers and families. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e451–e454. [Google Scholar] [CrossRef]
- Wadonda-Kabondo, N.; Sterne, J.A.; Golding, J.; Kennedy, C.T.; Archer, C.B.; Dunnill, M.G.; Team, A.S. Association of parental eczema, hayfever, and asthma with atopic dermatitis in infancy: Birth cohort study. Arch. Dis. Child. 2004, 89, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Kantor, R.; Kim, A.; Thyssen, J.P.; Silverberg, J.I. Association of atopic dermatitis with smoking: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2016, 75, 1119–1125.e1. [Google Scholar] [CrossRef]
- AlShatti, K.A.; Ziyab, A.H. Pet-keeping in relation to asthma, rhinitis, and eczema symptoms among adolescents in Kuwait: A cross-sectional study. Front. Pediatr. 2020, 8, 331. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Barbarot, S.; Gadkari, A.; Simpson, E.L.; Weidinger, S.; Mina-Osorio, P.; Rossi, A.B.; Brignoli, L.; Saba, G.; Guillemin, I.; et al. Atopic dermatitis in the pediatric population: A cross-sectional, international epidemiologic study. Ann. Allergy Asthma Immunol. 2021, 126, 417–428.e2. [Google Scholar] [CrossRef]
- Asher, M.I.; Keil, U.; Anderson, H.R.; Beasley, R.; Crane, J.; Martinez, F.; Mitchell, E.A.; Pearce, N.; Sibbald, B.; Stewart, A.W.; et al. International Study of Asthma and Allergies in Childhood (ISAAC): Rationale and methods. Eur. Respir. J. 1995, 8, 483–491. [Google Scholar] [CrossRef]
- Charman, C.R.; Venn, A.J.; Williams, H.C. The patient-oriented eczema measure: Development and initial validation of a new tool for measuring atopic eczema severity from the patients’ perspective. Arch. Dermatol. 2004, 140, 1513–1519. [Google Scholar] [CrossRef]
- Charman, C.R.; Venn, A.J.; Ravenscroft, J.C.; Williams, H.C. Translating Patient-Oriented Eczema Measure (POEM) scores into clinical practice by suggesting severity strata derived using anchor-based methods. Br. J. Dermatol. 2013, 169, 1326–1332. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, J.I.; Chiesa Fuxench, Z.C.; Gelfand, J.M.; Margolis, D.J.; Boguniewicz, M.; Fonacier, L.; Grayson, M.H.; Simpson, E.L.; Ong, P.Y. Content and construct validity, predictors, and distribution of self-reported atopic dermatitis severity in US adults. Ann. Allergy Asthma Immunol. 2018, 121, 729–734.e4. [Google Scholar] [CrossRef]
- Lawson, V.; Lewis-Jones, M.S.; Finlay, A.Y.; Reid, P.; Owens, R.G. The family impact of childhood atopic dermatitis: The dermatitis family impact questionnaire. Br. J. Dermatol. 1998, 138, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, F.D.; Chen, S.; Langan, S.M.; Prather, A.A.; McCulloch, C.E.; Kidd, S.A.; Cabana, M.D.; Chren, M.M.; Abuabara, K. Assessment of sleep disturbances and exhaustion in mothers of children with atopic dermatitis. JAMA Dermatol. 2019, 155, 556–563. [Google Scholar] [CrossRef]
- Cheng, B.T.; Silverberg, J.I. Association of pediatric atopic dermatitis and psoriasis with school absenteeism and parental work absenteeism: A cross-sectional United States population-based study. J. Am. Acad. Dermatol. 2021, 85, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto-Hanada, K.; Yang, L.; Ishitsuka, K.; Ayabe, T.; Mezawa, H.; Konishi, M.; Shoda, T.; Matsumoto, K.; Saito, H.; Ohya, Y.; et al. Allergic profiles of mothers and fathers in the Japan Environment and Children’s Study (JECS): A nationwide birth cohort study. World Allergy Organ. J. 2017, 10, 24. [Google Scholar] [CrossRef]
- Shinohara, M.; Matsumoto, K. Fetal tobacco smoke exposure in the third trimester of pregnancy is associated with atopic eczema/dermatitis syndrome in infancy. Pediatr. Allergy Immunol. Pulmonol. 2017, 30, 155–162. [Google Scholar] [CrossRef]
