Distinct Clinical and Prognostic Features of Myelodysplastic Syndrome in Patients from the Middle East, North Africa, and Beyond: A Systemic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Geographical Definitions
2.3. Study Selection
2.4. Quality Assessment
2.5. Data Extraction and Statistical Analysis
3. Results
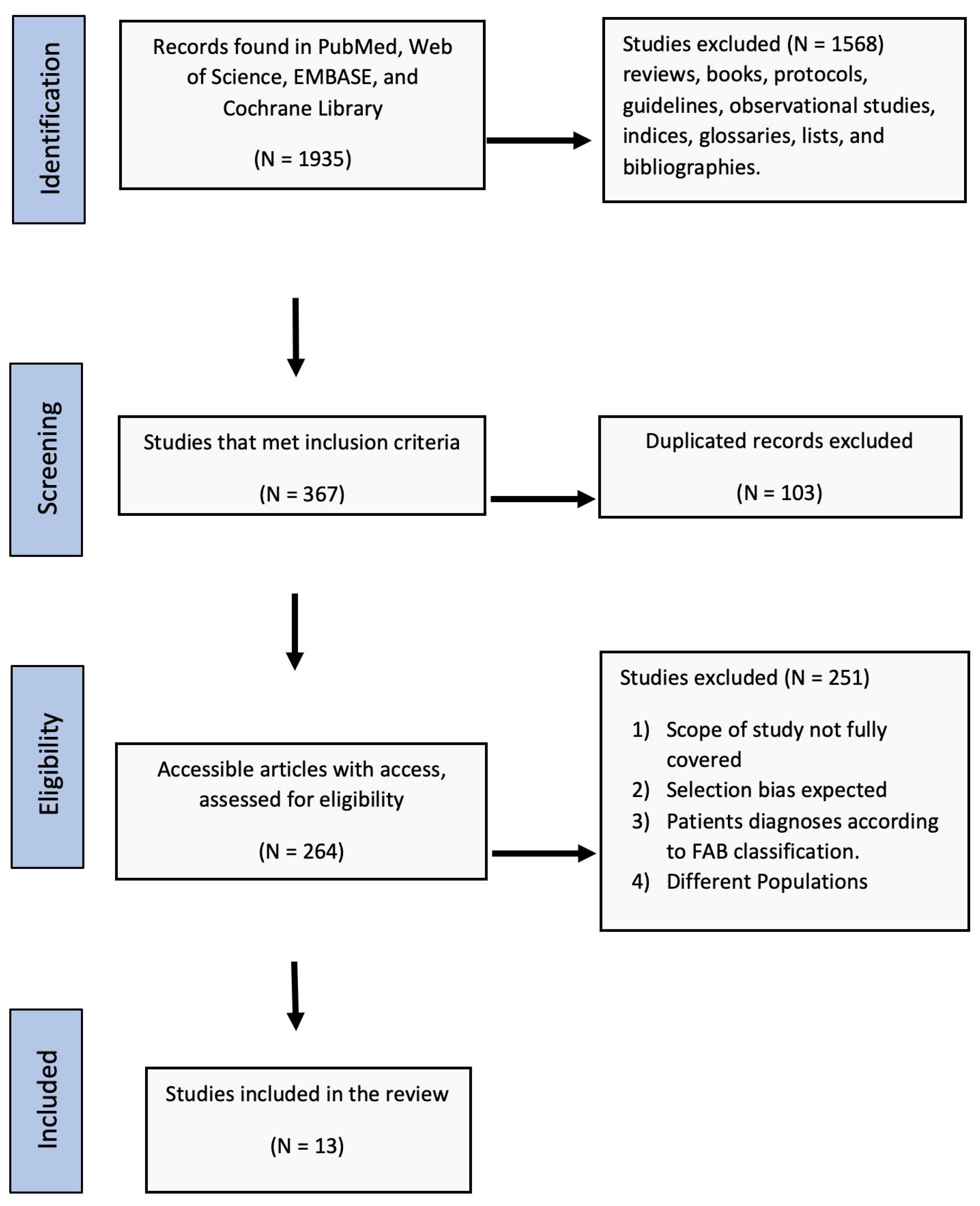
3.1. Identification and Selection of Records
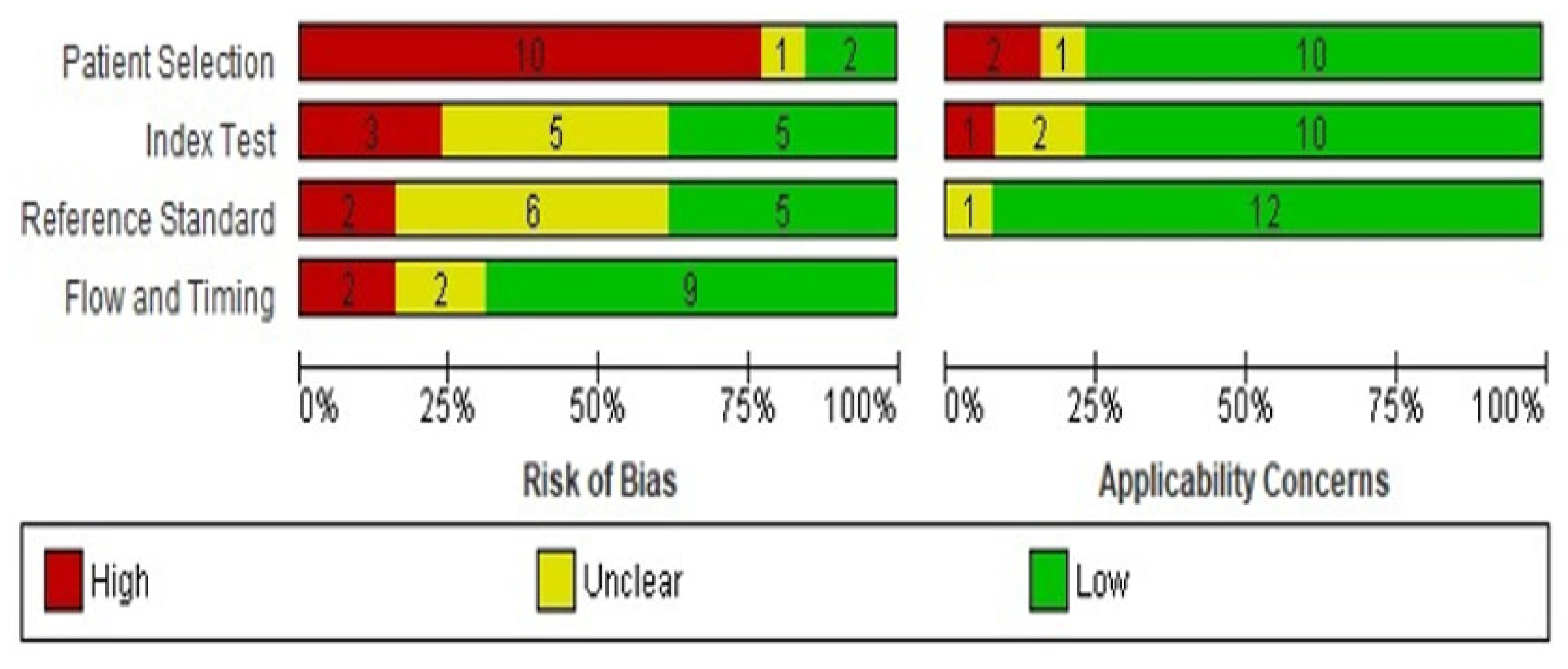
3.2. Quality of the Eligible Studies
3.3. Narrative Systematic Review
3.3.1. Study Characteristics
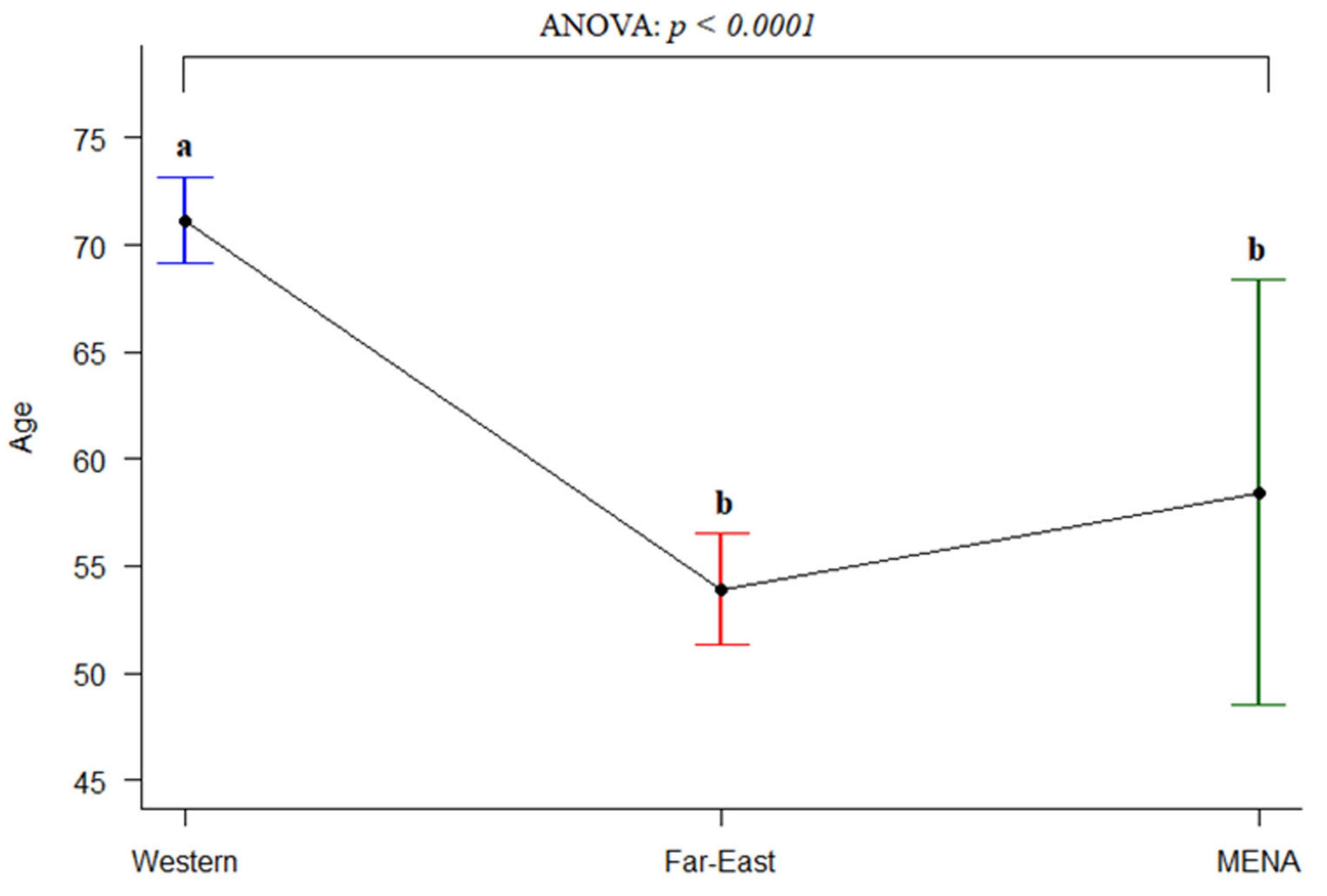
3.3.2. Gender and Age
3.3.3. Hematological and Laboratory Data
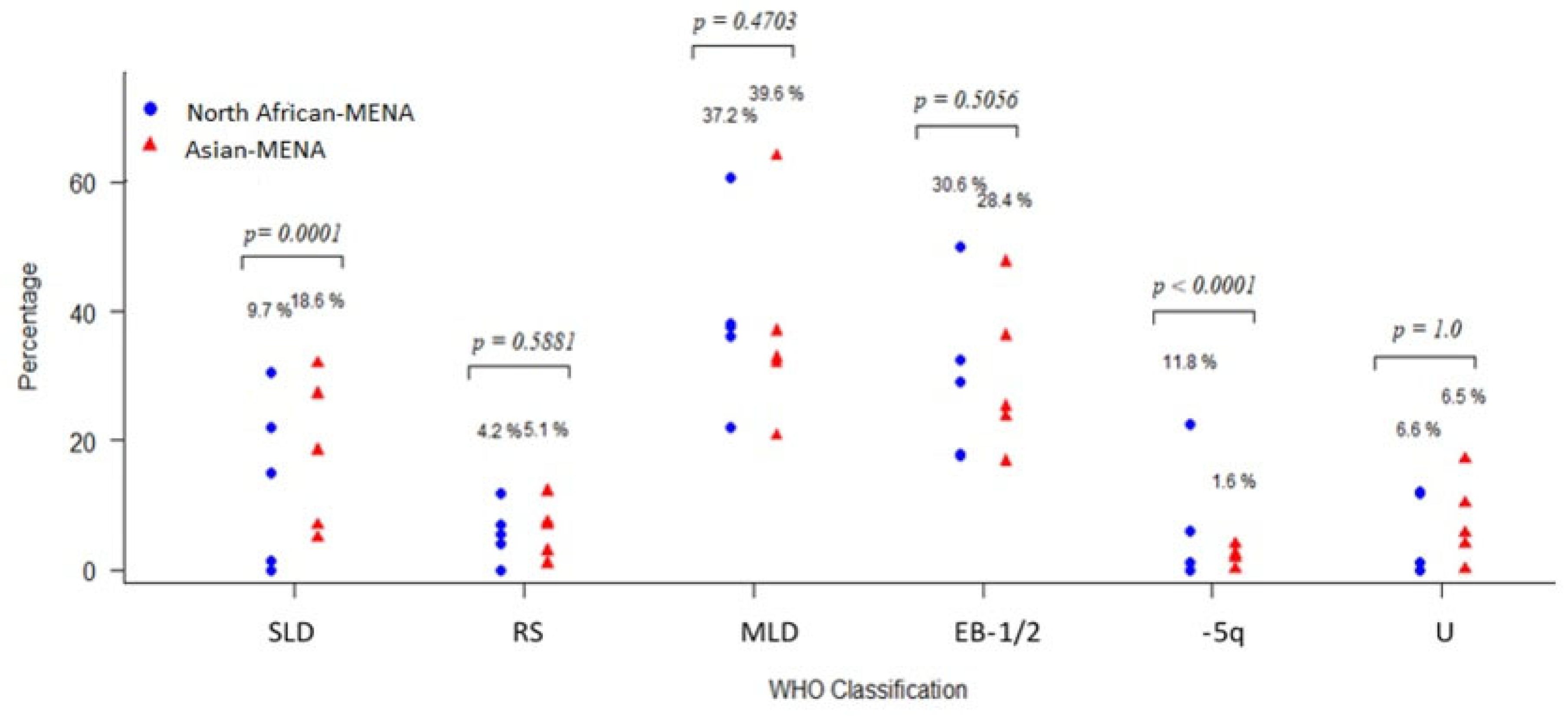
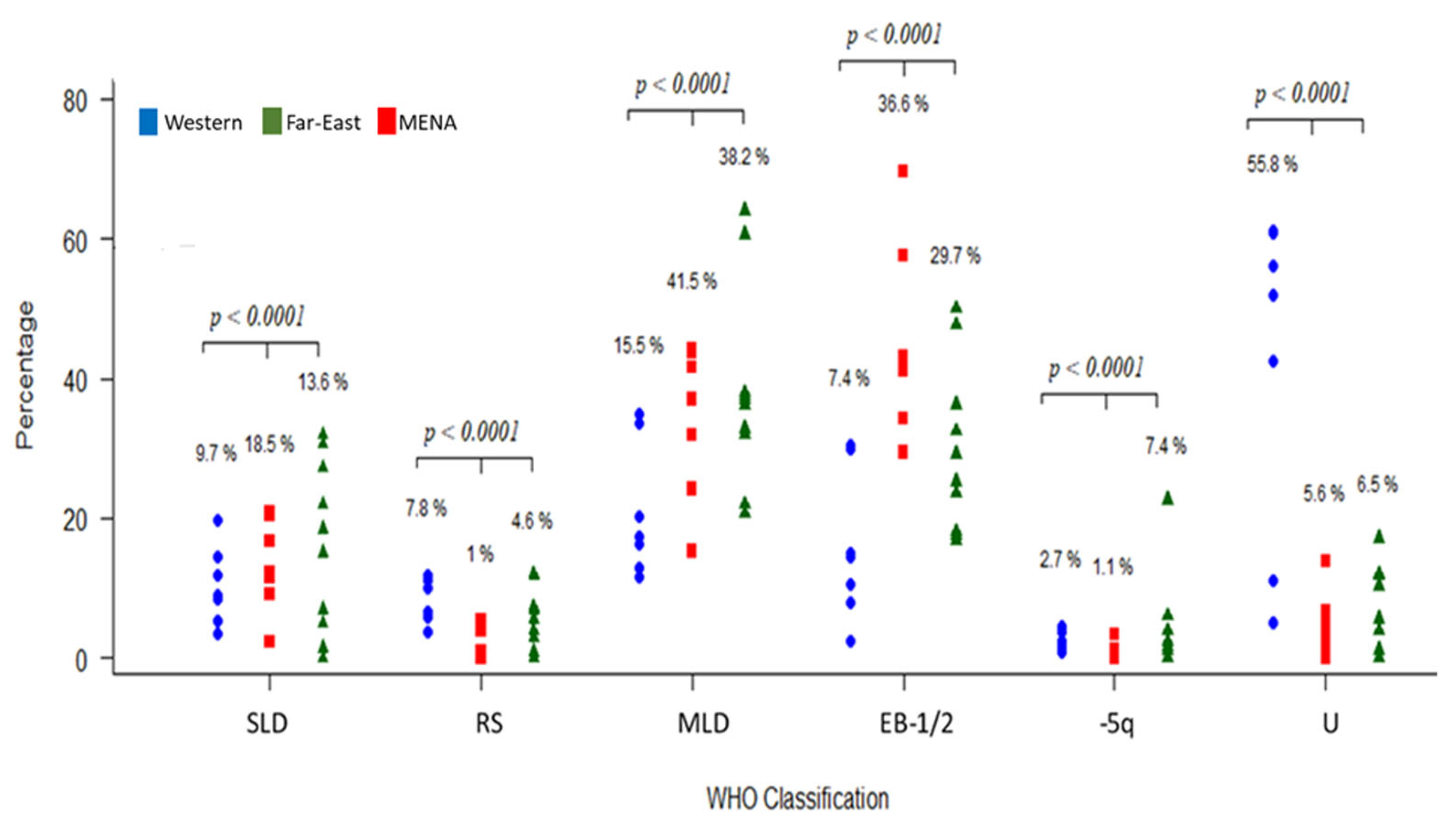
3.3.4. Patient Classification
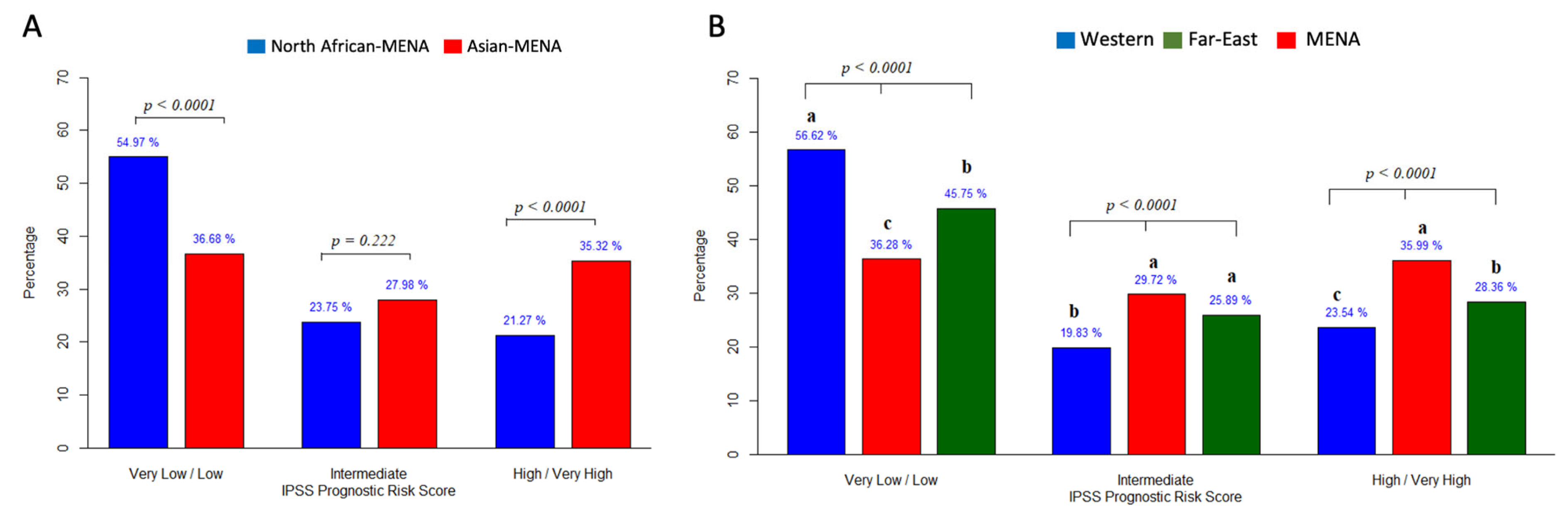
3.3.5. Prognosis
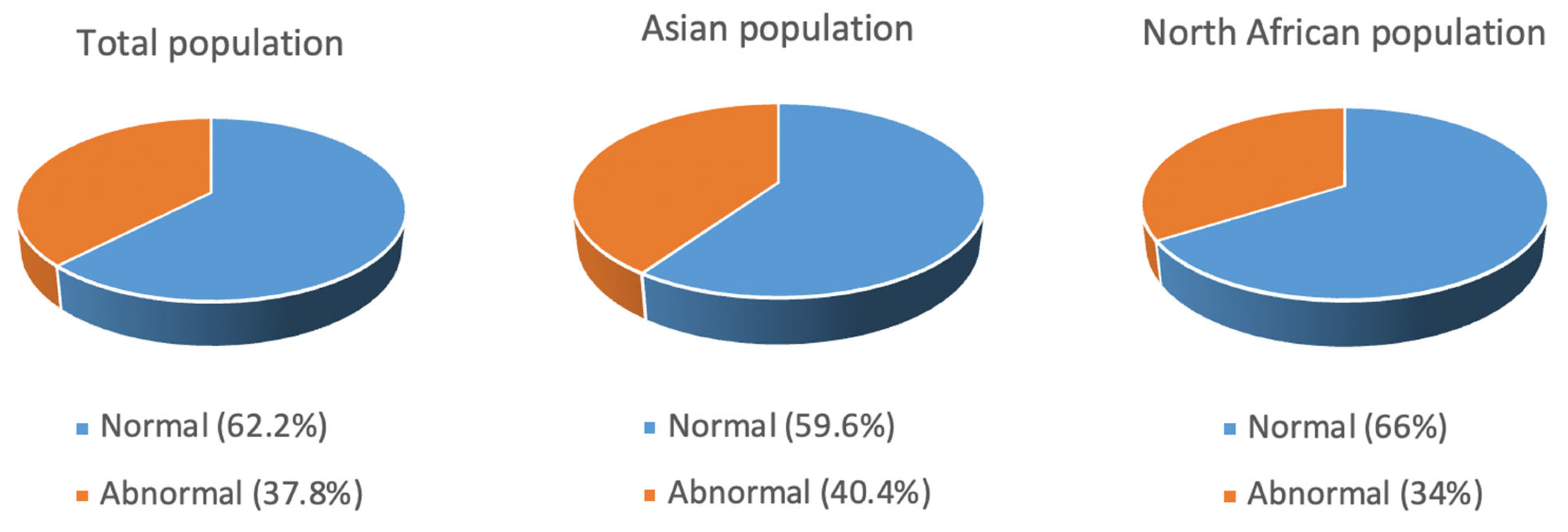
3.3.6. Karyotype
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sekeres, M.A.; Schoonen, W.M.; Kantarjian, H.; List, A.; Fryzek, J.; Paquette, R.; Maciejewski, J.P. Characteristics of US Patients with Myelodysplastic Syndromes: Results of Six Cross-Sectional Physician Surveys. J. Natl. Cancer Inst. 2008, 100, 1542–1551. [Google Scholar] [CrossRef]
- Kasprzak, A.; Kaivers, J.; Nachtkamp, K.; Haas, R.; Kobbe, G.; Gattermann, N.; Germing, U. Guidelines for Myelodysplastic Syndromes: Converting Evidence into Action? Int. J. Environ. Res. Public Health 2021, 18, 7629. [Google Scholar] [CrossRef]
- Mijovic, A.; Abdallah, A.; Pearce, L.; Tobal, K.; Mufti, G.J. Effects on Erythropoiesis of Alemtuzumab-Containing Reduced Intensity and Standard Conditioning Regimens. Br. J. Haematol. 2008, 142, 444–452. [Google Scholar] [CrossRef]
