Evaluation of Renal Fibrosis Using Magnetization Transfer Imaging at 1.5T and 3T in a Porcine Model of Renal Artery Stenosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Protocol
2.2. MRI Studies
2.3. MTI Parameters
2.4. Image Analysis
2.5. Statistical Analysis
3. Results
3.1. Animal Characteristics
3.2. MTR Comparison between Normal, STK, and CLK Kidneys
3.3. MTR Comparison between 3T and 1.5T
3.4. MTR Reproducibility
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chronic Kidney Disease in the United States, 2021; Center for Disease Control: Prevention, GA, USA, 2021.
- Saran, R.; Robinson, B.; Abbott, K.C.; Agodoa, L.Y.C.; Bragg-Gresham, J.; Balkrishnan, R.; Bhave, N.; Dietrich, X.; Ding, Z.; Eggers, P.W.; et al. US Renal Data System 2018 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am. J. Kidney Dis. 2019, 73, A7–A8. [Google Scholar] [CrossRef]
- Klinkhammer, B.M.; Goldschmeding, R.; Floege, J.; Boor, P. Treatment of Renal Fibrosis-Turning Challenges into Opportunities. Adv. Chronic. Kidney Dis. 2017, 24, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Leung, G.; Kirpalani, A.; Szeto, S.G.; Deeb, M.; Foltz, W.; Simmons, C.A.; Yuen, D.A. Could MRI Be Used To Image Kidney Fibrosis? A Review of Recent Advances and Remaining Barriers. Clin. J. Am. Soc. Nephrol. 2017, 12, 1019–1028. [Google Scholar] [CrossRef]
- Panizo, S.; Martínez-Arias, L.; Alonso-Montes, C.; Cannata, P.; Martín-Carro, B.; Fernández-Martín, J.L.; Naves-Díaz, M.; Carrillo-López, N.; Cannata-Andía, J.B. Fibrosis in Chronic Kidney Disease: Pathogenesis and Consequences. Int. J. Mol. Sci. 2021, 22, 408. [Google Scholar] [CrossRef] [PubMed]
- Bledsoe, G.; Shen, B.; Yao, Y.; Zhang, J.J.; Chao, L.; Chao, J. Reversal of renal fibrosis, inflammation, and glomerular hypertrophy by kallikrein gene delivery. Hum. Gene Ther. 2006, 17, 545–555. [Google Scholar] [CrossRef]
- Tampe, D.; Zeisberg, M. Potential approaches to reverse or repair renal fibrosis. Nat. Rev. Nephrol. 2014, 10, 226–237. [Google Scholar] [CrossRef]
- Corapi, K.M.; Chen, J.L.; Balk, E.M.; Gordon, C.E. Bleeding complications of native kidney biopsy: A systematic review and meta-analysis. Am. J. Kidney Dis. 2012, 60, 62–73. [Google Scholar] [CrossRef]