- Sugiyama, T.; Sugiyama, K.; Toda, M.; Yukawa, T.; Makino, S.; Fukuda, T. Risk factors for asthma allergic diseases among 13–14-year-old school children in Japan. Allergol. Int. 2002, 51, 139–150. [Google Scholar] [CrossRef]
- Tanaka, K.; Miyake, Y.; Furukawa, S.; Arakawa, M. Pre- and postnatal smoking exposure and risk of atopic eczema in young Japanese children: A prospective prebirth cohort study. Nicotine Tob. Res. 2017, 19, 804–809. [Google Scholar] [CrossRef]
- Jing, D.; Li, J.; Tao, J.; Wang, X.; Shan, S.; Kang, X.; Wu, B.; Zhang, Y.; Xiao, Y.; Chen, X.; et al. Associations of second-hand smoke exposure with hand eczema and atopic dermatitis among college students in China. Sci. Rep. 2020, 10, 17400. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Sim, S.; Choi, H.G. Atopic dermatitis is associated with active and passive cigarette smoking in adolescents. PLoS ONE 2017, 12, e0187453. [Google Scholar] [CrossRef]
- Lee, A.; Lee, S.Y.; Lee, K.S. Association of secondhand smoke exposure with allergic multimorbidity in Korean adolescents. Sci. Rep. 2020, 10, 16409. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Ohya, Y.; Tanaka, K.; Yokoyama, T.; Sasaki, S.; Fukushima, W.; Ohfuji, S.; Saito, K.; Kiyohara, C.; Hirota, Y.; et al. Home environment and suspected atopic eczema in Japanese infants: The Osaka maternal and child health study. Pediatr. Allergy Immunol. 2007, 18, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Kurosaka, F.; Nakatani, Y.; Terada, T.; Tanaka, A.; Ikeuchi, H.; Hayakawa, A.; Konohana, A.; Oota, K.; Nishio, H. Current cat ownership may be associated with the lower prevalence of atopic dermatitis, allergic rhinitis, and Japanese cedar pollinosis in schoolchildren in Himeji, Japan. Pediatr. Allergy Immunol. 2006, 17, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Katoh, N.; Ohya, Y.; Ikeda, M.; Ebihara, T.; Katayama, I.; Saeki, H.; Shimojo, N.; Tanaka, A.; Nakahara, T.; Nagao, M.; et al. Japanese guidelines for atopic dermatitis 2020. Allergol. Int. 2020, 69, 356–369. [Google Scholar] [CrossRef]
- Furue, M.; Hashimoto-Hachiya, A.; Tsuji, G. Aryl hydrocarbon receptor in atopic dermatitis and psoriasis. Int. J. Mol. Sci. 2019, 20, 5424. [Google Scholar] [CrossRef]
- Napolitano, M.; Fabbrocini, G.; Martora, F.; Picone, V.; Morelli, P.; Patruno, C. Role of aryl hydrocarbon receptor activation in inflammatory chronic skin diseases. Cells 2021, 10, 3559. [Google Scholar] [CrossRef]
| Criteria | Description |
|---|---|
| 1 | An itchy rash that has come and gone for ≥6 months |
| 2 | The itchy rash has appeared at any time in the past 12 months |
| 3 | The itchy rash has affected any of the following places at any time:
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saeki, H.; Ohya, Y.; Nawata, H.; Arima, K.; Inukai, M.; Rossi, A.B.; Bego-Le-Bagousse, G. Impact of the Family and Household Environment on Pediatric Atopic Dermatitis in Japan. J. Clin. Med. 2023, 12, 2988. https://doi.org/10.3390/jcm12082988
Saeki H, Ohya Y, Nawata H, Arima K, Inukai M, Rossi AB, Bego-Le-Bagousse G. Impact of the Family and Household Environment on Pediatric Atopic Dermatitis in Japan. Journal of Clinical Medicine. 2023; 12(8):2988. https://doi.org/10.3390/jcm12082988
Chicago/Turabian StyleSaeki, Hidehisa, Yukihiro Ohya, Hisakatsu Nawata, Kazuhiko Arima, Miho Inukai, Ana B. Rossi, and Gaelle Bego-Le-Bagousse. 2023. "Impact of the Family and Household Environment on Pediatric Atopic Dermatitis in Japan" Journal of Clinical Medicine 12, no. 8: 2988. https://doi.org/10.3390/jcm12082988
APA StyleSaeki, H., Ohya, Y., Nawata, H., Arima, K., Inukai, M., Rossi, A. B., & Bego-Le-Bagousse, G. (2023). Impact of the Family and Household Environment on Pediatric Atopic Dermatitis in Japan. Journal of Clinical Medicine, 12(8), 2988. https://doi.org/10.3390/jcm12082988