- Garcia-Manero, G.; Chien, K.S.; Montalban-Bravo, G. Myelodysplastic Syndromes: 2021 Update on Diagnosis, Risk Stratification and Management. Am. J. Hematol. 2020, 95, 1399–1420. [Google Scholar] [CrossRef]
- Cogle, C.R. Incidence and Burden of the Myelodysplastic Syndromes. Curr. Hematol. Malig. Rep. 2015, 10, 272–281. [Google Scholar] [CrossRef]
- Porwit, A.; Saft, L. The AML–MDS Interface—Leukemic Transformation in Myelodysplastic Syndromes. J. Hematop. 2011, 4, 69–79. [Google Scholar] [CrossRef]
- Shi, J.; Shao, Z.; Liu, H.; Bai, J.; Cao, Y.; He, G.; Tu, M.; Wang, X.; Hao, Y.; Yang, T.; et al. Transformation of Myelodysplastic Syndromes into Acute Myeloid Leukemias. Chin. Med. J. 2004, 117, 963–967. [Google Scholar]
- Komrokji, R.S.; Padron, E.; Lancet, J.E.; List, A.F. Prognostic Factors and Risk Models in Myelodysplastic Syndromes. Clin. Lymphoma Myeloma Leuk. 2013, 13, S295–S299. [Google Scholar] [CrossRef]
- Craig, B.M.; Rollison, D.E.; List, A.F.; Cogle, C.R. Underreporting of Myeloid Malignancies by United States Cancer Registries. Cancer Epidemiol. Biomark. Prev. 2012, 21, 474–481. [Google Scholar] [CrossRef]
- Hamed, N.A.M. What Is New in the 2016 Revision to WHO Classification of Myelodysplastic Syndromes? Cancer Ther. Oncol. Int. J. 2017, 4, 555645. [Google Scholar] [CrossRef]
- Dumper, M.; Stanley, B.E. (Eds.) Cities of the Middle East and North Africa: A Historical Encyclopedia; ABC-CLIO: Santa Barbara, CA, USA, 2007; ISBN 978-1-57607-919-5. [Google Scholar]
- AlMozain, N.; Mashi, A.; Alneami, Q.; Al-Omran, A.; Bakshi, N.; Owaidah, T.; Khalil, S.; Khogeer, H.; Hashmi, S.; Al-Sweedan, S.; et al. Spectrum of Myelodysplastic Syndrome in Patients Evaluated for Cytopenia(s). A Report from a Reference Centre in Saudi Arabia. Hematol. Oncol. Stem Cell Ther. 2020; in press. [Google Scholar] [CrossRef]
