Modulating Influence of State Anxiety on the Effect of Midazolam on Postsurgical Pain
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Protocol
2.3. Variables
2.4. Data Collection
2.5. Study Size
2.6. Statistical Analysis
3. Results
3.1. Participants
3.2. Outcome Data
3.3. Impact of Midazolam on Postsurgical Pain
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walker, E.M.; Bell, M.; Cook, T.M.; Grocott, M.P.W.; Moonesinghe, S.R.; Central SNAP-1 Organisation; National Study Groups. Patient reported outcome of adult perioperative anaesthesia in the United Kingdom: A cross-sectional observational study. Br. J. Anaesth. 2016, 117, 758–766, Erratum in Br. J. Anaesth. 2017, 119, 552. [Google Scholar] [CrossRef] [PubMed]
- Sobol-Kwapinska, M.; Bąbel, P.; Plotek, W.; Stelcer, B. Psychological correlates of acute postsurgical pain: A systematic review and meta-analysis. Eur. J. Pain 2016, 20, 1573–1586. [Google Scholar] [CrossRef]
- Yang, M.M.H.; Hartley, R.L.; Leung, A.; Ronksley, P.E.; Jetté, N.; Casha, S.; Riva-Cambrin, J. Preoperative predictors of poor acute postoperative pain control: A systematic review and meta-analysis. BMJ Open 2019, 9, e025091. [Google Scholar] [CrossRef] [PubMed]
- Pinto, P.R.; Vieira, A.; Pereira, D.; Almeida, A. Predictors of Acute Postsurgical Pain After Inguinal Hernioplasty. J. Pain 2017, 18, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Conway, A.; Chang, K.; Mafeld, S.; Sutherland, J. Midazolam for sedation before procedures in adults and children: A systematic review update. Syst. Rev. 2021, 10, 69. [Google Scholar] [CrossRef]
- Song, S.W.; Jin, Y.; Lim, H.; Lee, J.; Lee, K.H. Effect of intramuscular midazolam premedication on patient satisfaction in women undergoing general anaesthesia: A randomised control trial. BMJ Open 2022, 12, e059915. [Google Scholar] [CrossRef]
- Hasani, A.; Maloku, H.; Sallahu, F.; Gashi, V.; Ozgen, S.U. Preemptive analgesia with midazolam and diclofenac for hernia repair pain. Hernia 2011, 15, 267–272. [Google Scholar] [CrossRef]
- Ho, K.M.; Ismail, H. Use of intrathecal midazolam to improve perioperative analgesia: A meta-analysis. Anaesth. Intensiv. Care 2008, 36, 365–373. [Google Scholar] [CrossRef]
- Tatsuo, M.A.; Salgado, J.V.; Yokoro, C.M.; Duarte, I.D.; Francischi, J.N. Midazolam-induced hyperalgesia in rats: Modulation via GABA(A) receptors at supraspinal level. Eur. J. Pharmacol. 1999, 370, 9–15. [Google Scholar] [CrossRef]
- Frölich, M.A.; Zhang, K.; Ness, T.J. Effect of sedation on pain perception. Anesthesiology 2013, 118, 611–621. [Google Scholar] [CrossRef]
- Fernandes, A.; Alves, L.; Coimbra, L.; Gouveia, F.; Marcos, A.; Amaro, L.; Larmann, J.; Dahlem, C. Does intravenous midazolam induce hyperalgesia? A retrospective observational study in ambulatory surgery. Rev. Soc. Port. Anestesiol. 2019, 28, 154–160. [Google Scholar]
