Ovarian Cancer and Parkinson’s Disease: A Bidirectional Mendelian Randomization Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Standard Protocol Approvals, Registrations, and Patient Consents
2.2. Selection of Genetic Instrumental Variables
2.3. Ovarian Cancer Data
2.4. Parkinson’s Disease Population
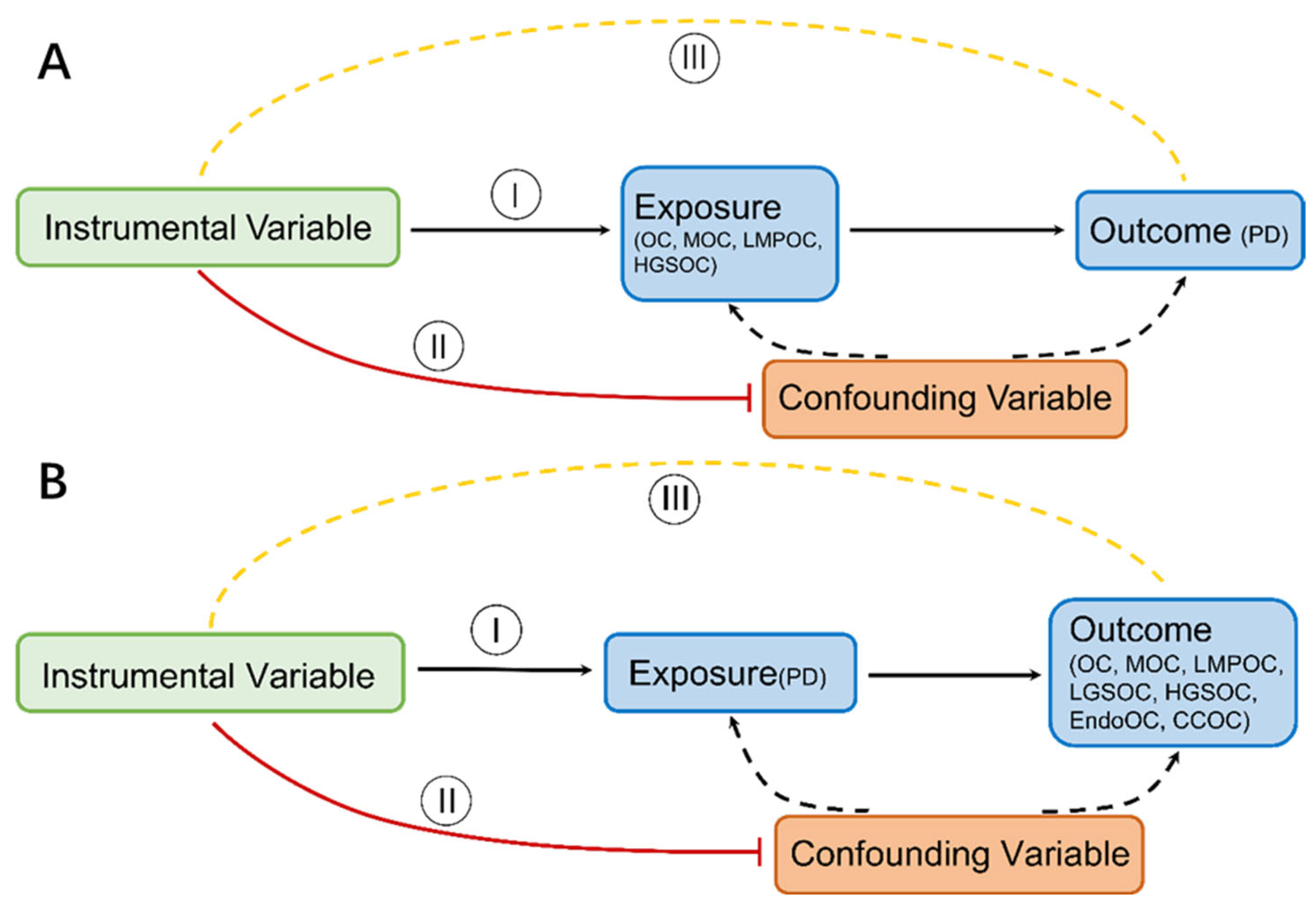
2.5. Mendelian Randomization
3. Results
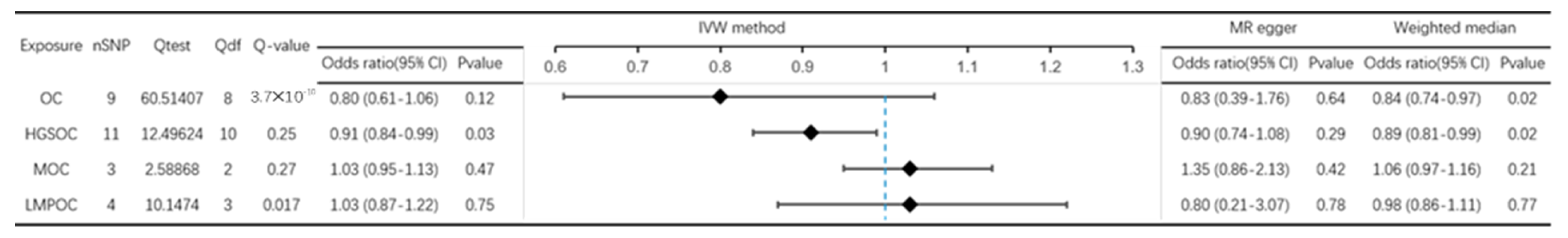
3.1. Effect of OC on PD
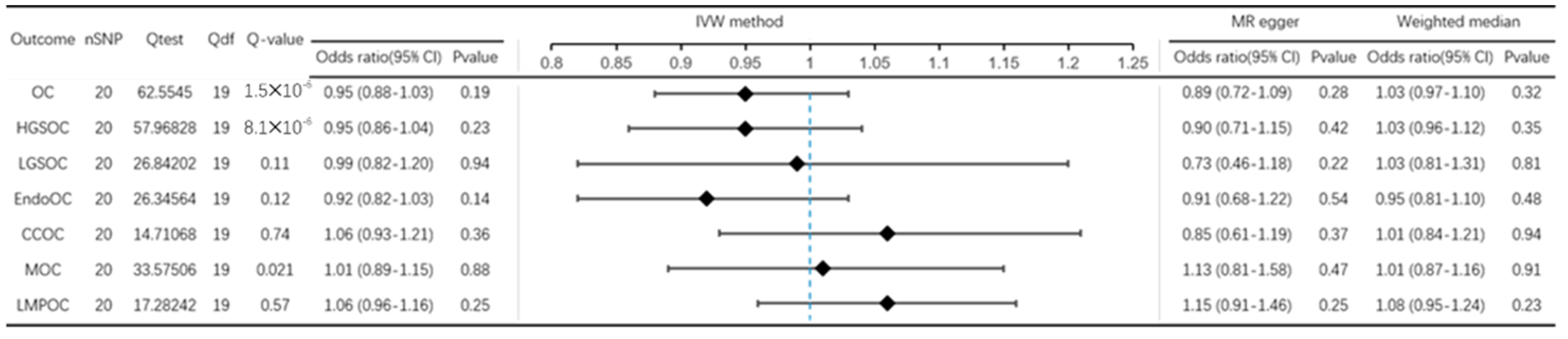
3.2. Effect of PD on OC
4. Discussion
4.1. Study Strengths
4.2. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seo, J.; Park, M. Molecular crosstalk between cancer and neurodegenerative diseases. Cell. Mol. Life Sci. 2019, 77, 2659–2680. [Google Scholar] [CrossRef] [PubMed]
- Crusz, S.M.; Balkwill, F.R. Inflammation and cancer: Advances and new agents. Nat. Rev. Clin. Oncol. 2015, 12, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.C. Multiple-mutation theory of carcinogenesis. Nature 1958, 181, 651–652. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Gao, H.M.; Hong, J.S. Why neurodegenerative diseases are progressive: Uncontrolled inflammation drives disease progression. Trends Immunol. 2008, 29, 357–365. [Google Scholar] [CrossRef]
- Ganguly, G.; Chakrabarti, S.; Chatterjee, U.; Saso, L. Proteinopathy, oxidative stress and mitochondrial dysfunction: Cross talk in Alzheimer’s disease and Parkinson's disease. Drug Des. Dev. Ther. 2017, 11, 797–810. [Google Scholar] [CrossRef]
- Walker, L.C.; LeVine, H., 3rd. Corruption and spread of pathogenic proteins in neurodegenerative diseases. J. Biol. Chem. 2012, 287, 33109–33115. [Google Scholar] [CrossRef]
- Avila, J. Common mechanisms in neurodegeneration. Nat. Med. 2010, 16, 1372. [Google Scholar] [CrossRef]
- Filippou, P.S.; Outeiro, T.F. Cancer and Parkinson’s Disease: Common Targets, Emerging Hopes. Mov. Disord. 2021, 36, 340–346. [Google Scholar] [CrossRef]
- Majd, S.; Power, J.; Majd, Z. Alzheimer’s Disease and Cancer: When Two Monsters Cannot Be Together. Front. Neurosci. 2019, 13, 155. [Google Scholar] [CrossRef]
