Augmented Reality Support for Anterior Decompression and Fusion Using Floating Method for Cervical Ossification of the Posterior Longitudinal Ligament
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Designs
2.2. Participants
2.3. Surgical Technique of ADF with Microscopic AR Support
2.3.1. Surgical Indications of ADF using the Floating Method for Cervical OPLL
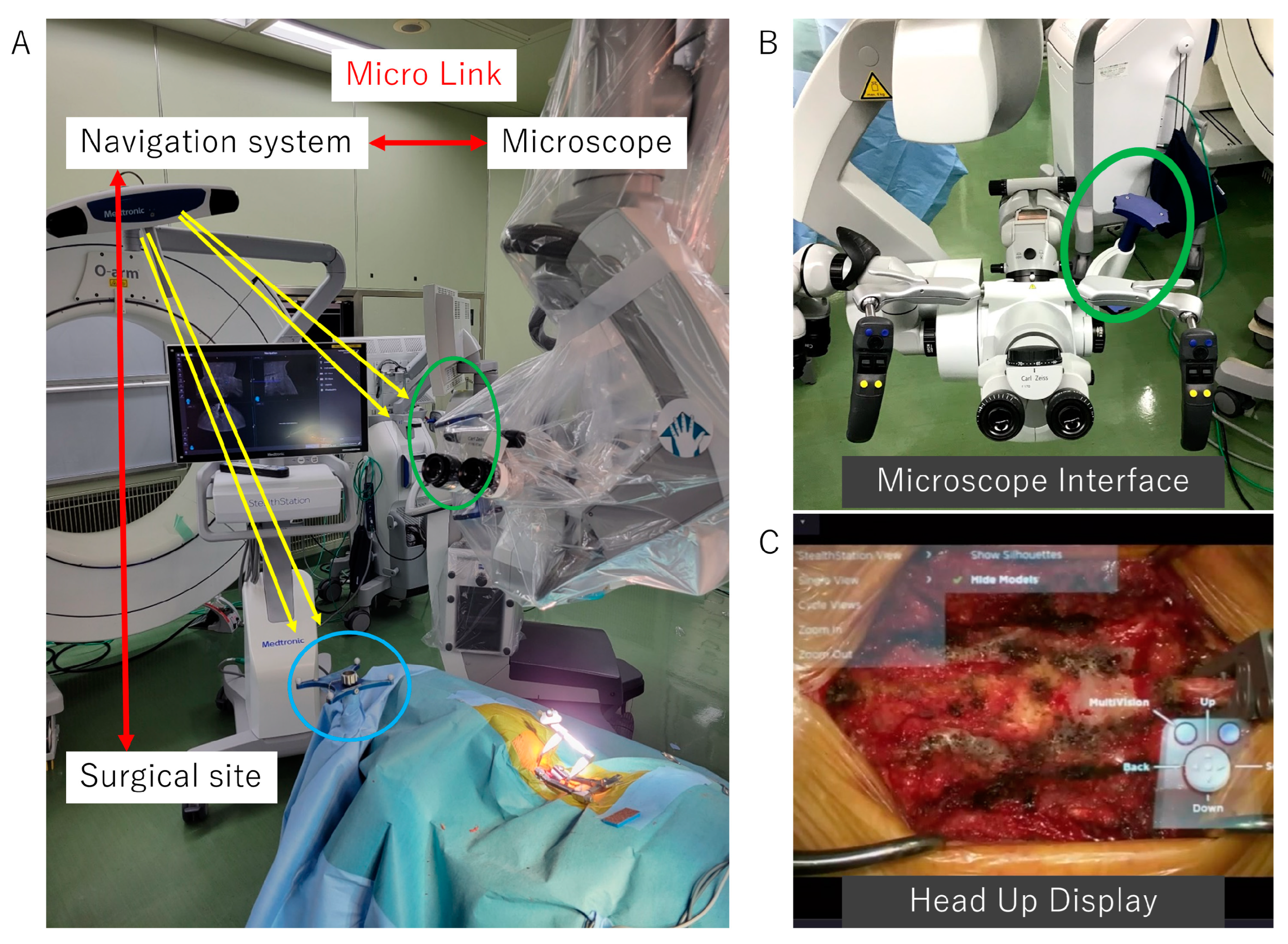
2.3.2. Surgical Approaches to the Cervical Spine and Preparation for Intraoperative Navigation
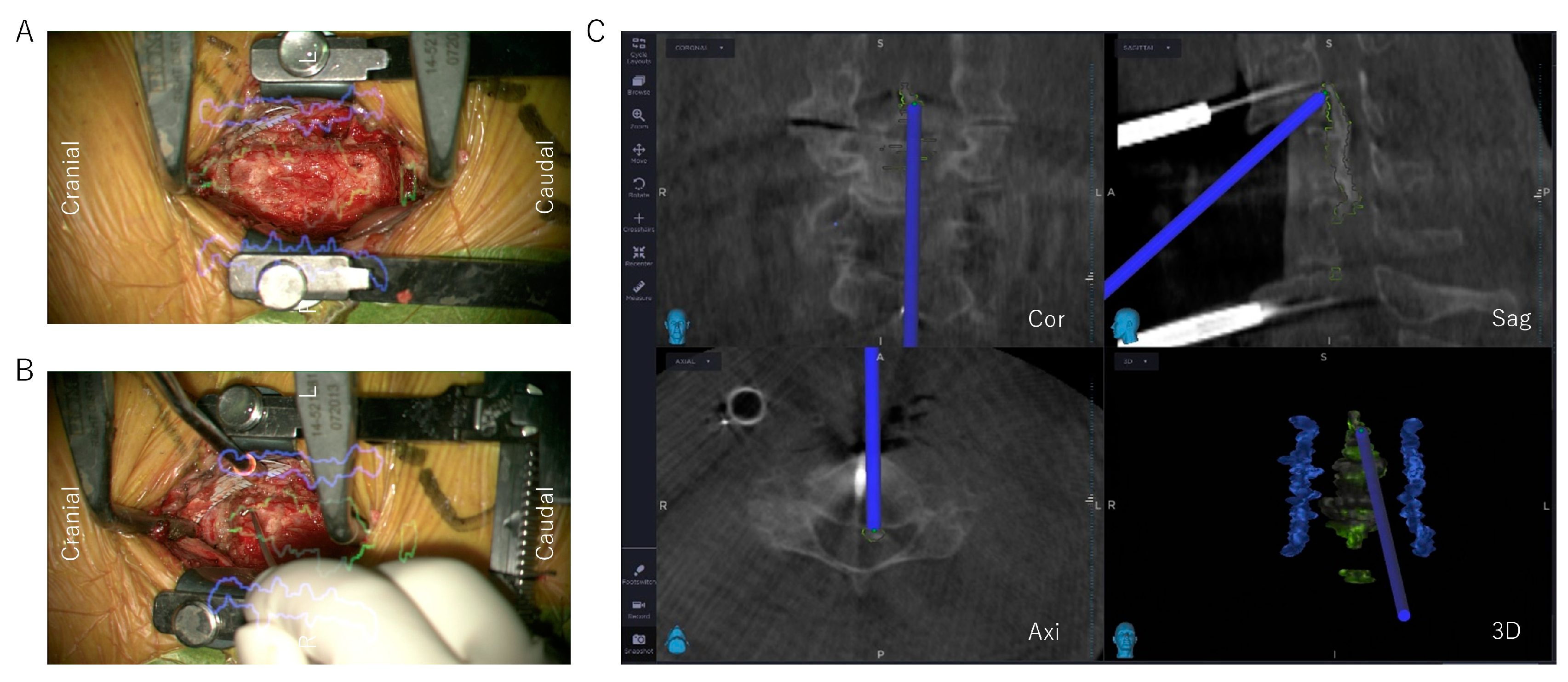
2.3.3. Reconstructed 3D Images of the OPLL Using Anatomical Mapping Software
2.3.4. Decompression and OPLL Floating Method with Microscopic AR Support
2.4. Clinical Assessments
2.5. Radiographic Assessments
2.6. Statistical Analysis
3. Results
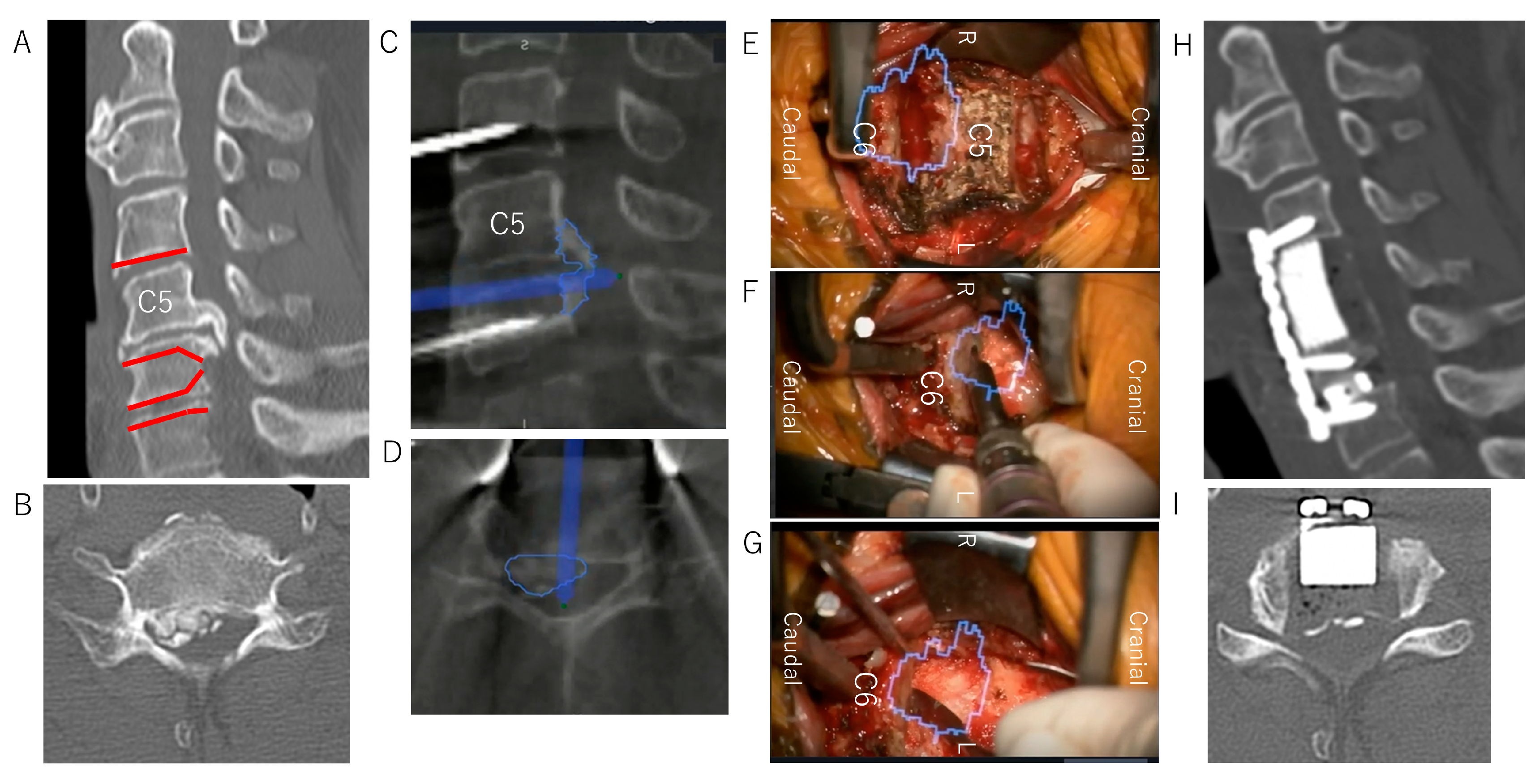
Case Presentation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lex, J.R.; Koucheki, R.; Toor, J.; Backstein, D.J. Clinical applications of augmented reality in orthopaedic surgery: A comprehensive narrative review. Int. Orthop. 2023, 47, 375–391. [Google Scholar] [CrossRef] [PubMed]
- Hersh, A.; Mahapatra, S.; Weber-Levine, C.; Awosika, T.; Theodore, J.N.; Zakaria, H.M.; Liu, A.; Witham, T.F.; Theodore, N. Augmented Reality in Spine Surgery: A Narrative Review. HSS J. Musculoskelet. J. Hosp. Spec. Surg. 2021, 17, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Hirai, T.; Yoshii, T.; Nagoshi, N.; Takeuchi, K.; Mori, K.; Ushio, S.; Iwanami, A.; Yamada, T.; Seki, S.; Tsuji, T.; et al. Distribution of ossified spinal lesions in patients with severe ossification of the posterior longitudinal ligament and prediction of ossification at each segment based on the cervical OP index classification: A multicenter study (JOSL CT study). BMC Musculoskelet. Disord. 2018, 19, 107. [Google Scholar] [CrossRef] [PubMed]
- Smith, Z.A.; Buchanan, C.C.; Raphael, D.; Khoo, L.T. Ossification of the posterior longitudinal ligament: Pathogenesis, management, and current surgical approaches. A review. Neurosurg. Focus 2011, 30, E10. [Google Scholar] [CrossRef]