- Kain, Z.N.; Sevarino, F.; Pincus, S.; Alexander, G.M.; Wang, S.M.; Ayoub, C.; Kosarussavadi, B. Attenuation of the preoperative stress response with midazolam: Effects on postoperative outcomes. Anesthesiology 2000, 93, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.K.; Seymour, R.A.; Tan, J.M. Sedation with midazolam leads to reduced pain after dental surgery. Anesth. Analg. 2004, 98, 1289–1293. [Google Scholar] [CrossRef]
- Auffret, Y.; Gouillou, M.; Jacob, G.R.; Robin, M.; Jenvrin, J.; Soufflet, F.; Alavi, Z. Does midazolam enhance pain control in prehospital management of traumatic severe pain? Am. J. Emerg. Med. 2014, 32, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Kain, Z.N.; Sevarino, F.B.; Rinder, C.; Pincus, S.; Alexander, G.; Ivy, M.; Heninger, G. Preoperative anxiolysis and postoperative recovery in women undergoing abdominal hysterectomy. Anesthesiology 2001, 94, 415–422. [Google Scholar] [CrossRef]
- Prosenz, J.; Gustorff, B. Midazolam as an active placebo in 3 fentanyl-validated nociceptive pain models. Pain 2017, 158, 1264–1271. [Google Scholar] [CrossRef]
- Day, M.A.; Rich, M.A.; Thorn, B.E.; Berbaum, M.L.; Mangieri, E.A. A placebo-controlled trial of midazolam as an adjunct to morphine patient-controlled analgesia after spinal surgery. J. Clin. Anesth. 2014, 26, 300–308. [Google Scholar] [CrossRef]
- HerniaSurge Group. International guidelines for groin hernia management. Hernia 2018, 22, 1–165. [Google Scholar] [CrossRef]
- Schug, S.A.; Lavand’homme, P.; Barke, A.; Korwisi, B.; Rief, W.; Treede, R.-D.; IASP Taskforce for the Classification of Chronic Pain. The IASP classification of chronic pain for ICD-11: Chronic postsurgical or posttraumatic pain. Pain 2019, 160, 45–52. [Google Scholar] [CrossRef]
- Theunissen, M.; Peters, M.L.; Schouten, E.G.W.; Fiddelers, A.A.A.; Willemsen, M.G.A.; Pinto, P.R.; Gramke, H.-F.; Marcus, M.A.E. Validation of the surgical fear questionnaire in adult patients waiting for elective surgery. PLoS ONE 2014, 9, e100225, Erratum in PLoS ONE 2016, 11, e0162737. [Google Scholar] [CrossRef]
- Facco, E.; Stellini, E.; Bacci, C.; Manani, G.; Pavan, C.; Cavallin, F.; Zanette, G. Validation of visual analogue scale for anxiety (VAS-A) in preanesthesia evaluation. Minerva Anestesiol. 2013, 79, 1389–1395. [Google Scholar]
- Azevedo, L.; Pereira, A.C.; Dias, C.; Agualusa, L.; Lemos, L.; Romão, J.; Patto, T.; Vaz-Serra, S.; Abrunhosa, R.; Carvalho, C.J.; et al. Tradução, Adaptação Cultural e Estudo Multicêntrico de Validação de Instrumentos para Rastreio e Avaliação do Impacto da Dor Crónica. Associação Portuguesa para o Estudo da Dor. 2007, Volume 15, pp. 1–57. Available online: https://www.aped-dor.org/socios/material_bibliografico/diversos_Questionarios_Dor-Rev_DOR_Volume15-n4-2007.pdf (accessed on 1 February 2018).