- Morris, L.G.; Veeriah, S.; Chan, T.A. Genetic determinants at the interface of cancer and neurodegenerative disease. Oncogene 2010, 29, 3453–3464. [Google Scholar] [CrossRef]
- Feng, D.D.; Cai, W.; Chen, X. The associations between Parkinson's disease and cancer: The plot thickens. Transl. Neurodegener. 2015, 4, 20. [Google Scholar] [CrossRef]
- Ibáñez, K.; Boullosa, C.; Tabarés-Seisdedos, R.; Baudot, A.; Valencia, A. Molecular evidence for the inverse comorbidity between central nervous system disorders and cancers detected by transcriptomic meta-analyses. PLoS Genet. 2014, 10, e1004173. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021. [Google Scholar] [CrossRef]
- Jayson, G.C.; Kohn, E.C.; Kitchener, H.C.; Ledermann, J.A. Ovarian cancer. Lancet 2014, 384, 1376–1388. [Google Scholar] [CrossRef]
- Guo, J.Z.; Xiao, Q.; Gao, S.; Li, X.Q.; Wu, Q.J.; Gong, T.T. Review of Mendelian Randomization Studies on Ovarian Cancer. Front. Oncol. 2021, 11, 681396. [Google Scholar] [CrossRef]
- Matak, L.; Mikuš, M.; Ćorić, M.; Spagnol, G.; Matak, M.; Vujić, G. Comparison end-to-end anastomosis with ostomy after secondary surgical cytoreduction for recurrent high-grade serous ovarian cancer: Observational single-center study. Arch. Gynecol. Obstet. 2023. [Google Scholar] [CrossRef]
- Reid, B.M.; Permuth, J.B.; Sellers, T.A. Epidemiology of ovarian cancer: A review. Cancer Biol. Med. 2017, 14, 9–32. [Google Scholar] [CrossRef]
- Penninkilampi, R.; Eslick, G.D. Perineal Talc Use and Ovarian Cancer: A Systematic Review and Meta-Analysis. Epidemiology 2018, 29, 41–49. [Google Scholar] [CrossRef]
- Mhyre, T.R.; Boyd, J.T.; Hamill, R.W.; Maguire-Zeiss, K.A. Parkinson’s disease. Subcell. Biochem. 2012, 65, 389–455. [Google Scholar] [CrossRef]
- Global, regional, and national burden of neurological disorders, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [CrossRef] [PubMed]
