Effectiveness and Safety of a Mixture of Nobiletin and Tangeretin in Nocturia Patients: A Randomized, Placebo-Controlled, Double-Blind, Crossover Study
Abstract
1. Introduction
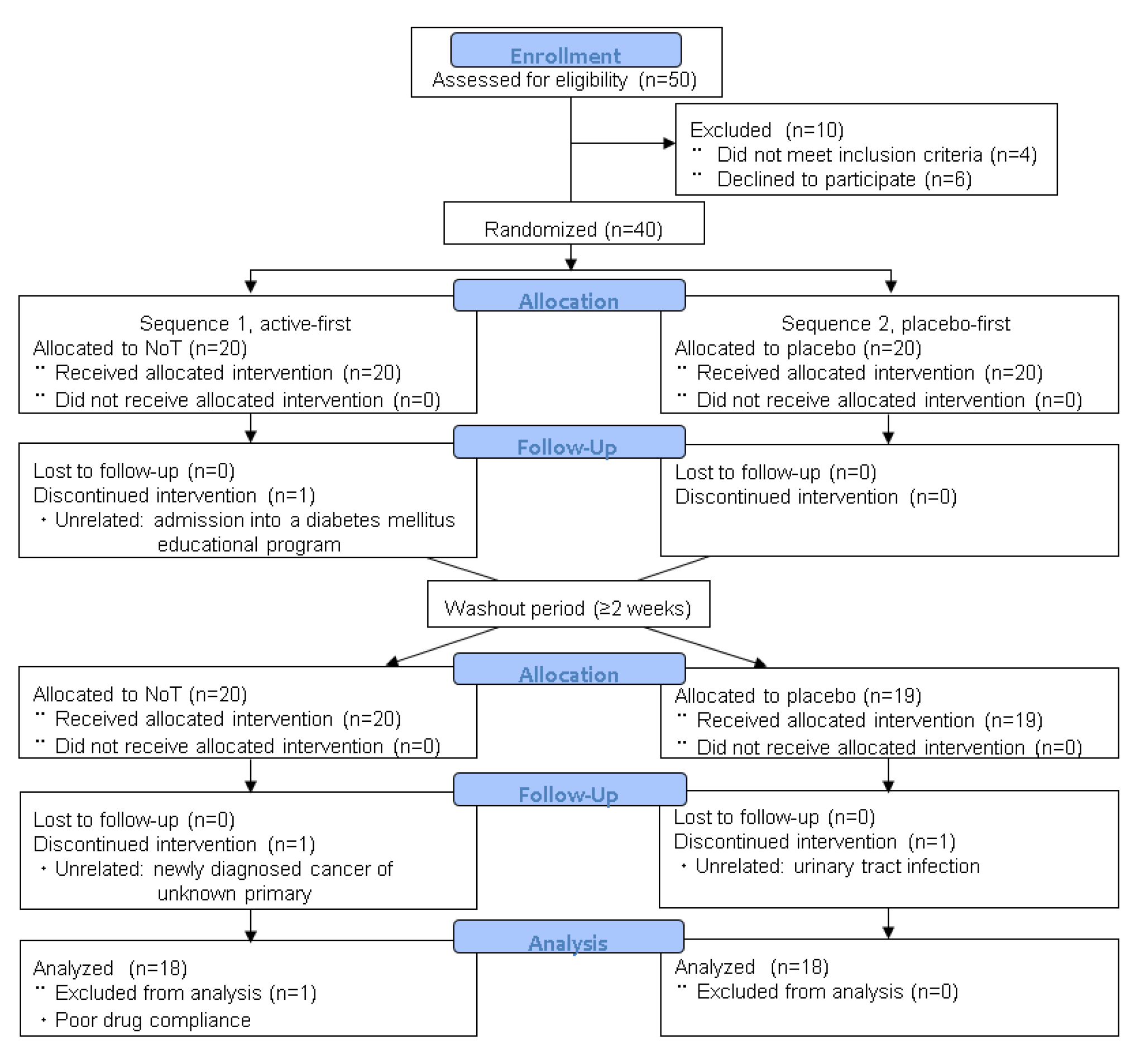
2. Materials and Methods
2.1. Study Design
2.2. Primary Endpoint Measure
2.3. Secondary Endpoint Measures
2.4. Statistical Analysis
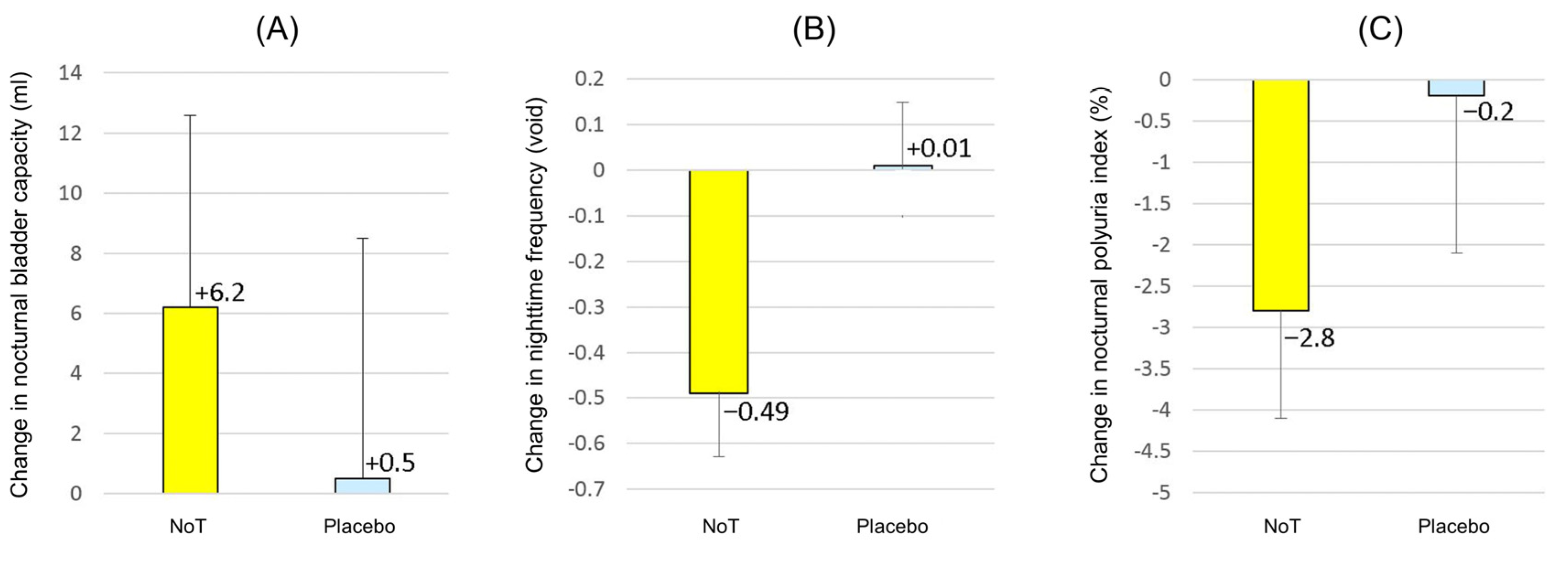
3. Results
3.1. Patient Features and Efficacy Measures
3.2. Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hashim, H.; Blanker, M.H.; Drake, M.J.; Djurhuus, J.C.; Meijlink, J.; Morris, V.; Petros, P.; Wen, J.G.; Wein, A. International Continence Society (ICS) report on the terminology for nocturia and nocturnal lower urinary tract function. Neurourol. Urodyn. 2019, 38, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Andersson, K.E.; Van Kerrebroeck, P. Pharmacotherapy for Nocturia. Curr. Urol. Rep. 2018, 19, 8. [Google Scholar] [CrossRef]
- Suvada, K.; Plantinga, L.; Vaughan, C.P.; Markland, A.D.; Mirk, A.; Burgio, K.L.; Erni, S.M.; Ali, M.K.; Okosun, I.; Young, H.; et al. Comorbidities, Age, and Polypharmacy Limit the Use by US Older Adults with Nocturia of the Only FDA-approved Drugs for the Symptom. Clin. Ther. 2020, 42, e259–e274. [Google Scholar] [CrossRef] [PubMed]
- Negoro, H.; Kanematsu, A.; Doi, M.; Suadicani, S.O.; Matsuo, M.; Imamura, M.; Okinami, T.; Nishikawa, N.; Oura, T.; Matsui, S.; et al. Involvement of urinary bladder Connexin43 and the circadian clock in coordination of diurnal micturition rhythm. Nat. Commun. 2012, 3, 809. [Google Scholar] [CrossRef]
- Negoro, H.; Kanematsu, A.; Yoshimura, K.; Ogawa, O. Chronobiology of Micturition: Putative Role of the Circadian Clock. J. Urol. 2013, 190, 843–849. [Google Scholar] [CrossRef]
- Birder, L.A.; Van Kerrebroeck, P.E.V. Pathophysiological Mechanisms of Nocturia and Nocturnal Polyuria: The Contribution of Cellular Function, the Urinary Bladder Urothelium, and Circadian Rhythm. Urology 2019, 133S, 14–23. [Google Scholar] [CrossRef]
- Mohawk, J.A.; Green, C.B.; Takahashi, J.S. Central and peripheral circadian clocks in mammals. Annu. Rev. Neurosci. 2012, 35, 445–462. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Nohara, K.; Park, N.; Park, Y.S.; Guillory, B.; Zhao, Z.; Garcia, J.M.; Koike, N.; Lee, C.C.; Takahashi, J.S.; et al. The Small Molecule Nobiletin Targets the Molecular Oscillator to Enhance Circadian Rhythms and Protect against Metabolic Syndrome. Cell Metab. 2016, 23, 610–621. [Google Scholar] [CrossRef]