- Sawhney, M.; Watt-Watson, J.; McGillion, M. A Pain Education Intervention for Patients Undergoing Ambulatory Inguinal Hernia Repair: A Randomized Controlled Trial. Can. J. Nurs. Res. 2017, 49, 108–117. [Google Scholar] [CrossRef]
- Sigel, E.; Steinmann, M.E. Structure, function, and modulation of GABA(A) receptors. J. Biol. Chem. 2012, 287, 40224–40231. [Google Scholar] [CrossRef] [PubMed]
- Sigel, E.; Ernst, M. The Benzodiazepine Binding Sites of GABAA Receptors. Trends Pharmacol. Sci. 2018, 39, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Shafer, A.; Fish, M.P.; Gregg, K.M.; Seavello, J.; Kosek, P. Preoperative anxiety and fear: A comparison of assessments by patients and anesthesia and surgery residents. Anesth. Analg. 1996, 83, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Badner, N.H.; Nielson, W.R.; Munk, S.; Kwiatkowska, C.; Gelb, A.W. Preoperative anxiety: Detection and contributing factors. Can. J. Anaesth. 1990, 37, 444–447. [Google Scholar] [CrossRef]

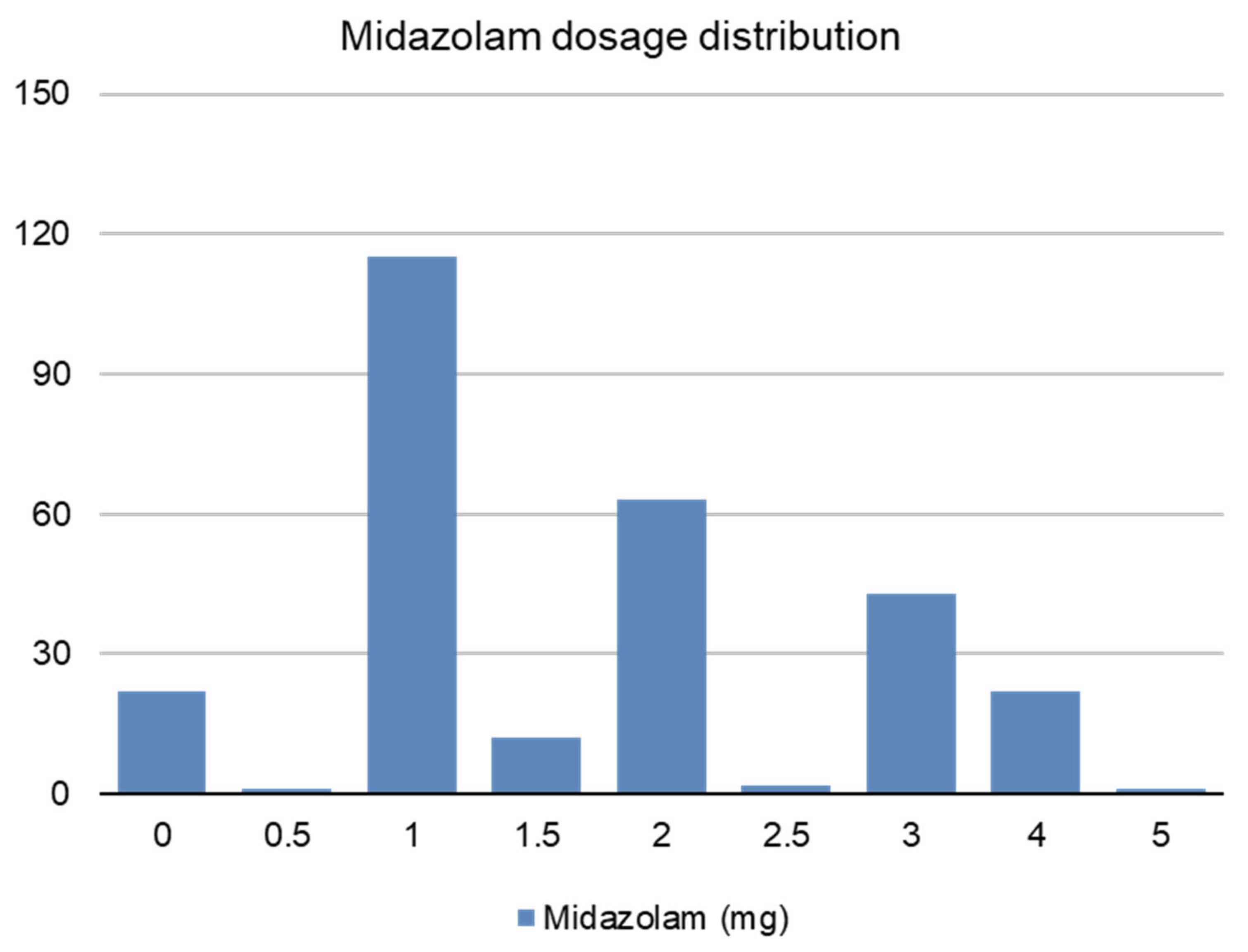
| n = 281 n (%) | Midazolam μg.Kg−1 | p | Missing Cases n (%) | ||
|---|---|---|---|---|---|
| Age (years) | <48 | 65 (23%) | 20.3 (12.5) | 0.078 & | 0 (0) |
| 49–66 | 151 (54%) | 25.1 (14.7) | |||
| >67 | 65 (23%) | 24.2 (15.2) | |||
| BMI (Kg.m−2) | <26 | 126 (46%) | 23.7 (15.0) | 0.913 # | 4 (1) |
| ≥26 | 151 (54%) | 23.9 (14.0) | |||
| Sex | Male | 250 (89%) | 24.2 (14.6) | 0.171 # | 0 (0) |
| Female | 31 (11%) | 20.4 (12.5) | |||
| Smoker | Yes | 51 (18%) | 24.6 (13.7) | 0.655 # | 0 (0) |
| No | 230 (82%) | 23.6 (14.6) | |||
| Chronic benzodiazepine | Yes | 20 (7%) | 26.7 (14.0) | 0.347 # | 0 (0) |
| No | 261 (93%) | 23.5 (14.5) | |||
| Education | <5 years | 155 (55%) | 24.9 (14.6) | 0.270 & | 0 (0) |
| 5–9 years | 57 (20%) | 23.2 (13.8) | |||
| >9 years | 69 (25%) | 21.6 (14.4) | |||
| Working status | Active | 163(58%) | 24.0 (13.9) | 0.925 # | 2 (1) |
| Not active | 116 (42%) | 23.7 (15.1) | |||