- Furness, P.N.; Philpott, C.M.; Chorbadjian, M.T.; Nicholson, M.L.; Bosmans, J.L.; Corthouts, B.L.; Bogers, J.J.; Schwarz, A.; Gwinner, W.; Haller, H.; et al. Protocol biopsy of the stable renal transplant: A multicenter study of methods and complication rates. Transplantation 2003, 76, 969–973. [Google Scholar] [CrossRef]
- Gerth, J.; Busch, M.; Illner, N.; Traut, M.; Gröne, H.J.; Wolf, G. Are tissue samples from two different anatomical areas of the kidney necessary for adequate diagnosis? Clin. Nephrol. 2010, 74, 258–265. [Google Scholar] [CrossRef]
- Varnell, C.D., Jr.; Stone, H.K.; Welge, J.A. Bleeding Complications after Pediatric Kidney Biopsy: A Systematic Review and Meta-Analysis. Clin. J. Am. Soc. Nephrol. 2019, 14, 57–65. [Google Scholar] [CrossRef]
- Cohen, E.P.; Olson, J.D.; Tooze, J.A.; Bourland, J.D.; Dugan, G.O.; Cline, J.M. Detection and quantification of renal fibrosis by computerized tomography. PLoS ONE 2020, 15, e0228626. [Google Scholar] [CrossRef]
- Jaschke, W.; Cogan, M.G.; Sievers, R.; Gould, R.; Lipton, M.J. Measurement of renal blood flow by cine computed tomography. Kidney Int. 1987, 31, 1038–1042. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, J.; Tang, K.; Quan, C.; Tian, Y.; Li, H.; Ao, G.; Qiu, L. Determination of Glomerular Filtration Rate with CT Measurement of Renal Clearance of Iodinated Contrast Material versus 99mTc-DTPA Dynamic Imaging “Gates” Method: A Validation Study in Asymmetrical Renal Disease. Radiology 2017, 282, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Xu, Y.; Liu, W.; Shao, P.; Lv, Q.; Yang, G.; Tang, L. Measurement of Glomerular Filtration Rate Using Multiphasic Computed Tomography in Patients With Unilateral Renal Tumors: A Feasibility Study. Front. Physiol. 2019, 10, 1209. [Google Scholar] [CrossRef] [PubMed]
- Bentley, M.D.; Lerman, L.O.; Hoffman, E.A.; Fiksen-Olsen, M.J.; Ritman, E.L.; Romero, J.C. Measurement of renal perfusion and blood flow with fast computed tomography. Circ. Res. 1994, 74, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Ferguson, C.M.; Lerman, L.O. Noninvasive assessment of renal fibrosis by magnetic resonance imaging and ultrasound techniques. Transl. Res. 2019, 209, 105–120. [Google Scholar] [CrossRef]