- Maaroufi, H.E.; Ababou, M.; Hammani, A.; Ahchouch, S.; Jennane, S.; Mahtat, M.; Mikdmae, M.; Messaoudi, N.; Doghmi, K. A monocentric study on the management of patients with myelodysplastic syndromes in Morocco. Pan Afr. Med. J. 2020, 37, 300. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Jiang, Y.; Eveillard, J.-R.; Couturier, M.-A.; Soubise, B.; Chen, J.-M.; Gao, S.; Basinko, A.; Morel, F.; Douet-Guilbert, N.; Troadec, M.-B. Asian Population Is More Prone to Develop High-Risk Myelodysplastic Syndrome, Concordantly with Their Propensity to Exhibit High-Risk Cytogenetic Aberrations. Cancers 2021, 13, 481. [Google Scholar] [CrossRef]
- Whiting, P.F. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann. Intern. Med. 2011, 155, 529. [Google Scholar] [CrossRef]
- Abdallah, A.M.; Carlus, S.J.; Al-Mazroea, A.H.; Alluqmani, M.; Almohammadi, Y.; Bhuiyan, Z.A.; Al-Harbi, K.M. Digenic Inheritance of LAMA4 and MYH7 Mutations in Patient with Infantile Dilated Cardiomyopathy. Medicina 2019, 55, 17. [Google Scholar] [CrossRef]
- McGrath, S.; Zhao, X.; Steele, R.; Thombs, B.D.; Benedetti, A.; The DEPRESsion Screening Data (DEPRESSD) Collaboration; Levis, B.; Riehm, K.E.; Saadat, N.; Levis, A.W.; et al. Estimating the Sample Mean and Standard Deviation from Commonly Reported Quantiles in Meta-Analysis. Stat. Methods Med. Res. 2020, 29, 2520–2537. [Google Scholar] [CrossRef]
- Talbi, T.F.; Abbadi, M.R.; Menouer, S.; Belakhel, S.; Djouadi, K.; Ardjoun, F.Z. PB2093 Clinical, Paraclinical Characteristics and Outcome in Algerian Myelodysplastic Syndromes: A Single Center Experience. HemaSphere 2019, 3, 943. [Google Scholar] [CrossRef]