- Tetreault, L.; Nakashima, H.; Kato, S.; Kryshtalskyj, M.; Nagoshi, N.; Nouri, A.; Singh, A.; Fehlings, M.G. A Systematic Review of Classification Systems for Cervical Ossification of the Posterior Longitudinal Ligament. Glob. Spine J. 2019, 9, 85–103. [Google Scholar] [CrossRef] [PubMed]
- Inamasu, J.; Guiot, B.H.; Sachs, D.C. Ossification of the posterior longitudinal ligament: An update on its biology, epidemiology, and natural history. Neurosurgery 2006, 58, 1027–1039. [Google Scholar] [CrossRef]
- Yamaura, I.; Kurosa, Y.; Matuoka, T.; Shindo, S. Anterior floating method for cervical myelopathy caused by ossification of the posterior longitudinal ligament. Clin. Orthop. Relat. Res. 1999, 359, 27–34. [Google Scholar] [CrossRef]
- Matsuoka, T.; Yamaura, I.; Kurosa, Y.; Nakai, O.; Shindo, S.; Shinomiya, K. Long-term results of the anterior floating method for cervical myelopathy caused by ossification of the posterior longitudinal ligament. Spine 2001, 26, 241–248. [Google Scholar] [CrossRef]
- Yoshii, T.; Sakai, K.; Hirai, T.; Yamada, T.; Inose, H.; Kato, T.; Enomoto, M.; Tomizawa, S.; Kawabata, S.; Arai, Y.; et al. Anterior decompression with fusion versus posterior decompression with fusion for massive cervical ossification of the posterior longitudinal ligament with a ≥50% canal occupying ratio: A multicenter retrospective study. Spine J. Off. J. N. Am. Spine Soc. 2016, 16, 1351–1357. [Google Scholar] [CrossRef]
- Yoshii, T.; Egawa, S.; Hirai, T.; Kaito, T.; Mori, K.; Koda, M.; Chikuda, H.; Hasegawa, T.; Imagama, S.; Yoshida, M.; et al. A systematic review and meta-analysis comparing anterior decompression with fusion and posterior laminoplasty for cervical ossification of the posterior longitudinal ligament. J. Orthop. Sci. Off. J. Jpn. Orthop. Assoc. 2020, 25, 58–65. [Google Scholar] [CrossRef]
- Yoshii, T.; Morishita, S.; Egawa, S.; Sakai, K.; Kusano, K.; Tsutsui, S.; Hirai, T.; Matsukura, Y.; Wada, K.; Katsumi, K.; et al. Prospective Investigation of Surgical Outcomes after Anterior Decompression with Fusion and Laminoplasty for the Cervical Ossification of the Posterior Longitudinal Ligament: A Propensity Score Matching Analysis. J. Clin. Med. 2022, 11, 7012. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, T.; Yamada, T.; Hirai, T.; Taniyama, T.; Kato, T.; Enomoto, M.; Inose, H.; Sumiya, S.; Kawabata, S.; Shinomiya, K.; et al. Dynamic changes in spinal cord compression by cervical ossification of the posterior longitudinal ligament evaluated by kinematic computed tomography myelography. Spine 2014, 39, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Koda, M.; Mochizuki, M.; Konishi, H.; Aiba, A.; Kadota, R.; Inada, T.; Kamiya, K.; Ota, M.; Maki, S.; Takahashi, K.; et al. Comparison of clinical outcomes between laminoplasty, posterior decompression with instrumented fusion, and anterior decompression with fusion for K-line (-) cervical ossification of the posterior longitudinal ligament. Eur. Spine J. Off. Publ. Eur. Spine Soc. Eur. Spinal Deform. Soc. Eur. Sect. Cerv. Spine Res. Soc. 2016, 25, 2294–2301. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, M.; Okuda, S.; Miyauchi, A.; Sakaura, H.; Mukai, Y.; Yonenobu, K.; Yoshikawa, H. Surgical strategy for cervical myelopathy due to ossification of the posterior longitudinal ligament: Part 2: Advantages of anterior decompression and fusion over laminoplasty. Spine 2007, 32, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Okawa, A.; Takahashi, M.; Arai, Y.; Kawabata, S.; Enomoto, M.; Kato, T.; Hirai, T.; Shinomiya, K. Five-year follow-up evaluation of surgical treatment for cervical myelopathy caused by ossification of the posterior longitudinal ligament: A prospective comparative study of anterior decompression and fusion with floating method versus laminoplasty. Spine 2012, 37, 367–376. [Google Scholar] [CrossRef]
- Sakai, K.; Yoshii, T.; Arai, Y.; Hirai, T.; Torigoe, I.; Inose, H.; Tomori, M.; Sakaki, K.; Matsukura, Y.; Okawa, A. Impact of preoperative cervical sagittal alignment for cervical myelopathy caused by ossification of the posterior longitudinal ligament on surgical treatment. J. Orthop. Sci. Off. J. Jpn. Orthop. Assoc. 2022, 27, 1208–1214. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, K.; Ma, X.; Yin, Q.; Wu, Z.; Xia, H.; Wang, Z. Systematic review of cohort studies comparing surgical treatment for multilevel ossification of posterior longitudinal ligament: Anterior vs posterior approach. Orthopedics 2011, 34, e397–e402. [Google Scholar] [CrossRef]