- Morrell, G.R.; Zhang, J.L.; Lee, V.S. Magnetic Resonance Imaging of the Fibrotic Kidney. J. Am. Soc. Nephrol. 2017, 28, 2564–2570. [Google Scholar] [CrossRef]
- Jiang, K.; Ferguson, C.M.; Woollard, J.R.; Landes, V.L.; Krier, J.D.; Zhu, X.; Nayak, K.S.; Lerman, L.O. Magnetization Transfer Imaging Is Unaffected by Decreases in Renal Perfusion in Swine. Investig. Radiol. 2019, 54, 681–688. [Google Scholar] [CrossRef]
- Afarideh, M.; Jiang, K.; Ferguson, C.M.; Woollard, J.R.; Glockner, J.F.; Lerman, L.O. Magnetization Transfer Imaging Predicts Porcine Kidney Recovery After Revascularization of Renal Artery Stenosis. Investig. Radiol. 2021, 56, 86–93. [Google Scholar] [CrossRef]
- Jiang, K.; Ferguson, C.M.; Ebrahimi, B.; Tang, H.; Kline, T.L.; Burningham, T.A.; Mishra, P.K.; Grande, J.P.; Macura, S.I.; Lerman, L.O. Noninvasive Assessment of Renal Fibrosis with Magnetization Transfer MR Imaging: Validation and Evaluation in Murine Renal Artery Stenosis. Radiology 2017, 283, 77–86. [Google Scholar] [CrossRef]
- Jiang, K.; Fang, Y.; Ferguson, C.M.; Tang, H.; Mishra, P.K.; Macura, S.I.; Lerman, L.O. Quantitative Magnetization Transfer Detects Renal Fibrosis in Murine Kidneys With Renal Artery Stenosis. J. Magn. Reson. Imaging 2021, 53, 884–893. [Google Scholar] [CrossRef]
- Kline, T.L.; Irazabal, M.V.; Ebrahimi, B.; Hopp, K.; Udoji, K.N.; Warner, J.D.; Korfiatis, P.; Mishra, P.K.; Macura, S.I.; Venkatesh, S.K.; et al. Utilizing magnetization transfer imaging to investigate tissue remodeling in a murine model of autosomal dominant polycystic kidney disease. Magn. Reson. Med. 2016, 75, 1466–1473. [Google Scholar] [CrossRef]
- Wang, F.; Wang, S.; Zhang, Y.; Li, K.; Harris, R.C.; Gore, J.C.; Zhang, M.Z. Noninvasive quantitative magnetization transfer MRI reveals tubulointerstitial fibrosis in murine kidney. NMR Biomed. 2019, 32, e4128. [Google Scholar] [CrossRef] [PubMed]