- Bekadja, M.; Ahmed Nacer, R.; Hamladji, R.; Boudjerra, N.; Belhani, M.; Ardjoun, F.; Abad, M.; Touhami, H.; Ait Ali, H.; Zouaoui, Z.; et al. Epidemiology and Clinical Features of Adults Myelodysplastic Syndromes in Algeria: A Population-Based Study. Review of the Algerian Myelodysplastic Syndromes Study Group. Blood 2015, 126, 5253. [Google Scholar] [CrossRef]
- El Menshawy, N.; El-Ashwah, S.; Ebrahim, M.; Mortada, M.; Ramez, A.; Attia, D. TERT Genotype Polymorphism: A Glance of Change Egyptian MDS Outcomes. Asian Pac. J. Cancer Prev. 2021, 22, 1547–1555. [Google Scholar] [CrossRef]
- Elnahass, Y.; Youssif, L. Cytogenetic Features in Primary Myelodysplastic Syndrome Egyptian Patients. J. Adv. Res. 2018, 10, 77–83. [Google Scholar] [CrossRef]
- Paridar, M.; Zibara, K.; Ahmadi, S.E.; Khosravi, A.; Soleymani, M.; Azizi, E.; Ghalesardi, O.K. Clinico-Hematological and Cytogenetic Spectrum of Adult Myelodysplastic Syndrome: The First Retrospective Cross-Sectional Study in Iranian Patients. Mol. Cytogenet. 2021, 14, 24. [Google Scholar] [CrossRef]
- Otrock, Z.K.; Chamseddine, N.; Salem, Z.M.; Wehbe, T.; Al-Ayoubi, M.; Dhaini, M.; Kattan, J.; Mokaddem, W.; Nasr, T.A.; Jradi, O.; et al. A Nationwide Non-Interventional Epidemiological Data Registry on Myelodysplastic Syndromes in Lebanon. Am. J. Blood Res. 2015, 5, 86–90. [Google Scholar]
- Elidrissi Errahhali, M.; Elidrissi Errahhali, M.; Boulouiz, R.; Ouarzane, M.; Bellaoui, M. Distribution and Features of Hematological Malignancies in Eastern Morocco: A Retrospective Multicenter Study over 5 Years. BMC Cancer 2016, 16, 159. [Google Scholar] [CrossRef]