- Yoshii, T.; Sakai, K.; Machino, M.; Furuya, T. Choice of Surgical Procedure for Cervical Ossification of the Posterior Longitudinal Ligament. J. Clin. Med. 2022, 11, 5396. [Google Scholar] [CrossRef]
- Egawa, S.; Yoshii, T.; Sakai, K.; Kusano, K.; Nakagawa, Y.; Hirai, T.; Kimura, A.; Furuya, T.; Kanchiku, T.; Nagamoto, Y.; et al. Prospective Investigation of Postoperative Complications in Anterior Decompression with Fusion for Severe Cervical Ossification of the Posterior Longitudinal Ligament: A Multi-institutional Study. Spine 2021, 46, 1621–1629. [Google Scholar] [CrossRef]
- Yoshii, T.; Hirai, T.; Yamada, T.; Inose, H.; Kato, T.; Sakai, K.; Enomoto, M.; Kawabata, S.; Arai, Y.; Okawa, A. Intraoperative evaluation using mobile computed tomography in anterior cervical decompression with floating method for massive ossification of the posterior longitudinal ligament. J. Orthop. Surg. Res. 2017, 12, 12. [Google Scholar] [CrossRef]
- Deinsberger, R.; Regatschnig, R.; Ungersböck, K. Intraoperative evaluation of bone decompression in anterior cervical spine surgery by three-dimensional fluoroscopy. Eur. Spine J. Off. Publ. Eur. Spine Soc. Eur. Spinal Deform. Soc. Eur. Sect. Cerv. Spine Res. Soc. 2005, 14, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Okawa, A.; Matsumoto, M.; Iwasaki, M.; Kawaguchi, Y. Computer-Aided Surgery for Ossification of the Spinal Ligaments. OPLL, 3rd ed.; Springer: Singapore, 2020; pp. 249–256. [Google Scholar] [CrossRef]
- Otomo, N.; Funao, H.; Yamanouchi, K.; Isogai, N.; Ishii, K. Computed Tomography-Based Navigation System in Current Spine Surgery: A Narrative Review. Medicina 2022, 58, 241. [Google Scholar] [CrossRef] [PubMed]
- Carl, B.; Bopp, M.; Saß, B.; Voellger, B.; Nimsky, C. Implementation of augmented reality support in spine surgery. Eur. Spine J. Off. Publ. Eur. Spine Soc. Eur. Spinal Deform. Soc. Eur. Sect. Cerv. Spine Res. Soc. 2019, 28, 1697–1711. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Sato, S.; Kato, K.; Hyakumachi, T.; Yanagibashi, Y.; Ito, M.; Abumi, K. A novel 3D guidance system using augmented reality for percutaneous vertebroplasty: Technical note. J. Neurosurg. Spine 2013, 19, 492–501. [Google Scholar] [CrossRef]