- Chade, A.R.; Zhu, X.Y.; Grande, J.P.; Krier, J.D.; Lerman, A.; Lerman, L.O. Simvastatin abates development of renal fibrosis in experimental renovascular disease. J. Hypertens. 2008, 26, 1651–1660. [Google Scholar] [CrossRef]
- Zhu, X.-Y.; Chade, A.R.; Rodriguez-Porcel, M.; Bentley, M.D.; Ritman, E.L.; Lerman, A.; Lerman, L.O. Cortical microvascular remodeling in the stenotic kidney: Role of increased oxidative stress. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1854–1859. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Ferguson, C.M.; Woollard, J.R.; Zhu, X.; Lerman, L.O. Magnetization Transfer Magnetic Resonance Imaging Noninvasively Detects Renal Fibrosis in Swine Atherosclerotic Renal Artery Stenosis at 3.0 T. Investig. Radiol. 2017, 52, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Hayashida, M.; Izumitani, S.; Fujimine, T.; Onishi, T.; Genba, K. Magnetisation transfer MR imaging of the kidney: Evaluation at 3.0 T in association with renal function. Eur. Radiol. 2013, 23, 2315–2319. [Google Scholar] [CrossRef]
- Jiang, K.; Ferguson, C.M.; Grimm, R.C.; Zhu, X.; Glockner, J.F.; Lerman, L.O. Reliable Assessment of Swine Renal Fibrosis Using Quantitative Magnetization Transfer Imaging. Investig. Radiol. 2022, 57, 334–342. [Google Scholar] [CrossRef]
- Marxer-Meier, A.; Hegyi, I.; Loffing, J.; Kaissling, B. Postnatal maturation of renal cortical peritubular fibroblasts in the rat. Anat. Embryol. 1998, 197, 143–153. [Google Scholar] [CrossRef]
- Krier, J.D.; Ritman, E.L.; Bajzer, Z.; Romero, J.C.; Lerman, A.; Lerman, L.O. Noninvasive measurement of concurrent single-kidney perfusion, glomerular filtration, and tubular function. Am. J. Physiol. Renal Physiol. 2001, 281, F630–F638. [Google Scholar] [CrossRef]
- Phal, P.M.; Usmanov, A.; Nesbit, G.M.; Anderson, J.C.; Spencer, D.; Wang, P.; Helwig, J.A.; Roberts, C.; Hamilton, B.E. Qualitative Comparison of 3-T and 1.5-T MRI in the Evaluation of Epilepsy. Am. J. Roentgenol. 2008, 191, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.J.; Kamel, I.R.; Macura, K.J.; Bluemke, D.A. 3.0-T MR Imaging of the Abdomen: Comparison with 1.5T. RadioGraphics 2008, 28, 1983–1998. [Google Scholar] [CrossRef] [PubMed]
