Ultrafiltration versus Diuretics on Prognostic Cardiac and Renal Biomarkers in Acute Decompensated Heart Failure: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
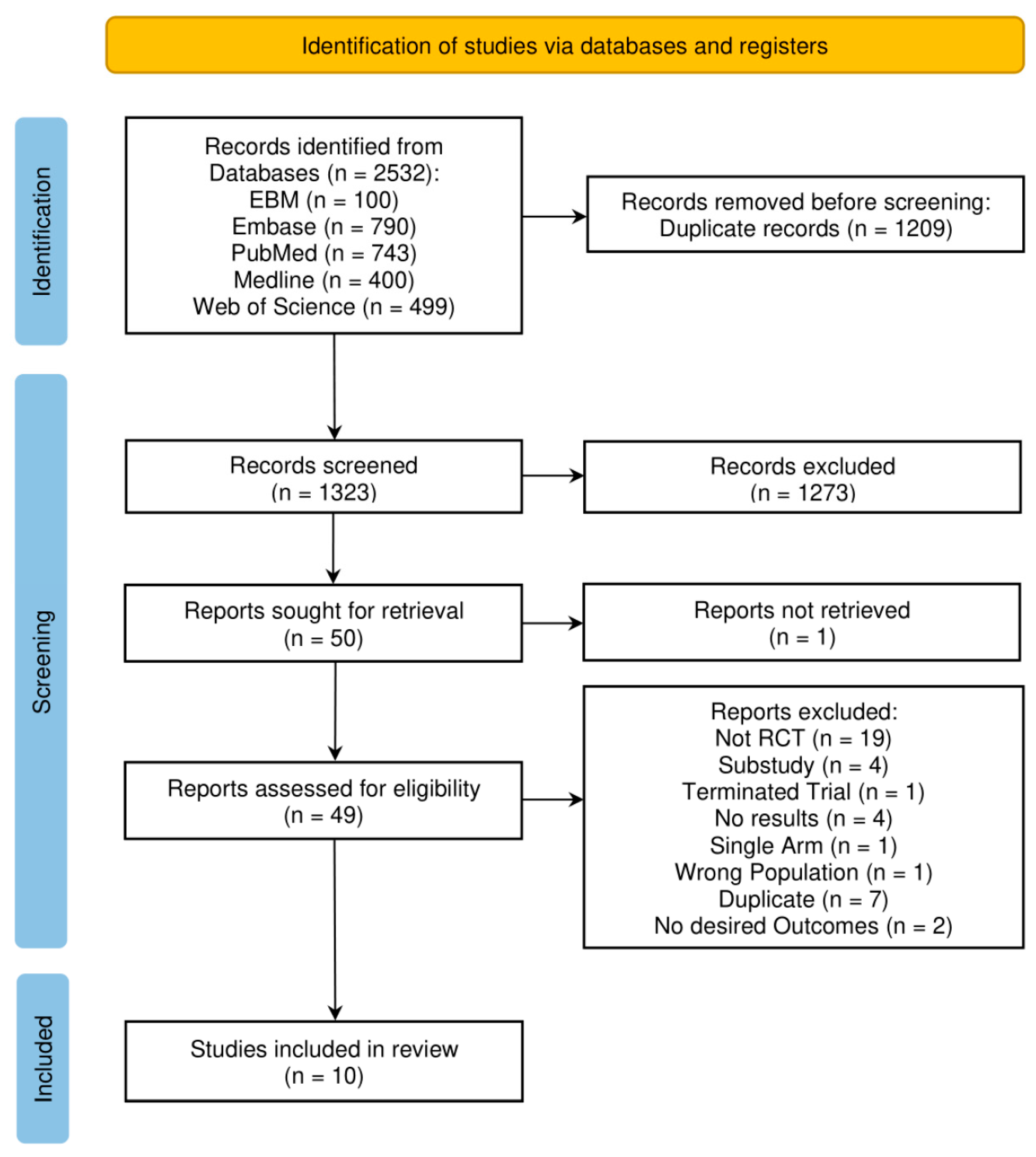
2. Materials and Methods
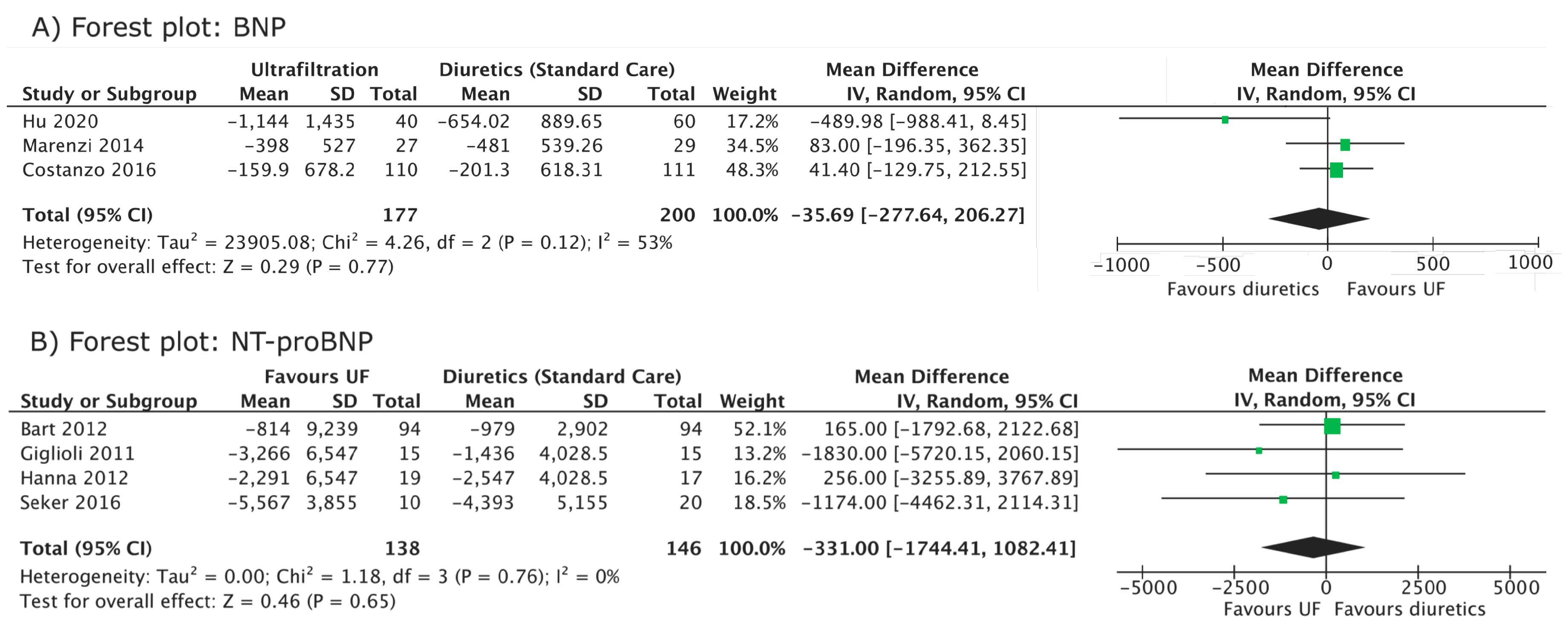
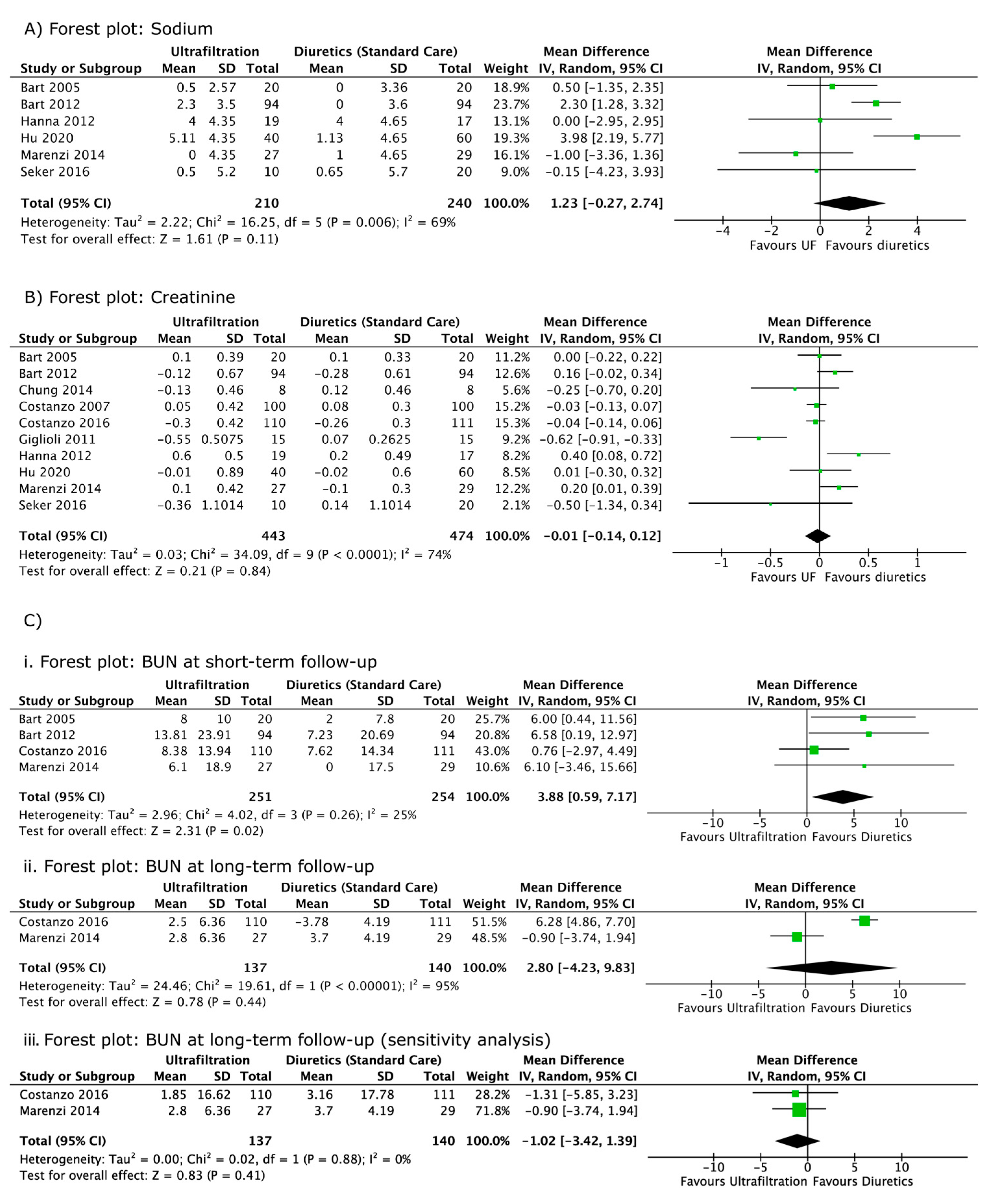
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2021, 42, 3599–3726. [Google Scholar]
- Grodin, J.L.; Carter, S.; Bart, B.A.; Goldsmith, S.R.; Drazner, M.H.; Tang, W.H.W. Direct comparison of ultrafiltration to pharmacological decongestion in heart failure: A per-protocol analysis of CARRESS-HF. Eur. J. Heart Fail. 2018, 20, 1148–1156. [Google Scholar] [CrossRef]
- Bart, B.A.; Boyle, A.; Bank, A.J.; Anand, I.; Olivari, M.T.; Kraemer, M.; Mackedanz, S.; Sobotka, P.A.; Schollmeyer, M.; Goldsmith, S.R. Ultrafiltration Versus Usual Care for Hospitalized Patients with Heart Failure: The Relief for Acutely Fluid-Overloaded Patients With Decompensated Congestive Heart Failure (RAPID-CHF) Trial. J. Am. Coll. Cardiol. 2005, 46, 2043–2046. [Google Scholar] [CrossRef] [PubMed]
- Giglioli, C.; Landi, D.; Cecchi, E.; Chiostri, M.; Gensini, G.F.; Valente, S.; Ciaccheri, M.; Castelli, G.; Romano, S.M. Effects of ultrafiltration vs. diuretics on clinical, biohumoral and haemodynamic variables in patients with decompensated heart failure: The ultradisco study. Eur. J. Heart Fail. 2011, 13, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, M.R.; Negoianu, D.; Jaski, B.E.; Bart, B.A.; Heywood, J.T.; Anand, I.S.; Smelser, J.M.; Kaneshige, A.M.; Chomsky, D.B.; Adler, E.D.; et al. Aquapheresis Versus Intravenous Diuretics and Hospitalizations for Heart Failure. JACC Heart Fail. 2016, 4, 95–105. [Google Scholar] [CrossRef]
- Hanna, M.A.; Tang, W.H.; Teo, B.W.; O’Neill, J.O.; Weinstein, D.M.; Lau, S.M.; Van Lente, F.; Starling, R.C.; Paganini, E.P.; Taylor, D.O. Extracorporeal Ultrafiltration vs Conventional Diuretic Therapy in Advanced Decompensated Heart Failure. Congest. Heart Fail. 2012, 18, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, M.R.; Guglin, M.E.; Saltzberg, M.T.; Jessup, M.L.; Bart, B.A.; Teerlink, J.R.; Jaski, B.E.; Fang, J.C.; Feller, E.D.; Haas, G.J.; et al. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. Commentary. J. Am. Coll. Cardiol. 2007, 49, 675–686. [Google Scholar] [CrossRef]
- Srivastava, M.; Harrison, N.; Caetano, A.F.S.; Tan, A.R.; Law, M. Ultrafiltration for acute heart failure. Cochrane Database Syst. Rev. 2022, 2022, CD013593. [Google Scholar]