- Dorsey, E.R.; Sherer, T.; Okun, M.S.; Bloem, B.R. The Emerging Evidence of the Parkinson Pandemic. J. Parkinsons Dis. 2018, 8, S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Deuschl, G.; Beghi, E.; Fazekas, F.; Varga, T.; Christoforidi, K.A.; Sipido, E.; Bassetti, C.L.; Vos, T.; Feigin, V.L. The burden of neurological diseases in Europe: An analysis for the Global Burden of Disease Study 2017. Lancet Public Health 2020, 5, e551–e567. [Google Scholar] [CrossRef] [PubMed]
- Macchi, Z.A.; Koljack, C.E.; Miyasaki, J.M.; Katz, M.; Galifianakis, N.; Prizer, L.P.; Sillau, S.H.; Kluger, B.M. Patient and caregiver characteristics associated with caregiver burden in Parkinson's disease: A palliative care approach. Ann. Palliat. Med. 2020, 9, S24–S33. [Google Scholar] [CrossRef]
- Global, regional, and national burden of Parkinson's disease, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 939–953. [CrossRef]
- Ascherio, A.; Schwarzschild, M.A. The epidemiology of Parkinson's disease: Risk factors and prevention. Lancet Neurol. 2016, 15, 1257–1272. [Google Scholar] [CrossRef]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, B. Association between Parkinson's Disease and Risk of Cancer: A PRISMA-compliant Meta-analysis. ACS Chem. Neurosci. 2019, 10, 4430–4439. [Google Scholar] [CrossRef]
- Lin, P.Y.; Chang, S.N.; Hsiao, T.H.; Huang, B.T.; Lin, C.H.; Yang, P.C. Association Between Parkinson Disease and Risk of Cancer in Taiwan. JAMA Oncol. 2015, 1, 633–640. [Google Scholar] [CrossRef]
- Leong, Y.Q.; Lee, S.W.H.; Ng, K.Y. Cancer risk in Parkinson disease: An updated systematic review and meta-analysis. Eur. J. Neurol. 2021, 28, 4219–4237. [Google Scholar] [CrossRef]
- Gala, H.; Tomlinson, I. The use of Mendelian randomisation to identify causal cancer risk factors: Promise and limitations. J. Pathol. 2020, 250, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, N.A.; Didelez, V.; Burton, P.R.; Tobin, M.D. Mendelian randomisation and causal inference in observational epidemiology. PLoS Med. 2008, 5, e177. [Google Scholar] [CrossRef] [PubMed]
- Phelan, C.M.; Kuchenbaecker, K.B.; Tyrer, J.P.; Kar, S.P.; Lawrenson, K.; Winham, S.J.; Dennis, J.; Pirie, A.; Riggan, M.J.; Chornokur, G.; et al. Identification of 12 new susceptibility loci for different histotypes of epithelial ovarian cancer. Nat. Genet. 2017, 49, 680–691. [Google Scholar] [CrossRef] [PubMed]