- Mascitelli, J.R.; Schlachter, L.; Chartrain, A.G.; Oemke, H.; Gilligan, J.; Costa, A.B.; Shrivastava, R.K.; Bederson, J.B. Navigation-Linked Heads-Up Display in Intracranial Surgery: Early Experience. Oper. Neurosurg. 2018, 15, 184–193. [Google Scholar] [CrossRef]
- Smith, G.W.; Robinson, R.A. The treatment of certain cervical-spine disorders by anterior removal of the intervertebral disc and interbody fusion. J. Bone Joint. Surg. Am. 1958, 40, 607–624. [Google Scholar] [CrossRef]
- Hirabayashi, K.; Watanabe, K.; Wakano, K.; Suzuki, N.; Satomi, K.; Ishii, Y. Expansive open-door laminoplasty for cervical spinal stenotic myelopathy. Spine 1983, 8, 693–699. [Google Scholar] [CrossRef]
- Tang, J.A.; Scheer, J.K.; Smith, J.S.; Deviren, V.; Bess, S.; Hart, R.A.; Lafage, V.; Shaffrey, C.I.; Schwab, F.; Ames, C.P. The impact of standing regional cervical sagittal alignment on outcomes in posterior cervical fusion surgery. Neurosurgery 2012, 71, 662–669. [Google Scholar] [CrossRef]
- Franken, R.J.; Gupta, S.C.; Banis, J.C., Jr.; Thomas, S.V.; Derr, J.W.; Klein, S.A.; Kon, M.; Barker, J.H. Microsurgery without a microscope: Laboratory evaluation of a three-dimensional on-screen microsurgery system. Microsurgery 1995, 16, 746–751. [Google Scholar] [CrossRef]
- Yoon, J.W.; Chen, R.E.; Kim, E.J.; Akinduro, O.O.; Kerezoudis, P.; Han, P.K.; Si, P.; Freeman, W.D.; Diaz, R.J.; Komotar, R.J.; et al. Augmented reality for the surgeon: Systematic review. Int. J. Med. Robot. Comput. Assist. Surg. MRCAS 2018, 14, e1914. [Google Scholar] [CrossRef]
- Wanivenhaus, F.; Neuhaus, C.; Liebmann, F.; Roner, S.; Spirig, J.M.; Farshad, M. Augmented reality-assisted rod bending in spinal surgery. Spine J. Off. J. N. Am. Spine Soc. 2019, 19, 1687–1689. [Google Scholar] [CrossRef] [PubMed]