- Kwok, C.S.; Wong, C.W.; Rushton, C.; Ahmed, F.; Cunnington, C.; Davies, S.J.; Patwala, A.; Mamas, M.A.; Satchithananda, D. Ultrafiltration for acute decompensated cardiac failure: A systematic review and meta-analysis. Int. J. Cardiol. 2017, 228, 122–128. [Google Scholar] [CrossRef]
- Bart, B.A.; Goldsmith, S.R.; Lee, K.L.; Givertz, M.M.; O′Connor, C.M.; Bull, D.A.; Redfield, M.M.; Deswal, A.; Rouleau, J.L.; LeWinter, M.M.; et al. Ultrafiltration in Decompensated Heart Failure with Cardiorenal Syndrome. N. Engl. J. Med. 2012, 367, 2296–2304. [Google Scholar] [CrossRef]
- Anand, I.S.; Fisher, L.D.; Chiang, Y.-T.; Latini, R.; Masson, S.; Maggioni, A.P.; Glazer, R.D.; Tognoni, G.; Cohn, J.N. Changes in Brain Natriuretic Peptide and Norepinephrine Over Time and Mortality and Morbidity in the Valsartan Heart Failure Trial (Val-HeFT). Circulation 2003, 107, 1278–1283. [Google Scholar] [CrossRef]
- Bodaghi, A.; Fattahi, N.; Ramazani, A. Biomarkers: Promising and valuable tools towards diagnosis, prognosis and treatment of Covid-19 and other diseases. Heliyon 2023, 9, e13323. [Google Scholar] [CrossRef]
- Han, S.W.; Ryu, K.H. Renal Dysfunction in Acute Heart Failure. Korean Circ. J. 2011, 41, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons, Ltd.: Newark, NJ, USA, 2019. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wan, Q.; Zhang, Y.; Zhou, J.; Li, M.; Jiang, L.; Yuan, F. Efficacy and safety of early ultrafiltration in patients with acute decompensated heart failure with volume overload: A prospective, randomized, controlled clinical trial. BMC Cardiovasc. Disord. 2020, 20, 447. [Google Scholar] [CrossRef]
- Marenzi, G.; Muratori, M.; Cosentino, E.R.; Rinaldi, E.R.; Donghi, V.; Milazzo, V.; Ferramosca, E.; Borghi, C.; Santoro, A.; Agostoni, P. Continuous ultrafiltration for congestive heart failure: The CUORE trial. J. Card. Fail. 2014, 20, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Noyes, J.; Booth, A.; Cargo, M.; Flemming, K.; Garside, R.; Hannes, K.; Harden, A.; Harris, J.; Lewin, S.; Pantoja, T.; et al. Cochrane Qualitative and Implementation Methods Group guidance series—Paper 1: Introduction. J. Clin. Epidemiol. 2018, 97, 35–38. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Deeks, J.J.; Higgins, J.P.; Altman, D.G. Analysing Data and Undertaking Meta-Analyses. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons, Ltd.: Chichester, UK, 2008; pp. 243–296. [Google Scholar]