- Shinozaki, A.; Misawa, K.; Ikeda, Y.; Haraguchi, A.; Kamagata, M.; Tahara, Y.; Shibata, S. Potent Effects of Flavonoid Nobiletin on Amplitude, Period, and Phase of the Circadian Clock Rhythm in PER2::LUCIFERASE Mouse Embryonic Fibroblasts. PLoS ONE 2017, 12, e0170904. [Google Scholar] [CrossRef]
- Mileykovskaya, E.; Yoo, S.-H.; Dowhan, W.; Chen, Z. Nobiletin: Targeting the Circadian Network to Promote Bioenergetics and Healthy Aging. Biochemistry 2020, 85, 1554–1559. [Google Scholar] [CrossRef]
- Evans, M.; Judy, W.V.; Wilson, D.; Rumberger, J.A.; Guthrie, N. Randomized, double-blind, placebo-controlled, clinical study on the effect of Diabetinol® on glycemic control of subjects with impaired fasting glucose. Diabetes Metab. Syndr. Obes. Targets Ther. 2015, 8, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Shirai, M.; Ono, K.; Teruya, T.; Yamano, A.; Tae Woo, J. Beneficial effects of a nobiletin-rich formulated supplement of Sikwasa (C. depressa) peel on cognitive function in elderly Japanese subjects; A multicenter, randomized, double-blind, placebo-controlled study. Food Sci Nutr. 2021, 9, 6844–6853. [Google Scholar] [CrossRef]
- Woo, J.T. Methods for high-purity nobiletin production from Citrus depressa and its various physiological functions. Jpn. Soc. Med. Use Funct. Foods 2017, 10, 334–344. (In Japanese) [Google Scholar]
- Scarpero, H.M.; Fiske, J.; Xue, X.; Nitti, V.W. American Urological Association Symptom Index for lower urinary tract symptoms in women: Correlation with degree of bother and impact on quality of life. Urology 2003, 61, 1118–1122. [Google Scholar] [CrossRef] [PubMed]
- Okamura, K.; Usami, T.; Nagahama, K.; Maruyama, S.; Mizuta, E. The relationships among filling, voiding subscores from International Prostate Symptom Score and quality of life in Japanese elderly men and women. Eur. Urol. 2002, 42, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Okamura, T.; Miura, K.; Kadowaki, T.; Ueshima, H.; Nakagawa, H.; Hashimoto, T. A simple method to estimate populational 24-h urinary sodium and potassium excretion using a casual urine specimen. J. Hum. Hypertens. 2002, 16, 97–103. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D.; for the CONSORT Group. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. J. Pharmacol. Pharmacother. 2010, 1, c332. [Google Scholar] [CrossRef]