| Preoperative pain | <4 | 216 (77%) | 23.9 (14.6) | 0.938 # | 1 (0) |
| ≥4 | 64 (23%) | 23.7 (13.9) | |||
| Anxiety SFQ | 0–12 | 87 (31%) | 23.0 (15.0) | 0.334 & | 6 (2) |
| 13–27 | 98 (35%) | 22.7 (14.3) | |||
| 28–80 | 90 (32%) | 25.6 (13.7) | |||
| Anesthesia | Spinal | 136 (49%) | 32.0 (14.5) | <0.001 # | 1 (0) |
| General | 144 (51%) | 15.9 (9.1) | |||
| Surgery | RR | 242 (88%) | 23.5 (14.5) | 0.245 # | 7 (2) |
| Lichtenstein | 32 (12%) | 26.7 (13.2) | |||
| Ambulatory unit | 1 | 90 (32%) | 16.6 (7.1) | <0.001 & | 0 (0) |
| 2 | 126 (45%) | 27.3 (14.1) | |||
| 3 | 65 (23%) | 26.5 (18.6) | |||
| Total n = 281 | Midazolam μg.Kg−1 | p # | Missing Cases n (%) | ||
|---|---|---|---|---|---|
| At 24 h | |||||
| Pain at REST | 2.2 (1.9) | 20 (7) | |||
| MOV pain | 4.5 (2.3) | 20 (7) | |||
| Average pain | 3.9 (2.0) | 4 (1) | |||
| Acetaminophen (g) | 1.7 (1.0) | 4 (1) | |||
| Ibuprofen (g) | 0.7 (0.4) | 4 (1) | |||
| Rescue analgesia | Yes | 30 (11%) | 20.1 (7.7) | 0.121 | 6 (2) |
| No | 245 (89%) | 24.4 (15.0) | |||
| Pain | <4 | 91 (33%) | 24.4 (14.6) | 0.726 | 4 (1) |
| ≥4 | 186 (67%) | 23.7 (14.3) | |||
| At 7 days | |||||
| Pain at REST | 1 [2] | 17 (6) | |||
| MOV pain | 2.9 (1.8) | 17 (6) | |||
| Average pain | 3.3 (1.7) | 9 (3) | |||
| Acetaminophen (g) | 10.1 (6.8) | 11 (4) | |||
| Ibuprofen (g) | 4.3 (2.3) | 11 (4) | |||
| Ongoing analgesia | Yes | 59 (22%) | 21.0 (12.3) | 0.089 | 9 (3) |
| No | 213 (78%) | 24.6 (14.8) | |||
| Pain | <4 | 125 (46%) | 24.2 (14.7) | 0.731 | 7 (2) |
| ≥4 | 149 (54%) | 23.5 (14.1) | |||
| Satisfaction | 10 [2] | 8 (3) | |||
| At 3 months | |||||
| Pain at REST | 0 [0] | 7 (2) | |||
| MOV pain | 1 [3] | 9 (3) | |||
| Average pain | 0 [2] | 8 (3) | |||
| CPSP | Yes | 72 (27%) | 22.8 (14.0) | 0.424 | 9 (3) |
| No | 200 (73%) | 24.3 (14.3) | |||
| Analgesic use | Yes | 10 (4%) | 24.6 (17.0) | 0.859 | 9 (3) |
| No | 262 (96%) | 23.7 (14.2) | |||