- Chung, E.S.; O′Brien, T.M.; Menon, S.; Bartone, C.; Mazur, W.; Kereiakes, D.J. A Pilot Study of Target Weight Guided Treatment in Acute Heart Failure Using Ultrafiltration or Usual Care: Effect on Sodium Removal. Korean Circ. J. 2014, 44, 156–161. [Google Scholar] [CrossRef]
- Şeker, A.; Kayataş, M.; Hüzmeli, C.; Candan, F.; yılmaz, M.B. Comparison of Ultrafiltration and Intravenous Diuretic Therapies in Patients Hospitalized for Acute Decompensated Biventricular Heart Failure. Turk Nefroloji Dializ ve Transplantasyon Dergisi 2016, 25, 79–87. [Google Scholar] [CrossRef]
- Sav, T.; Cecen, F.; Albayrak, E.S. The effects of ultrafiltration and diuretic therapies on oxidative stress markers in patients with cardio-renal syndrome. Minerva Urol. Nephrol. 2017, 69, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Kitai, T.; Grodin, J.L.; Kim, Y.; Tang, W.H.W. Impact of ultrafiltration on serum sodium homeostasis and its clinical implication in patients with acute heart failure, congestion, and worsening renal function. Circ. Heart Fail. 2017, 10, e003603. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, W.J.; Kohut, A.R.; Hasni, S.F.; Goldman, J.M.; Silverman, B.; Kelepouris, E.; Eisen, H.J.; Aggarwal, S. Readmission rate after ultrafiltration in acute decompensated heart failure: A systematic review and meta-analysis. Heart Fail. Rev. 2017, 22, 685–698. [Google Scholar] [CrossRef]
- Wobbe, B.; Wagner, J.; Szabó, D.K.; Rostás, I.; Farkas, N.; Garami, A.; Balaskó, M.; Hartmann, P.; Solymár, M.; Tenk, J.; et al. Ultrafiltration is better than diuretic therapy for volume-overloaded acute heart failure patients: A meta-analysis. Heart Fail. Rev. 2020, 26, 577–585. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Y.; Jin, H. Ultrafiltration for patients with acute decompensated heart failure: A systematic review and meta-analysis. Medicine 2021, 100, e28029. [Google Scholar] [CrossRef]
- Costanzo, M.R. Ultrafiltration in the Management of Heart Failure. US Cardiol. Rev. 2008, 5, 66–69. [Google Scholar] [CrossRef]
- Prabhakar, S.S. CHAPTER 8—Nephrology. In Medical Secrets, 5th ed.; Harward, M.P., Ed.; Mosby: Saint Louis, MO, USA, 2012; pp. 190–219. [Google Scholar]
- Kaissling, B.; Stanton, B.A. Adaptation of distal tubule and collecting duct to increased sodium delivery. I. Ultrastructure. Am. J. Physiol.-Ren. Physiol. 1988, 255, 1256–1268. [Google Scholar] [CrossRef] [PubMed]