- Gibby, J.T.; Swenson, S.A.; Cvetko, S.; Rao, R.; Javan, R. Head-mounted display augmented reality to guide pedicle screw placement utilizing computed tomography. Int. J. Comput. Assist. Radiol. Surg. 2019, 14, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Elmi-Terander, A.; Nachabe, R.; Skulason, H.; Pedersen, K.; Söderman, M.; Racadio, J.; Babic, D.; Gerdhem, P.; Edström, E. Feasibility and Accuracy of Thoracolumbar Minimally Invasive Pedicle Screw Placement With Augmented Reality Navigation Technology. Spine 2018, 43, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Molina, C.A.; Dibble, C.F.; Lo, S.L.; Witham, T.; Sciubba, D.M. Augmented reality-mediated stereotactic navigation for execution of en bloc lumbar spondylectomy osteotomies. J. Neurosurg. Spine 2021, 34, 700–705. [Google Scholar] [CrossRef]
- Umebayashi, D.; Yamamoto, Y.; Nakajima, Y.; Fukaya, N.; Hara, M. Augmented Reality Visualization-guided Microscopic Spine Surgery: Transvertebral Anterior Cervical Foraminotomy and Posterior Foraminotomy. Journal of the American Academy of Orthopaedic Surgeons. Glob. Res. Rev. 2018, 2, e008. [Google Scholar] [CrossRef]
- Sommer, F.; Hussain, I.; Kirnaz, S.; Goldberg, J.L.; Navarro-Ramirez, R.; McGrath, L.B., Jr.; Schmidt, F.A.; Medary, B.; Gadjradj, P.S.; Härtl, R. Augmented Reality to Improve Surgical Workflow in Minimally Invasive Transforaminal Lumbar Interbody Fusion—A Feasibility Study With Case Series. Neurospine 2022, 19, 574–585. [Google Scholar] [CrossRef]
- Yoshii, T.; Egawa, S.; Sakai, K.; Kusano, K.; Nakagawa, Y.; Hirai, T.; Wada, K.; Katsumi, K.; Fujii, K.; Kimura, A.; et al. Perioperative Complications in Posterior Surgeries for Cervical Ossification of the Posterior Longitudinal Ligament: A Prospective Nationwide Investigation. Clin. Spine Surg. 2021, 34, E594–E600. [Google Scholar] [CrossRef]
- Kimura, A.; Seichi, A.; Hoshino, Y.; Yamazaki, M.; Mochizuki, M.; Aiba, A.; Kato, T.; Uchida, K.; Miyamoto, K.; Nakahara, S.; et al. Perioperative complications of anterior cervical decompression with fusion in patients with ossification of the posterior longitudinal ligament: A retrospective, multi-institutional study. J. Orthop. Sci. Off. J. Jpn. Orthop. Assoc. 2012, 17, 667–672. [Google Scholar] [CrossRef]
- Liu, W.; Hu, L.; Chou, P.H.; Liu, M.; Kan, W.; Wang, J. Comparison of anterior decompression and fusion versus laminoplasty in the treatment of multilevel cervical ossification of the posterior longitudinal ligament: A systematic review and meta-analysis. Ther. Clin. Risk Manag. 2016, 12, 675–685. [Google Scholar] [CrossRef]