- Auton, A.; Brooks, L.D.; Durbin, R.M.; Garrison, E.P.; Kang, H.M.; Korbel, J.O.; Marchini, J.L.; McCarthy, S.; McVean, G.A.; Abecasis, G.R. A global reference for human genetic variation. Nature 2015, 526, 68–74. [Google Scholar] [CrossRef]
- Nalls, M.A.; Blauwendraat, C.; Vallerga, C.L.; Heilbron, K.; Bandres-Ciga, S.; Chang, D.; Tan, M.; Kia, D.A.; Noyce, A.J.; Xue, A.; et al. Expanding Parkinson’s disease genetics: Novel risk loci, genomic context, causal insights and heritable risk. Lancet Neurol. 2019, 2019, 388165. [Google Scholar] [CrossRef]
- Burgess, S.; Butterworth, A.; Thompson, S.G. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 2013, 37, 658–665. [Google Scholar] [CrossRef]
- Staley, J.R.; Burgess, S. Semiparametric methods for estimation of a nonlinear exposure-outcome relationship using instrumental variables with application to Mendelian randomization. Genet. Epidemiol. 2017, 41, 341–352. [Google Scholar] [CrossRef]
- Luo, J.; le Cessie, S.; van Heemst, D.; Noordam, R. Diet-Derived Circulating Antioxidants and Risk of Coronary Heart Disease: A Mendelian Randomization Study. J. Am. Coll. Cardiol. 2021, 77, 45–54. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef]
- Verbanck, M.; Chen, C.Y.; Neale, B.; Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef]
- Greco, M.F.; Minelli, C.; Sheehan, N.A.; Thompson, J.R. Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat. Med. 2015, 34, 2926–2940. [Google Scholar] [CrossRef]
- Devine, M.J.; Plun-Favreau, H.; Wood, N.W. Parkinson's disease and cancer: Two wars, one front. Nat. Rev. Cancer 2011, 11, 812–823. [Google Scholar] [CrossRef]
- Bruening, W.; Giasson, B.I.; Klein-Szanto, A.J.; Lee, V.M.; Trojanowski, J.Q.; Godwin, A.K. Synucleins are expressed in the majority of breast and ovarian carcinomas and in preneoplastic lesions of the ovary. Cancer 2000, 88, 2154–2163. [Google Scholar] [CrossRef]