- Cuthbert, J.J.; Bhandari, S.; Clark, A.L. Hypochloraemia in Patients with Heart Failure: Causes and Consequences. Cardiol. Ther. 2020, 9, 333–347. [Google Scholar] [CrossRef] [PubMed]
| Bart [3] | Costanzo [7] | Giglioli [4] | Bart [10] | Hanna [6] | Chung [21] | Marenzi [17] | Costanzo [5] | Şeker [22] | Hu [16] | |
|---|---|---|---|---|---|---|---|---|---|---|
| Year | 2005 | 2007 | 2011 | 2012 | 2012 | 2014 | 2014 | 2016 | 2016 | 2020 |
| Trial | RAPID-CHF | UNLOAD | ULTRADISCO | CARRESS-HF | - | - | CUORE | AVOID-HF | - | - |
| Country | USA | USA | Italy | Canada, USA | USA | USA | Italy | USA | Turkey | China |
| Sample size | 40 | 200 | 30 | 188 | 36 | 16 | 56 | 221 | 30 | 100 |
| Intervention (UF) cohort size | 20 | 100 | 15 | 94 | 19 | 8 | 27 | 110 | 10 | 40 |
| Diuretics cohort size | 20 | 100 | 15 | 94 | 17 | 8 | 29 | 111 | 20 | 60 |
| Mean ± SD age of UF cohort | 67.5 * | 62 ± 15 | 72.4 ± 14.1 | 68.9 ± 12.0 | 60 ± 9.1 | 69 ± 14 | 75 ± 8 | 67 ± 13 | 66.5 ± 9.8 | 70.6 ± 10.44 |
| Mean ± SD age of diuretics cohort | 69.5 * | 63 ± 14 | 65.8 ± 18.4 | 67.1 ± 13.7 | 59 ± 15.5 | 74 ± 12 | 73 ± 9 | 67 ± 13 | 66.8 ±10.2 | 73.52 ± 9.83 |
| % Male in UF | 70 | 70 | 87 | 78 | 84.2 | 87.5 | 81 | 69.1 | 60 | 55 |
| % Male in diuretics | 70 | 68 | 87 | 72 | 76.5 | 100 | 83 | 73 | 65 | 55 |
| Study ID | Protocol for Ultrafiltration (UF) Group | Protocol for Diuretics Group |
|---|---|---|
| Bart et al., 2005 (RAPID-CHF) [3] | System 100 was used for a single 8 h course with fluid removal rates determined by the attending physician (up to a maximum of 500 cc/h). For the duration of UF, diuretics were withheld. Additional courses of UF were allowed at the discretion of treating physicians after 24 h endpoints were assessed. The median cumulative dose of furosemide received during the first 24 h was 80 mg. The median volume of ultrafiltrate removed was 3213 mL. | Standard therapies as per local guidelines were given. The median cumulative dose of furosemide received during the first 24 h was 160 mg. |
| Costanzo et al., 2007 (UNLOAD trial) [7] | Aquadex System 100 was used with a blood flow ranging between 10 and 40 mL/min and with a total blood volume of 33 mL. The duration and rate (up to 500 mL/h) were decided by the treating physician. Intravenous diuretics were prohibited in the first 48 h after enrolment. Administration of IV vasoactive drugs were not prohibited, but