| Satisfaction | 10 [1] | 7 (2) | |||
| GRSI | 95 [20] | 7 (2) |
| Midazolam (mg) | Crude OR (95% CI) | Adjusted OR (95% CI) | ||
|---|---|---|---|---|
| Total sample | Total sample | SFQ 0–12 | SFQ 28–80 | |
| 24 h pain ≥ 4 * | 0.656 (0.494–0.873) | 0.630 (0.427–0.930) #,1 | 0.407 (0.144–1.153) | 0.753 (0.379–1.496) |
| 7 d pain ≥ 1 * | 0.990 (0.620–1.581) | 0.418 (0.175–0.997) #,2 | 0.261 (0.072–0.953) | 0.258 (0.003–20.016) |
| 7 d pain ≥ 4 * | 0.882 (0.690–1.127) | 0.764 (0.547–1.068) #,3 | 0.289 (0.117–0.713) | 0.955 (0.543–1.679) |
| 3 mo CPSP & | 0.925 (0.716–1.196) | 1.046 (0.742–1.474) #,4 | 0.828 (0.415–1.654) | 2.124 (1.026–4.397) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dahlem, C.; Monteiro, C.; Mendes, E.; Martinho, J.; Oliveira, C.; Bettencourt, M.; Coelho, M.; Neves, P.; Azevedo, L.; Granja, C. Modulating Influence of State Anxiety on the Effect of Midazolam on Postsurgical Pain. J. Clin. Med. 2023, 12, 2669. https://doi.org/10.3390/jcm12072669
Dahlem C, Monteiro C, Mendes E, Martinho J, Oliveira C, Bettencourt M, Coelho M, Neves P, Azevedo L, Granja C. Modulating Influence of State Anxiety on the Effect of Midazolam on Postsurgical Pain. Journal of Clinical Medicine. 2023; 12(7):2669. https://doi.org/10.3390/jcm12072669
Chicago/Turabian StyleDahlem, Caroline, Catarina Monteiro, Eunice Mendes, Joana Martinho, Carmen Oliveira, Margarida Bettencourt, Miguel Coelho, Paula Neves, Luís Azevedo, and Cristina Granja. 2023. "Modulating Influence of State Anxiety on the Effect of Midazolam on Postsurgical Pain" Journal of Clinical Medicine 12, no. 7: 2669. https://doi.org/10.3390/jcm12072669
APA StyleDahlem, C., Monteiro, C., Mendes, E., Martinho, J., Oliveira, C., Bettencourt, M., Coelho, M., Neves, P., Azevedo, L., & Granja, C. (2023). Modulating Influence of State Anxiety on the Effect of Midazolam on Postsurgical Pain. Journal of Clinical Medicine, 12(7), 2669. https://doi.org/10.3390/jcm12072669