- Buser, N.; Ivic, S.; Kessler, T.M.; Kessels, A.G.; Bachmann, L.M. Efficacy and Adverse Events of Antimuscarinics for Treating Overactive Bladder: Network Meta-analyses. Eur. Urol. 2012, 62, 1040–1060. [Google Scholar] [CrossRef]
- Yamaguchi, O.; Juul, K.V.; Falahati, A.; Yoshimura, T.; Imura, F.; Kitamura, M. Efficacy and safety of 25 and 50 μg desmopressin orally disintegrating tablets in Japanese patients with nocturia due to nocturnal polyuria: Results from two phase 3 studies of a multicenter randomized double-blind placebo-controlled parallel-group development program. LUTS Low. Urin. Tract Symptoms 2019, 12, 8–19. [Google Scholar]
- Van Leeuwen, J.H.S.; Castro, R.; Busse, M.; Bemelmans, B.L. The placebo effect in the pharmacologic treatment of patients with lower urinary tract symptoms. Eur. Urol. 2006, 50, 440–453. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, L.; Shi, W.; Liu, H.; Yang, J.; Yuan, X.; Wu, L. The Multifunctional Effects of Nobiletin and Its Metabolites In Vivo and In Vitro. Evid. Based Complement. Altern. Med. 2016, 2016, 2918796. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.R.; Albuquerque, T.; Quintela, T.; Costa, D. Circadian rhythm and disease: Relationship, new insights, and future perspectives. J. Cell. Physiol. 2022, 237, 3239–3256. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.G.; Pollock, D.M. Circadian regulation of renal function. Free. Radic. Biol. Med. 2018, 119, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, K.; Nakajima, A.; Guo, Y.; Ohizumi, Y. A Narrative Review of the Effects of Citrus Peels and Extracts on Human Brain Health and Metabolism. Nutrients 2022, 14, 1847. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Dong, L.; Qu, F.; He, H.; Sun, W.; Man, Y.; Jiang, H. Effects of glycyrrhizin on the pharmacokinetics of nobiletin in rats and its potential mechanism. Pharm. Biol. 2020, 58, 352–356. [Google Scholar] [CrossRef]
- Hashimoto, M.; Matsuzaki, K.; Maruyama, K.; Hossain, S.; Sumiyoshi, E.; Wakatsuki, H.; Kato, S.; Ohno, M.; Tanabe, Y.; Kuroda, Y.; et al. Perilla seed oil in combination with nobiletin-rich ponkan powder enhances cognitive function in healthy elderly Japanese individuals: A possible supplement for brain health in the elderly. Food Funct. 2022, 13, 2768–2781. [Google Scholar] [CrossRef]