- Okochi-Takada, E.; Nakazawa, K.; Wakabayashi, M.; Mori, A.; Ichimura, S.; Yasugi, T.; Ushijima, T. Silencing of the UCHL1 gene in human colorectal and ovarian cancers. Int. J. Cancer 2006, 119, 1338–1344. [Google Scholar] [CrossRef]
- Hu, Q.; Hao, P.; Liu, Q.; Dong, M.; Gong, Y.; Zhang, C.; Zhang, Y. Mendelian randomization studies on atherosclerotic cardiovascular disease: Evidence and limitations. Sci. China Life Sci. 2019, 62, 758–770. [Google Scholar] [CrossRef]
- Holmes, M.V.; Ala-Korpela, M.; Smith, G.D. Mendelian randomization in cardiometabolic disease: Challenges in evaluating causality. Nat. Rev. Cardiol. 2017, 14, 577–590. [Google Scholar] [CrossRef]
- Burgess, S.; Swanson, S.A.; Labrecque, J.A. Are Mendelian randomization investigations immune from bias due to reverse causation? Eur. J. Epidemiol. 2021, 36, 253–257. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA 2020, 323, 548–560. [Google Scholar] [CrossRef]
- Nonnekes, J.; Post, B.; Tetrud, J.W.; Langston, J.W.; Bloem, B.R. MPTP-induced parkinsonism: An historical case series. Lancet Neurol. 2018, 17, 300–301. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.-Z.; Xiao, Q.; Wu, L.; Chen, F.; Yin, J.-L.; Qin, X.; Gong, T.-T.; Wu, Q.-J. Ovarian Cancer and Parkinson’s Disease: A Bidirectional Mendelian Randomization Study. J. Clin. Med. 2023, 12, 2961. https://doi.org/10.3390/jcm12082961
Guo J-Z, Xiao Q, Wu L, Chen F, Yin J-L, Qin X, Gong T-T, Wu Q-J. Ovarian Cancer and Parkinson’s Disease: A Bidirectional Mendelian Randomization Study. Journal of Clinical Medicine. 2023; 12(8):2961. https://doi.org/10.3390/jcm12082961
Chicago/Turabian StyleGuo, Jian-Zeng, Qian Xiao, Lang Wu, Fa Chen, Jia-Li Yin, Xue Qin, Ting-Ting Gong, and Qi-Jun Wu. 2023. "Ovarian Cancer and Parkinson’s Disease: A Bidirectional Mendelian Randomization Study" Journal of Clinical Medicine 12, no. 8: 2961. https://doi.org/10.3390/jcm12082961
APA StyleGuo, J.-Z., Xiao, Q., Wu, L., Chen, F., Yin, J.-L., Qin, X., Gong, T.-T., & Wu, Q.-J. (2023). Ovarian Cancer and Parkinson’s Disease: A Bidirectional Mendelian Randomization Study. Journal of Clinical Medicine, 12(8), 2961. https://doi.org/10.3390/jcm12082961