- Morishita, S.; Yoshii, T.; Okawa, A.; Fushimi, K.; Fujiwara, T. Perioperative complications of anterior decompression with fusion versus laminoplasty for the treatment of cervical ossification of the posterior longitudinal ligament: Propensity score matching analysis using a nation-wide inpatient database. Spine J. Off. J. N. Am. Spine Soc. 2019, 19, 610–616. [Google Scholar] [CrossRef]

| Case No. | Age (years) | Sex (M/F) | Procedure of ADF Using the Floating Method | Operative Time (min) | Blood Loss (grams) | Intraoperative Complication | Perioperative Complication |
|---|---|---|---|---|---|---|---|
| 1 | 58 | M | C4–6ACCF C6/7ACDF | 292 | 30 | Intubation management (postoperative days 1–4) | |
| 2 | 54 | M | C3–5ACCF | 390 | 80 | CSF leakage | |
| 3 | 54 | M | C3/4AF C4/5/6/7ACDF | 312 | 35 | ||
| 4 | 63 | M | C4–6ACCF C6/7ACDF | 263 | 35 | ||
| 5 | 69 | F | C4/5ACDF C5–7ADF | 240 | 400 | ||
| 6 | 63 | M | C2–5ACCF C5/6ACDF | 461 | 29 | ||
| 7 | 78 | F | C3–6ACCF | 313 | 500 | CSF leakage | |
| 8 | 55 | M | C3/4ACDF C4–7ACCF | 476 | 56 | CSF leakage | |
| 9 | 84 | M | C3/4/5ACDF | 186 | 10 | ||
| 10 | 75 | M | C4–7ACCF | 270 | 100 | ||
| 11 | 63 | F | C3/4ACDF C4–7ACCF | 518 | 372 | ||
| 12 | 54 | M | C3/4ACDF C4–7ACCF | 444 | 630 | ||
| 13 | 46 | F | C4–7ACCF | 394 | 295 | CSF leakage | C5 palsy |
| 14 | 72 | M | C4/5ACDF C5–7ACCF | 345 | 10 |
| Non-AR (n = 53) | AR (n = 14) | p | |
|---|---|---|---|
| Clinical outcomes | |||
| Age (years) | 63.4 ± 8.7 | 63.5 ± 10.9 | 0.99 |
| Sex (M/F, M%) | 45/8 (84.9%) | 10/4 (71.4%) | 0.25 |
| Pre JOA (score) | 10.4 ± 2.4 | 11.7 ± 1.9 | 0.06 |
| Post JOA (score) | 14.1 ± 2.1 | 14.3 ± 1.4 | 0.74 |
| Recovery rate (%) | 56.2 ± 27.0 | 47.6 ± 31.6 | 0.64 |
| Operative outcomes | |||
| Number of operated levels | 3.2 ± 0.8 | 3.3 ± 0.7 | 0.69 |
| Number of corpectomies | 2.0 ± 0.7 | 1.4 ± 0.7 | 0.007 * |
| Operative time (min) | 295.6 ± 93.5 | 350.3 ± 99.2 | 0.071 |
| Intraoperative blood loss (g) | 302.1 ± 895.7 | 184.4 ± 211.2 | 0.63 |
| CSF leakage, n (%) | 8 (15.1%) | 4 (28.6%) | 0.19 |