patients requiring it 48 h post-randomisation were considered to have failed treatment. The mean rate of fluid removal was 241 mL/h for 12.3 ± 12 h. | Intravenous diuretics were used. The dose of diuretics had to be at least twice the dose of pre-hospitalisation. A total of 68 patients received bolus injections, and 32 received continuous infusion of intravenous diuretics. The mean dose of diuretics given daily was 181 ± 121 mg during the 48 h after randomisation. |
| Giglioli et al., 2011 (ULTRADISCO) [4] | PRISMA System was used (M 100 PRESET PRISMA filter) with an blood flow rate of 150 mL/h. Continuous UF technique was used, with the rate of fluid removal ranging from 100 to 300 mL/h, which was adjusted according to the response. The duration of UF differed according to the clinical condition of the patient. Intravenous diuretic therapy was discontinued during UF treatment. Administration of IV inotropes were not prohibited, but patients requiring it post-randomisation were considered to have failed treatment (no patients required inotropes). | Continuous infusion of furosemide at an initial dose of 250 mg/24 h. This was reduced or increased, depending on patient response. The maximum dose was 500 mg/24 h. Administration of IV inotropes were not prohibited, but patients requiring it post-randomisation were considered to have failed treatment (no patients required inotropes). |
| Bart et al., 2012 (CARRESS-HF) [10] | The Aquadex System was used with a fluid removal rate of 200 mL/h. Loop diuretics were withheld for the duration of UF treatment in the intervention group. Additional treatments (with vasodilators or positive inotropes) were discontinued after randomisation unless deemed necessary as life-saving therapies. | Intravenous diuretics were used, and the dose was adjusted to maintain a urinary output of 3 to 5 L/24 h. Treatment was continued by the treating physician until volume status (based on blood pressure, physical exam findings, haemodynamics, BUN, and creatinine) was optimised. The use of IV vasodilators and inotropic agents was allowed in patients that did not meet their target urine output. |