- Udayakumar, A.; Fawaz, N.; Pathare, A.; Asraf, S.; Al-Huneini, M.; Al-Farsi, K.; Al-Kindi, S.; Al-Khabouri, M. First Cytogenetic Profile of Omani Patients with de Novo Myelodysplastic Syndromes: Comparison with Data from Asia, Africa, Europe and North and South America. Sultan Qaboos Univ. Med. J. 2017, 17, e286–e292. [Google Scholar] [CrossRef]
- Uyanik, M.S.; Demir, A.M.; Kurt, I.; Maden, M.; Oz Puyan, F.; Gurkan, H.; Umit, E.G.; Pamuk, G.E. Could the Mosaic Pattern of Chromosomal Abnormality Predict Overall Survival of Patients with Myelodysplastic Syndrome? Hematol. Oncol. Stem Cell Ther. 2016, 9, 41–47. [Google Scholar] [CrossRef]
- Demirkan, F.; Alacacioglu, I.; Piskin, O.; Ozsan, H.G.; Akinci, B.; Ozcan, A.M.; Yavuzsen, T.; Yuksel, E.; Undar, B. The Clinical, Haematological and Morphological Profile of Patients with Myelodysplastic Syndromes: A Single Institution Experience from Turkey. Leuk. Lymphoma 2007, 48, 1372–1378. [Google Scholar] [CrossRef]
- Deviren, A.; Gürsel, İ.M.; Kuru, D.; Tarkan Argüden, Y.; Yilmaz, Ş.; Çirakoğlu, A.; Hacihanefioğlu, S. Cytogenetic Evaluation in 221 Untreated Patients with Myelodysplastic Syndrome. Turk. Klin. J. Med. Sci. 2012, 32, 15–23. [Google Scholar] [CrossRef]
- Dinmohamed, A.G.; Visser, O.; van Norden, Y.; Huijgens, P.C.; Sonneveld, P.; van de Loosdrecht, A.A.; Jongen-Lavrencic, M. Trends in Incidence, Initial Treatment and Survival of Myelodysplastic Syndromes: A Population-Based Study of 5144 Patients Diagnosed in the Netherlands from 2001 to 2010. Eur. J. Cancer 2014, 50, 1004–1012. [Google Scholar] [CrossRef]
- Moreno Berggren, D.; Folkvaljon, Y.; Engvall, M.; Sundberg, J.; Lambe, M.; Antunovic, P.; Garelius, H.; Lorenz, F.; Nilsson, L.; Rasmussen, B.; et al. Prognostic Scoring Systems for Myelodysplastic Syndromes (MDS) in a Population-Based Setting: A Report from the Swedish MDS Register. Br. J. Haematol. 