- Takeda, M.; Yokoyama, O.; Lee, S.W.; Murakami, M.; Morisaki, Y.; Viktrup, L. Tadalafil 5 mg once-daily therapy for men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia: Results from a randomized, double-blind, placebo-controlled trial carried out in Japan and Korea. Int. J. Urol. 2014, 21, 670–675. [Google Scholar] [CrossRef]
- Negoro, H.; Goto, T.; Akamatsu, S.; Terada, N.; Kobayashi, T.; Matsui, Y.; Yamamoto, T.; Omura, T.; Yonezawa, A.; Matsubara, K.; et al. Add-on effects of tadalafil in tamsulosin-treated patients with small benign prostatic enlargement: A randomized, placebo-controlled, double-blind, crossover study. Neurourol. Urodyn. 2020, 39, 237–242. [Google Scholar] [CrossRef]

| Sequence 1, Active-First | Sequence 2, Placebo-First | Overall Cohort | |
|---|---|---|---|
| n = 20 | n = 20 | n = 40 | |
| Age (y) | 73.8 ± 4.0 | 73.2 ± 6.7 | 73.5 ± 5.5 |
| Sex | 13 men, 7 women | 14 men, 6 women | 27 men, 13 women |
| Body mass index (kg/m2) | 23.4 ± 3.7 | 24.0 ± 3.6 | 23.7 ± 3.6 |
| Hypertension | 13 (65%) | 17 (85%) | 30 (75%) |
| Diabetes mellitus | 4 (20%) | 2 (10%) | 6 (15%) |
| Systolic blood pressure (mmHg) | 133.4 ± 19.1 | 134.9 ± 16.6 | 134.2 ± 17.9 |
| Diastolic blood pressure (mmHg) | 78.5 ± 12.1 | 79.9 ± 7.9 | 79.2 ± 10.2 |
| Heart rate (beats/min) | 74.9 ± 12.1 | 74.9 ± 12.0 | 74.9 ± 12.0 |
| Questionnaire scores | |||
| IPSS-total | 13.3 ± 6.8 | 10.1 ± 5.2 | 11.7 ± 6.3 |
| IPSS-Q7 | 3.05 ± 0.59 | 2.55 ± 1.07 | 2.80 ± 0.90 |
| IPSS-QOL | 4.5 ± 1.1 | 4.0 ± 1.2 | 4.2 ± 1.1 |
| PSQI | 8.6 ± 2.9 | 8.8 ± 4.5 | 8.7 ± 3.8 |
| Frequency–volume chart variables | |||
| Nocturnal bladder capacity (mL) | 192.5 ± 66.1 | 205.5 ± 64.4 | 199.0 ± 65.6 |
| Nighttime frequency (void) | 2.85 ± 0.72 | 2.72 ± 1.06 | 2.78 ± 0.91 |
| 24 h urine volume (mL) | 1483 ± 341 | 1647 ± 348 | 1565 ± 354 |
| Nocturnal polyuria index (%) | 43.9 ± 15.2 | 41.0 ± 12.4 | 42.5 ± 13.9 |
| Nocturnal urine volume (mL) | 658 ± 298 | 673 ± 244 | 666 ± 272 |
| Maximum voided volume (mL) | 307.5 ± 87.1 | 301.5 ± 76.0 | 304.5 ± 81.8 |
| Nocturia index * | 2.19 ± 0.81 | 2.30 ± 0.86 | 2.24 ± 0.84 |