| Hanna et al., 2012 [6] | NxStage System One was used with a blood flow rate of 200 to 300 mL/min. UF rate was set at 400 mL/h for 6 h and then decreased to 200 mL/h (changes were permitted if clinically indicated). All diuretics except for spironolactone (≤25 mg/dL) were stopped during UF treatment. Intravenous vasoactive medication was used under the discretion of the treating physician based on haemodynamic targets. Vasoactive doses were only reduced and never increased. | IV diuretics were used at doses and frequencies designated by the treating clinician. Intravenous vasoactive medication was used under the discretion of the treating physician based on haemodynamic targets. |
| Chung et al., 2014 [21] | The Aquadex 100 system was to achieve a target weight removal that was established by the heart failure service. The mean UF rate was 162 mL/h. Loop diuretics were discontinued in patients in the UF group after randomisation. | Continuous intravenous furosemide infusions were given to achieve the removal of a target weight established by the heart failure service.The mean daily dose of furosemide was 212 mg. |
| Marenzi et al., 2014 (CUORE Trial) [17] | A simplified Device (Peristaltic pump and polysulphone filter) was used with a blood flow rate from 40 to 100 mL/min and a total extracorporeal blood volume of 100 mL. The session duration and UF rate (100–500 mL/h) were determined by the treating physician. The number of sessions varied between one or two sessions. Single-session UF was performed in twenty patients, while seven patients required double-daily sessions. The mean time of UF treatment was 19 ± 10 h. Intravenous diuretics that were initiated before randomisation were allowed to be continued throughout the duration of treatment. The mean dosage of intravenous furosemide given was 194 ± 175 mg/day. | Intravenous loop diuretics were used according to guideline recommendations under the supervision of an experienced HF cardiologist. Intravenous diuretics that were initiated before randomisation were allowed to be continued throughout the duration of treatment. The mean dosage of intravenous furosemide given was 153 ± 115 mg/day. |
| Costanzo et al., 2016 (AVOID-HF) [5] | The Aquadex FlexFlow System was used at an initial rate between 150 and 250 cc/h, which was determined by the patient’s initial systolic blood pressure. The therapy was adjusted according to the patient’s response. UF was administered at an average rate of 138 ± 47 mL/h for a mean duration of 80 ± 53 h. Diuretics were withheld for the duration of treatment. Vasoactive drugs were not used, except as a rescue therapy. | Mixed intravenous bolus and infusion of loop diuretics were used according to guidelines and adjusted according to the patient’s response (vital signs and renal function). A mean daily dose of 271.26 ± 263.06 mg of furosemide-equivalent loop diuretic was given. Vasoactive drugs were not used, except as a rescue therapy. |