2018, 181, 614–627. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Xu, X.; Lin, G. Cytogenetic Features and Prognosis Analysis in Chinese Patients with Myelodysplastic Syndrome: A Multicenter Study. Ann. Hematol. 2010, 89, 535–544. [Google Scholar] [CrossRef]
- Yao, C.-Y.; Hou, H.-A.; Lin, T.-Y.; Lin, C.-C.; Chou, W.-C.; Tseng, M.-H.; Chiang, Y.-C.; Liu, M.-C.; Liu, C.-W.; Kuo, Y.-Y.; et al. Distinct Mutation Profile and Prognostic Relevance in Patients with Hypoplastic Myelodysplastic Syndromes (h-MDS). Oncotarget 2016, 7, 63177–63188. [Google Scholar] [CrossRef]
- Qu, S.; Xu, Z.; Zhang, Y.; Qin, T.; Zhang, T.; Cui, R.; Xiao, Z. Impacts of Cytogenetic Categories in the Revised International Prognostic Scoring System on the Prognosis of Primary Myelodysplastic Syndromes: Results of a Single-Center Study. Leuk. Lymphoma 2012, 53, 940–946. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Tuechler, H.; Sanz, G.; Schanz, J.; Garcia-Manero, G.; Solé, F.; Bennett, J.M.; Bowen, D.; Fenaux, P.; Dreyfus, F.; et al. Differing Clinical Features between Japanese and Caucasian Patients with Myelodysplastic Syndromes: Analysis from the International Working Group for Prognosis of MDS. Leuk. Res. 2018, 73, 51–57. [Google Scholar] [CrossRef]
- Rollison, D.E.; Howlader, N.; Smith, M.T.; Strom, S.S.; Merritt, W.D.; Ries, L.A.; Edwards, B.K.; List, A.F. Epidemiology of Myelodysplastic Syndromes and Chronic Myeloproliferative Disorders in the United States, 2001–2004, Using Data from the NAACCR and SEER Programs. Blood 2008, 112, 45–52. [Google Scholar] [CrossRef]
- Cancer of the Endometrium—Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/corp.html (accessed on 3 April 2020).