| Nerve palsy, n (%) | 10 (18.9%) | 1 (7.1%) | 0.48 |
| VA injury, n (%) | 0 (0%) | 0 (0%) | - |
| Impingement of floating OPLL, n (%) | 7 (13.2%) | 1 (7.1%) | 0.47 |
| Graft dislodgement, n (%) | 9 (17.0%) | 0 (0%) | 0.18 |
| Reoperation, n (%) | 10 (18.9%) | 0 (0%) | 0.078 |
| Radiographic outcomes | |||
| Pre C2–7 angle (°) | 5.4 ± 12.7 | 1.0 ± 11.1 | 0.091 |
| Pre CSVA (mm) | 30.5 ± 20.3 | 24.6 ± 14.4 | 0.31 |
| Pre C7 slope (°) | 26.9 ± 8.6 | 21.8 ± 2.3 | 0.054 |
| Post C2–7 angle (°) | 7.9 ± 9.9 | 4.1 ± 10.6 | 0.22 |
| Post CSVA (mm) | 25.7 ± 18.7 | 23.9 ± 13.5 | 0.74 |
| Post C7 slope (°) | 25.0 ± 1.2 | 20.2 ± 2.3 | 0.091 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onuma, H.; Sakai, K.; Arai, Y.; Torigoe, I.; Tomori, M.; Sakaki, K.; Hirai, T.; Egawa, S.; Kobayashi, Y.; Okawa, A.; et al. Augmented Reality Support for Anterior Decompression and Fusion Using Floating Method for Cervical Ossification of the Posterior Longitudinal Ligament. J. Clin. Med. 2023, 12, 2898. https://doi.org/10.3390/jcm12082898
Onuma H, Sakai K, Arai Y, Torigoe I, Tomori M, Sakaki K, Hirai T, Egawa S, Kobayashi Y, Okawa A, et al. Augmented Reality Support for Anterior Decompression and Fusion Using Floating Method for Cervical Ossification of the Posterior Longitudinal Ligament. Journal of Clinical Medicine. 2023; 12(8):2898. https://doi.org/10.3390/jcm12082898
Chicago/Turabian StyleOnuma, Hiroaki, Kenichiro Sakai, Yoshiyasu Arai, Ichiro Torigoe, Masaki Tomori, Kyohei Sakaki, Takashi Hirai, Satoru Egawa, Yutaka Kobayashi, Atsushi Okawa, and et al. 2023. "Augmented Reality Support for Anterior Decompression and Fusion Using Floating Method for Cervical Ossification of the Posterior Longitudinal Ligament" Journal of Clinical Medicine 12, no. 8: 2898. https://doi.org/10.3390/jcm12082898
APA StyleOnuma, H., Sakai, K., Arai, Y., Torigoe, I., Tomori, M., Sakaki, K., Hirai, T., Egawa, S., Kobayashi, Y., Okawa, A., & Yoshii, T. (2023). Augmented Reality Support for Anterior Decompression and Fusion Using Floating Method for Cervical Ossification of the Posterior Longitudinal Ligament. Journal of Clinical Medicine, 12(8), 2898. https://doi.org/10.3390/jcm12082898