- Pohmann, R.; Shajan, G.; Balla, D.Z. Contrast at high field: Relaxation times, magnetization transfer and phase in the rat brain at 16.4T. Magn. Reson. Med. 2011, 66, 1572–1581. [Google Scholar] [CrossRef] [PubMed]

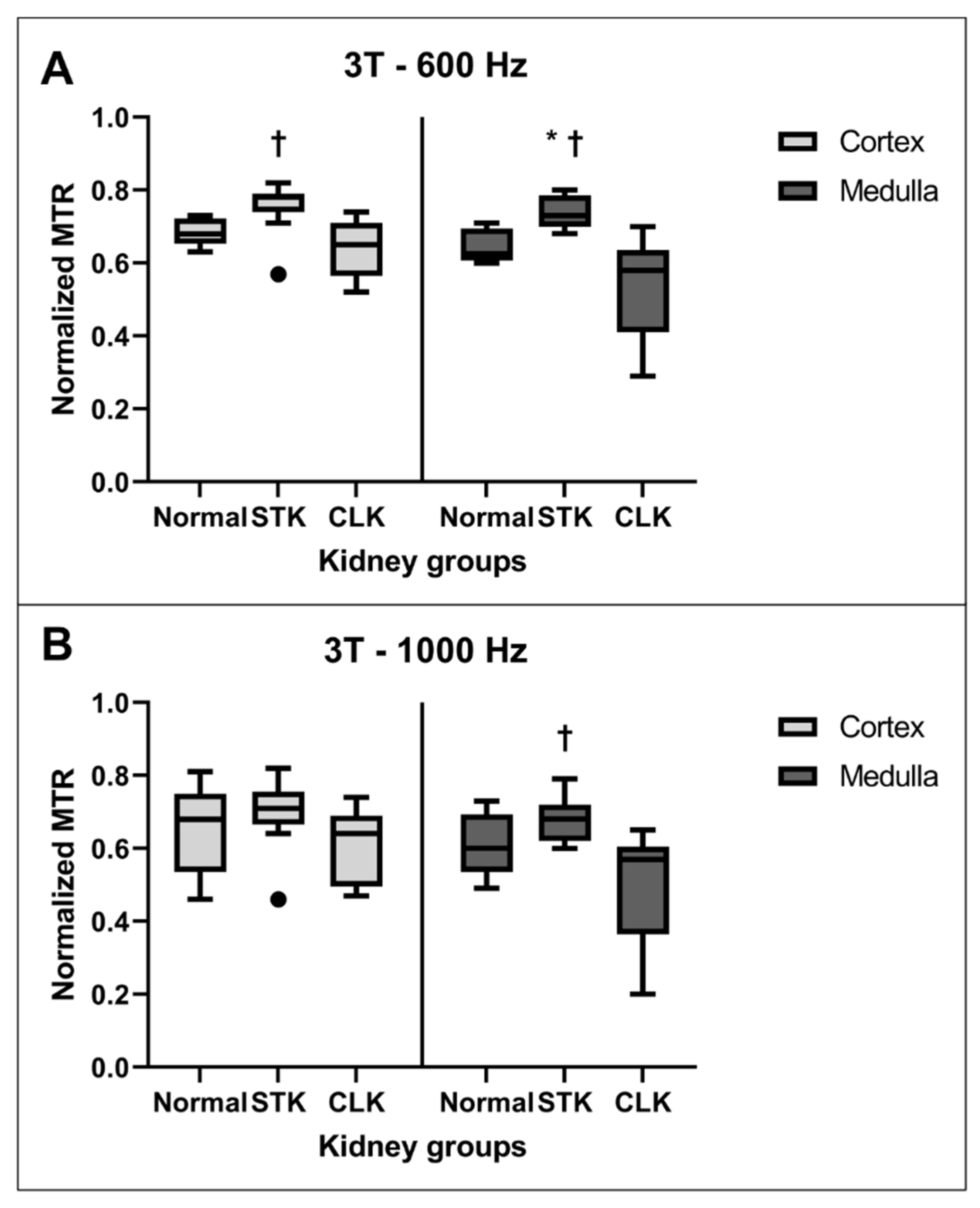


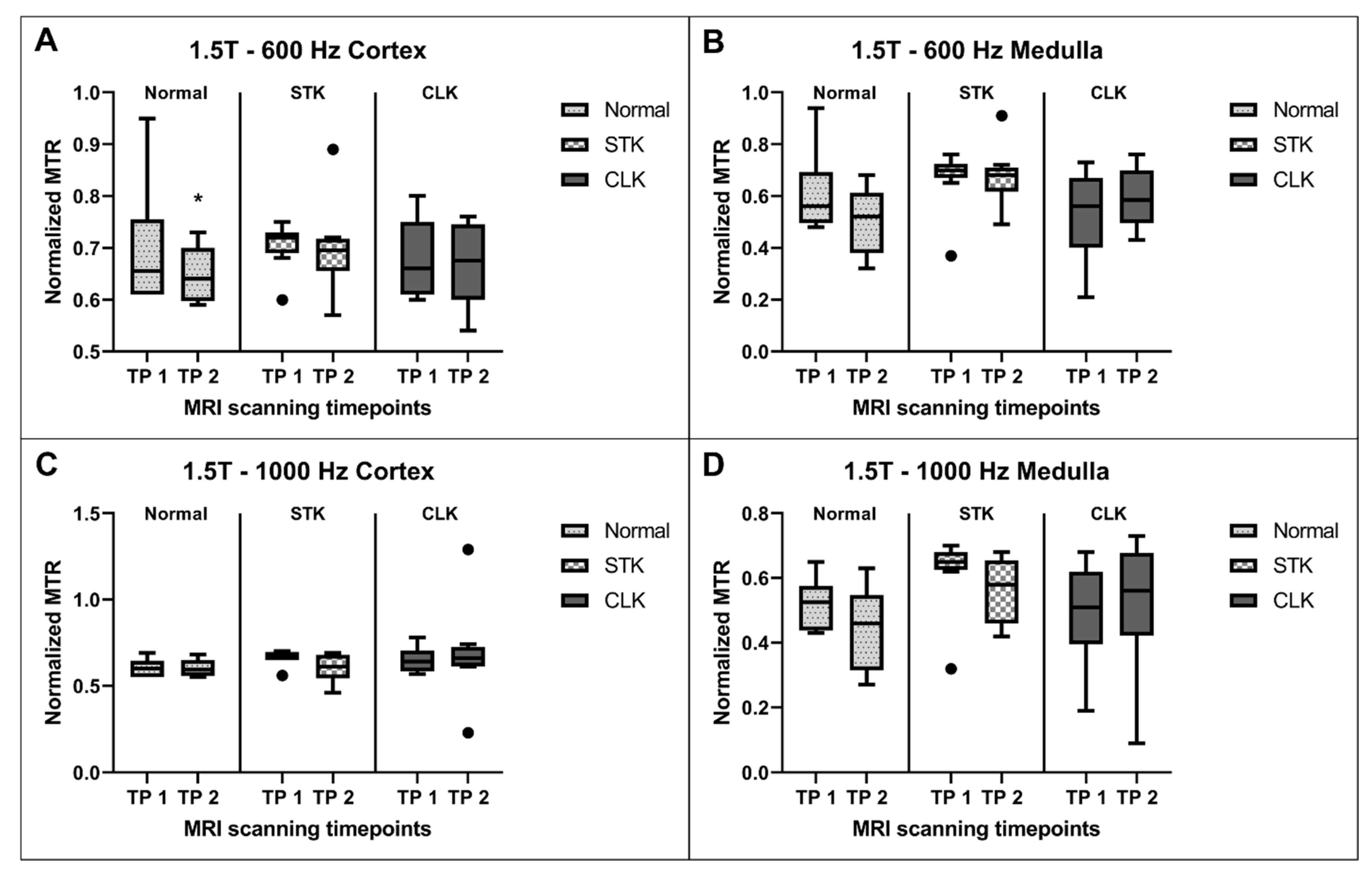
| Region | Kidneys | 3T—600 Hz | 1.5T—600 Hz | 3T—1000 Hz | 1.5T—1000 Hz |
|---|---|---|---|---|---|
| Cortex | Normal | 0.68 ± 0.04 | 0.69 ± 0.13 | 0.65 ± 0.13 | 0.60 ± 0.05 |
| Stenotic | 0.75 ± 0.08 | 0.71 ± 0.04 | 0.70 ± 0.10 | 0.67 ± 0.04 | |
| Contralateral | 0.64 ± 0.08 | 0.69 ± 0.07 | 0.61 ± 0.10 | 0.66 ± 0.07 | |
| Medulla | Normal | 0.64 ± 0.04 | 0.61 ± 0.17 | 0.61 ± 0.09 | 0.52 ± 0.08 |
| Stenotic | 0.74 ± 0.04 | 0.67 ± 0.12 | 0.68 ± 0.06 | 0.62 ± 0.11 | |
| Contralateral | 0.53 ± 0.15 | 0.52 ± 0.17 | 0.50 ± 0.17 | 0.50 ± 0.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gandhi, D.B.; Al Saeedi, M.; Krier, J.D.; Jiang, K.; Glockner, J.F.; Lerman, L.O. Evaluation of Renal Fibrosis Using Magnetization Transfer Imaging at 1.5T and 3T in a Porcine Model of Renal Artery Stenosis. J. Clin. Med. 2023, 12, 2956. https://doi.org/10.3390/jcm12082956
Gandhi DB, Al Saeedi M, Krier JD, Jiang K, Glockner JF, Lerman LO. Evaluation of Renal Fibrosis Using Magnetization Transfer Imaging at 1.5T and 3T in a Porcine Model of Renal Artery Stenosis. Journal of Clinical Medicine. 2023; 12(8):2956. https://doi.org/10.3390/jcm12082956
Chicago/Turabian StyleGandhi, Deep B., Mina Al Saeedi, James D. Krier, Kai Jiang, James F. Glockner, and Lilach O. Lerman. 2023. "Evaluation of Renal Fibrosis Using Magnetization Transfer Imaging at 1.5T and 3T in a Porcine Model of Renal Artery Stenosis" Journal of Clinical Medicine 12, no. 8: 2956. https://doi.org/10.3390/jcm12082956
APA StyleGandhi, D. B., Al Saeedi, M., Krier, J. D., Jiang, K., Glockner, J. F., & Lerman, L. O. (2023). Evaluation of Renal Fibrosis Using Magnetization Transfer Imaging at 1.5T and 3T in a Porcine Model of Renal Artery Stenosis. Journal of Clinical Medicine, 12(8), 2956. https://doi.org/10.3390/jcm12082956