- Bonadies, N.; Feller, A.; Rovo, A.; Ruefer, A.; Blum, S.; Gerber, B.; Stuessi, G.; Benz, R.; Cantoni, N.; Holbro, A.; et al. Trends of Classification, Incidence, Mortality, and Survival of MDS Patients in Switzerland between 2001 and 2012. Cancer Epidemiol. 2017, 46, 85–92. [Google Scholar] [CrossRef]
- McQuilten, Z.K.; Wood, E.M.; Polizzotto, M.N.; Campbell, L.J.; Wall, M.; Curtis, D.J.; Farrugia, H.; McNeil, J.J.; Sundararajan, V. Underestimation of Myelodysplastic Syndrome Incidence by Cancer Registries: Results from a Population-Based Data Linkage Study: MDS Incidence Data Linkage Study. Cancer 2014, 120, 1686–1694. [Google Scholar] [CrossRef]
- Park, E.-H.; Lee, H.; Won, Y.-J.; Ju, H.Y.; Oh, C.-M.; Ingabire, C.; Kong, H.-J.; Park, B.-K.; Yoon, J.Y.; Eom, H.-S.; et al. Nationwide Statistical Analysis of Myeloid Malignancies in Korea: Incidence and Survival Rate from 1999 to 2012. Blood Res. 2015, 50, 204. [Google Scholar] [CrossRef]
- Wang, W.; Wang, H.; Wang, X.-Q.; Lin, G.-W. First Report of Incidence of Adult Myelodysplastic Syndrome in China. Ann. Hematol. 2012, 91, 1321–1322. [Google Scholar] [CrossRef]
- Li, X.; Xiao, Z.; Chang, C.; Xu, F.; Wu, L.; He, Q.; Xu, Z.; Song, L.; Zhang, Z.; Zhou, L.; et al. Distinct Clinical and Experimental Characteristics in the Patients Younger than 60 Years Old with Myelodysplastic Syndromes. PLoS ONE 2013, 8, e57392. [Google Scholar] [CrossRef]
- Avgerinou, C.; Alamanos, Y.; Zikos, P.; Lampropoulou, P.; Melachrinou, M.; Labropoulou, V.; Tavernarakis, I.; Aktypi, A.; Kaiafas, P.; Raptis, C.; et al. The Incidence of Myelodysplastic Syndromes in Western Greece Is Increasing. Ann. Hematol. 2013, 92, 877–887. [Google Scholar] [CrossRef]
- Domenica Cappellini, M.; Motta, I. Anemia in Clinical Practice—Definition and Classification: Does Hemoglobin Change with Aging? Semin. Hematol. 2015, 52, 261–269. [Google Scholar] [CrossRef]
- Chen, B.; Zhao, W.-L.; Jin, J.; Xue, Y.-Q.; Cheng, X.; Chen, X.-T.; Cui, J.; Chen, Z.-M.; Cao, Q.; Yang, G.; et al. Clinical and Cytogenetic Features of 508 Chinese Patients with Myelodysplastic Syndrome and Comparison with Those in Western Countries. Leukemia 2005, 19, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Chaubey, R.; Sazawal, S.; Dada, R.; Mahapatra, M.; Saxena, R. Cytogenetic Profile of Indian Patients with de Novo Myelodysplastic Syndromes. Indian J. Med. Res. 2011, 134, 452–457. [Google Scholar] [PubMed]
- Ehsan, A.; Aziz, M. Clinico-Haematological Characteristics in Pakistani Patients of Primary Myelodysplastic Syndrome According to World Health Organization Classification. J. Coll. Physicians Surg. Pak. JCPSP 2010, 20, 232–236. [Google Scholar] [PubMed]
- Mahmood, R.; Altaf, C.; Ahmed, P.; Khan, S.A.; Malik, H.S. Myelodysplastic Syndrome in Pakistan: Clinicohematological Characteristics, Cytogenetic Profile, and Risk Stratification. Turk. J. Hematol. 2018, 35, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Pozdnyakova, O.; Miron, P.M.; Tang, G.; Walter, O.; Raza, A.; Woda, B.; Wang, S.A. Cytogenetic Abnormalities in a Series of 1029 Patients with Primary Myelodysplastic Syndromes: A Report from the US with a Focus on Some Undefined Single Chromosomal Abnormalities. Cancer 2008, 113, 3331–3340. [Google Scholar] [CrossRef]
- Li, L.; Liu, X.-P.; Nie, L.; Yu, M.-H.; Zhang, Y.; Qin, T.-J.; Xiao, Z.-J. Unique Cytogenetic Features of Primary Myelodysplastic Syndromes in Chinese Patients. Leuk. Res. 2009, 33, 1194–1198. [Google Scholar] [CrossRef]
- Akhtar, M.; Al Hyassat, S.; Elaiwy, O.; Rashid, S.; Al-Nabet, A.D.M.H. Classification of Endometrial Carcinoma: New Perspectives Beyond Morphology. Adv. Anat. Pathol. 2019, 26, 421–427. [Google Scholar] [CrossRef]
- Haase, D.; Germing, U.; Schanz, J.; Pfeilstöcker, M.; Nösslinger, T.; Hildebrandt, B.; Kundgen, A.; Lübbert, M.; Kunzmann, R.; Giagounidis, A.A.N.; et al. New Insights into the Prognostic Impact of the Karyotype in MDS and Correlation with Subtypes: Evidence from a Core Dataset of 2124 Patients. Blood 2007, 110, 4385–4395. [Google Scholar] [CrossRef]
- Gmidène, A.; Sennana, H.; Fenaux, P.; Laatiri, A.; Zarrouk, M.; Bouaziz, H.; Harrabi, I.; Saad, A. Cytogenetic Abnormalities in Tunisian de Novo Myelodysplastic Syndrome: A Comparison with Other Populations. Leuk. Res. 2008, 32, 1824–1829. [Google Scholar] [CrossRef]