| Şeker et al., 2016 [22] | UF with a maximum rate of 500 cc/h. The rate of blood flow was set to 50–100 mL/min. The duration and rate of UF were determined by the clinician. The mean UF duration was 20.5 ± 4.6 h. All forms of intravenous and oral diuretics were withheld for the duration of UF. | Maximum tolerable IV furosemide dose was used as bolus or continuous infusion. The mean daily dose of furosemide given was 164.1 ± 51.3 mg. |
| Hu et al., 2020 [16] | The FQ-16 type HF ultrafiltration dehydration device was used, with a blood flow rate of 25–40ml/min. The initial UF rate was set to 200–300ml/h, with a mean UF duration of 10.8h/day. The rate and duration of UF were adjusted by the physician depending on the condition of the patient based on blood pressure monitoring. | IV loop diuretics (mean torasemide dose = 20 mg/day) were used along with vasopressin V2 receptor antagonist (mean tolvaptan dose = 10 mg/day). |
| First Author | Bart [3] | Bart [10] | Chung [21] | Costanzo [7] | Costanzo [5] | Giglioli [4] | Hanna [6] | Hu [16] | Marenzi [17] | Şeker [22] |
|---|---|---|---|---|---|---|---|---|---|---|
| Year | 2005 | 2012 | 2014 | 2007 | 2016 | 2011 | 2012 | 2020 | 2014 | 2016 |
| Change in sodium level in UF (mmol/L) | −0.5 ± 2.57 (48 h) | −2.3 ± 3.5 (96 h) | - | - | - | - | −4 ± 4.35 * (96 h) | 5.11 ± 4.35 * (8 days/EoT) | 0 ± 4.35 * (Discharge) | −0.5 ± 5.2 (96 h) |
| Change in sodium level in diuretics (mmol/L) | 0 ± 3.36 (48 h) | 0.0 ± 3.6 (96 h) | - | - | - | - | −4 ± 4.65 * (96 h) | 1.13 ± 4.65 * (8 days/EoT) | 1 ± 4.65 * (Discharge) | 0.65 ± 5.7 (96 h) |
| Change in creatinine in UF (mg/dL) | 0.1 ± 0.39 (48 h) | −0.12 ± 0.67 (2 months) | −0.13 ± 0.46 (discharge) | 0.05 ± 0.42 (3 months) † | −0.30 ± 0.42 (3 months) | −0.55 ± 0.5075 (36 h) ‡ | 0.6 ± 0.5 (96 h) § | −0.01 ± 0.89 (8 days/EoT) || | 0.1 ± 0.42 (12 months) † | −0.36 ± 1.1014 (3 month) |
| Change in creatinine in diuretics (mg/dL) | 0.1 ± 0.33 (48 h) | −0.28 ± 0.61 (2 months) | 0.12 ± 0.46 (discharge) | 0.08 ± 0.30 (3 months) † | −0.26 ± 0.30 (3 months) | 0.07 ± 0.2625 (36 h) ‡ | 0.2 ± 0.49 (96 h) § | −0.02 ± 0.60 (8 days/EoT) || | −0.1 ± 0.30 (12 months) † | 0.14 ± 1.1014 (3 month) |