| Nocturnal bladder capacity index † | 1.66 ± 0.54 | 1.42 ± 0.56 | 1.54 ± 0.57 |
| Hours of undisturbed sleep (h) | 2.31 ± 0.71 | 2.40 ± 0.70 | 2.35 ± 0.71 |
| Laboratory data | |||
| Post-void residual (mL) | 19.8 ± 25.5 | 27.5 ± 31.2 | 23.6 ± 28.8 |
| Serum creatinine (mg/dL) | 0.90 ± 0.23 | 0.89 ± 0.20 | 0.89 ± 0.21 |
| BNP (pg/mL) | 40.5 ± 33.9 | 35.6 ± 33.5 | 38.1 ± 33.8 |
| Estimated daily salt intake (g/day) | 8.79 ± 2.38 | 8.77 ± 2.99 | 8.78 ± 2.70 |
| Change from Baseline | ||||
|---|---|---|---|---|
| Questionnaire Scores | NoT | Placebo | Difference | p-Value |
| n = 36 | n = 36 | |||
| IPSS-total | −0.64 ± 3.20 | −0.68 ± 2.99 | 0.04 | 0.88 |
| IPSS-Q7 | −0.22 ± 0.75 | −0.21 ± 0.80 | −0.01 | 0.89 |
| IPSS-QOL | −0.19 ± 0.99 | −0.06 ± 1.11 | −0.13 | 0.49 |
| PSQI | −0.5 ± 1.9 | −0.2 ± 2.0 | −0.3 | 0.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ito, H.; Negoro, H.; Kono, J.; Hayata, N.; Miura, T.; Manabe, Y.; Miyazaki, Y.; Mishina, M.; Woo, J.T.; Sakane, N.; et al. Effectiveness and Safety of a Mixture of Nobiletin and Tangeretin in Nocturia Patients: A Randomized, Placebo-Controlled, Double-Blind, Crossover Study. J. Clin. Med. 2023, 12, 2757. https://doi.org/10.3390/jcm12082757
Ito H, Negoro H, Kono J, Hayata N, Miura T, Manabe Y, Miyazaki Y, Mishina M, Woo JT, Sakane N, et al. Effectiveness and Safety of a Mixture of Nobiletin and Tangeretin in Nocturia Patients: A Randomized, Placebo-Controlled, Double-Blind, Crossover Study. Journal of Clinical Medicine. 2023; 12(8):2757. https://doi.org/10.3390/jcm12082757
Chicago/Turabian StyleIto, Haruki, Hiromitsu Negoro, Jin Kono, Naoki Hayata, Takayoshi Miura, Yumi Manabe, Yu Miyazaki, Mutsuki Mishina, Je Tae Woo, Naoki Sakane, and et al. 2023. "Effectiveness and Safety of a Mixture of Nobiletin and Tangeretin in Nocturia Patients: A Randomized, Placebo-Controlled, Double-Blind, Crossover Study" Journal of Clinical Medicine 12, no. 8: 2757. https://doi.org/10.3390/jcm12082757
APA StyleIto, H., Negoro, H., Kono, J., Hayata, N., Miura, T., Manabe, Y., Miyazaki, Y., Mishina, M., Woo, J. T., Sakane, N., & Okuno, H. (2023). Effectiveness and Safety of a Mixture of Nobiletin and Tangeretin in Nocturia Patients: A Randomized, Placebo-Controlled, Double-Blind, Crossover Study. Journal of Clinical Medicine, 12(8), 2757. https://doi.org/10.3390/jcm12082757