- Voso, M.T.; Fenu, S.; Latagliata, R.; Buccisano, F.; Piciocchi, A.; Aloe-Spiriti, M.A.; Breccia, M.; Criscuolo, M.; Andriani, A.; Mancini, S.; et al. Revised International Prognostic Scoring System (IPSS) Predicts Survival and Leukemic Evolution of Myelodysplastic Syndromes Significantly Better Than IPSS and WHO Prognostic Scoring System: Validation by the Gruppo Romano Mielodisplasie Italian Regional Database. J. Clin. Oncol. 2013, 31, 2671–2677. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S. Clinical, Hematological, and Cytogenetic Profile of Adult Myelodysplastic Syndrome in a Tertiary Care Center. J. Blood Med. 2017, 8, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, A.M.; Alnuzha, A.; Al-Mazroea, A.H.; Eldardear, A.E.; AlSamman, A.Y.; Almohammadi, Y.; Al-Harbi, K.M. IL10 Promoter Polymorphisms Are Associated with Rheumatic Heart Disease in Saudi Arabian Patients. Pediatr. Cardiol. 2016, 37, 99–105. [Google Scholar] [CrossRef]
- Carraway, H.E.; Saygin, C. Therapy for Lower-Risk MDS. Hematol. Am. Soc. Hematol. Educ. Program 2020, 2020, 426–433. [Google Scholar] [CrossRef] [PubMed]
| Included | Excluded | |
|---|---|---|
| Classification | WPSS and IPSS | FAB |
| Population | MENA population | Other populations |
| Intervention | None | None |
| Comparator | Patient at presentation assessment | None |
| Study Design | Prospective and retrospective cohort report, case-control | RCTs, case reports |
| Primary Outcome | Disease severity at presentation | Irrelevant |
| Others | Published research articles | Unpublished data, review articles |
| Authors and Year of Study | Country | Number of Patients | Gender | Age Mean | SD | |||
|---|---|---|---|---|---|---|---|---|
| M | F | Ratio (M:F) | ||||||
| 1 | Talbi et al. (2019) [19] | Algeria | 243 | 126 | 117 | 1.1 | NA | NA |
| 2 | Bekadja et al. (2015) [20] | Algeria | 85 | 55 | 30 | 1.8 | 53.3 | 19.9 |
| 3 | El-Menshawy et al. (2021) [21] | Egypt | 100 | 54 | 46 | 1.2 | 56 | 11.0 |
| 4 | Elnahass et al. (2018) [22] | Egypt | 50 | 29 | 21 | 1.4 | 56 | 12.03 |
| 5 | Paridar et al. (2021) [23] | Iran | 103 | 75 | 28 | 2.68 | 66 | 5.5 |
| 6 | Otrock et al. (2015) [24] | Lebanon | 58 | 29 | 29 | 1 | 71 | 10.0 |
| 7 | Errahhali et al. (2016) [25] | Morocco | 20 | 8 | 12 | 0.67 | 52.8 | NA |
| 8 | El Maaroufi et al. (2020) [13] | Morocco | 76 | 43 | 33 | 1.3 | 65.7 | 14.0 |
| 9 | Udayakumar et al. (2017) [26] | Oman | 36 | 18 | 18 | 1 | 63 | 12.7 |
| 10 | AlMozain (2020) [12] | Saudi Arabia | 82 | 50 | 32 | 1.6 | 50 | 18.0 |
| 11 | Uyanik et al. (2015) [27] | Turkey | 119 | 59 | 60 | 1 | 66.3 | 12.3 |
| 12 | Demirkan et al. (2007) [28] | Turkey | 113 | 76 | 37 | 2.1 | 64.7 | 12.4 |
| 13 | Deviren et al. (2012) [29] | Turkey | 221 | 132 | 89 | 1.5 | 48.1 | 16.7 |
| Total for North African MENA | 574 | 315 | 259 | 1.3 | 57.3 | |||
| Total for Asian MENA | 732 | 439 | 293 | 1.5 | 59.0 | |||
| Overall total | 1306 | 754 | 552 | 1.4 | 58.4 | |||
| Clinical Parameter | All Patients (Total n = 359) n (%) | North African MENA (Total n = 264) n (%) | Asian MENA (Total n = 95) n (%) | |
|---|---|---|---|---|
| Cytopenia | None | 1 (0.3) | 0 | 1 (1.1) |
| Unicytopenia | 106 (38.2) | 85 (32.2) | 52 (54.7) | |
| Bicytopenia | 99 (27.6) | 84 (31.8) | 15 (15.8) | |
| Pancytopenia | 122 (34) | 95 (36) | 27 (28.4) | |
| All Patients (Total n = 191) n (%) | North African MENA (Total n = 124) n (%) | Asian MENA (Total n = 67) n (%) | ||
| Bone marrow blasts | <5% | 134 (70.2) | 90 (72.5) | 44 (65.6) |
| 5–9% | 35 (18.3) | 21 (17) | 14 (21) | |
| 10–19% | 22 (11.5) | 13 (10.5) | 9 (13.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Haidose, A.; Yassin, M.A.; Ahmed, M.N.; Kunhipurayil, H.H.; Al-Harbi, A.A.; Aljaberi, M.A.; Abbasi, S.A.; Kordasti, S.; Abdallah, A.M. Distinct Clinical and Prognostic Features of Myelodysplastic Syndrome in Patients from the Middle East, North Africa, and Beyond: A Systemic Review. J. Clin. Med. 2023, 12, 2832. https://doi.org/10.3390/jcm12082832
Al-Haidose A, Yassin MA, Ahmed MN, Kunhipurayil HH, Al-Harbi AA, Aljaberi MA, Abbasi SA, Kordasti S, Abdallah AM. Distinct Clinical and Prognostic Features of Myelodysplastic Syndrome in Patients from the Middle East, North Africa, and Beyond: A Systemic Review. Journal of Clinical Medicine. 2023; 12(8):2832. https://doi.org/10.3390/jcm12082832
Chicago/Turabian StyleAl-Haidose, Amal, Mohamed A. Yassin, Muna N. Ahmed, Hasna H. Kunhipurayil, Asrar A. Al-Harbi, Musheer A. Aljaberi, Saddam A. Abbasi, Shahram Kordasti, and Atiyeh M. Abdallah. 2023. "Distinct Clinical and Prognostic Features of Myelodysplastic Syndrome in Patients from the Middle East, North Africa, and Beyond: A Systemic Review" Journal of Clinical Medicine 12, no. 8: 2832. https://doi.org/10.3390/jcm12082832
APA StyleAl-Haidose, A., Yassin, M. A., Ahmed, M. N., Kunhipurayil, H. H., Al-Harbi, A. A., Aljaberi, M. A., Abbasi, S. A., Kordasti, S., & Abdallah, A. M. (2023). Distinct Clinical and Prognostic Features of Myelodysplastic Syndrome in Patients from the Middle East, North Africa, and Beyond: A Systemic Review. Journal of Clinical Medicine, 12(8), 2832. https://doi.org/10.3390/jcm12082832