| Change in BUN in UF (mg/dL) | 8 ± 10.0 (48 h) | 13.81 ± 23.91 (7 days) | - | - | 2.5 ± 6.36 (3 months) | - | - | - | 6.1 ± 18.9 (Discharge) # | - |
| Change in BUN in diuretics (mg/dL) | 2 ± 7.8 (48 h) | 7.23 ± 20.69 (7 days) | - | - | −3.78 ± 4.19 (3 months) | - | - | - | 0 ± 17.5 (Discharge) # | - |
| Change in BNP in UF (pg/mL) | - | - | - | - | −159.9 ± 678.2 (3 months) | - | - | −1144 ± 1435 (EoT) | −398 ± 527 (Discharge) ** | - |
| Change in BNP in diuretics (pg/mL) | - | - | - | - | −201.3 ± 618.31 (3 months) | - | - | −654.02 ± 889.65 (EoT) | −481 ± 539.26 (Discharge) ** | - |
| Change in NT-proBNP in UF (pg/mL) | - | −814 ± 9239 (96 h) | - | - | - | −3266 ± 6547 (36 h) * | −2291 ± 6547 (48 h) * | - | - | −5567 ± 3855 (96 h) |
| Change in NT-proBNP in diuretics (pg/mL) | - | −979 ± 2902 (96 h) | - | - | - | −1436 ± 4028.5 (36 h) * | -2547 ± 4028.5 (48 h) * | - | - | −4393 ± 5155 (96 h) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tay, K.L.-Y.; Osman, A.R.; Yeoh, E.E.X.; Luangboriboon, J.; Lau, J.F.; Chan, J.J.A.; Yousif, M.; Tse, B.Y.H.; Horgan, G.; Gamble, D.T.; et al. Ultrafiltration versus Diuretics on Prognostic Cardiac and Renal Biomarkers in Acute Decompensated Heart Failure: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 2793. https://doi.org/10.3390/jcm12082793
Tay KL-Y, Osman AR, Yeoh EEX, Luangboriboon J, Lau JF, Chan JJA, Yousif M, Tse BYH, Horgan G, Gamble DT, et al. Ultrafiltration versus Diuretics on Prognostic Cardiac and Renal Biomarkers in Acute Decompensated Heart Failure: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2023; 12(8):2793. https://doi.org/10.3390/jcm12082793
Chicago/Turabian StyleTay, Kirsty Luo-Yng, Abdel Rahman Osman, Esyn Ee Xin Yeoh, Jasmine Luangboriboon, Jie Fei Lau, Joanne Jia An Chan, Majed Yousif, Benjamin Yi Hong Tse, Graham Horgan, David T. Gamble, and et al. 2023. "Ultrafiltration versus Diuretics on Prognostic Cardiac and Renal Biomarkers in Acute Decompensated Heart Failure: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 12, no. 8: 2793. https://doi.org/10.3390/jcm12082793
APA StyleTay, K. L.-Y., Osman, A. R., Yeoh, E. E. X., Luangboriboon, J., Lau, J. F., Chan, J. J. A., Yousif, M., Tse, B. Y. H., Horgan, G., Gamble, D. T., & Myint, P. K. (2023). Ultrafiltration versus Diuretics on Prognostic Cardiac and Renal Biomarkers in Acute Decompensated Heart Failure: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 12(8), 2793. https://doi.org/10.3390/jcm12082793







