High-Tech Home-Based Rehabilitation after Stroke: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
- (a)
- What are the effect sizes of VR, RDs, and games in home-based rehabilitation?
- (b)
- Does the effect size differ depending on the type of technique applied?
- (c)
- Does the effect size on the physical function of the stroke survivor differ depending on the target limb?
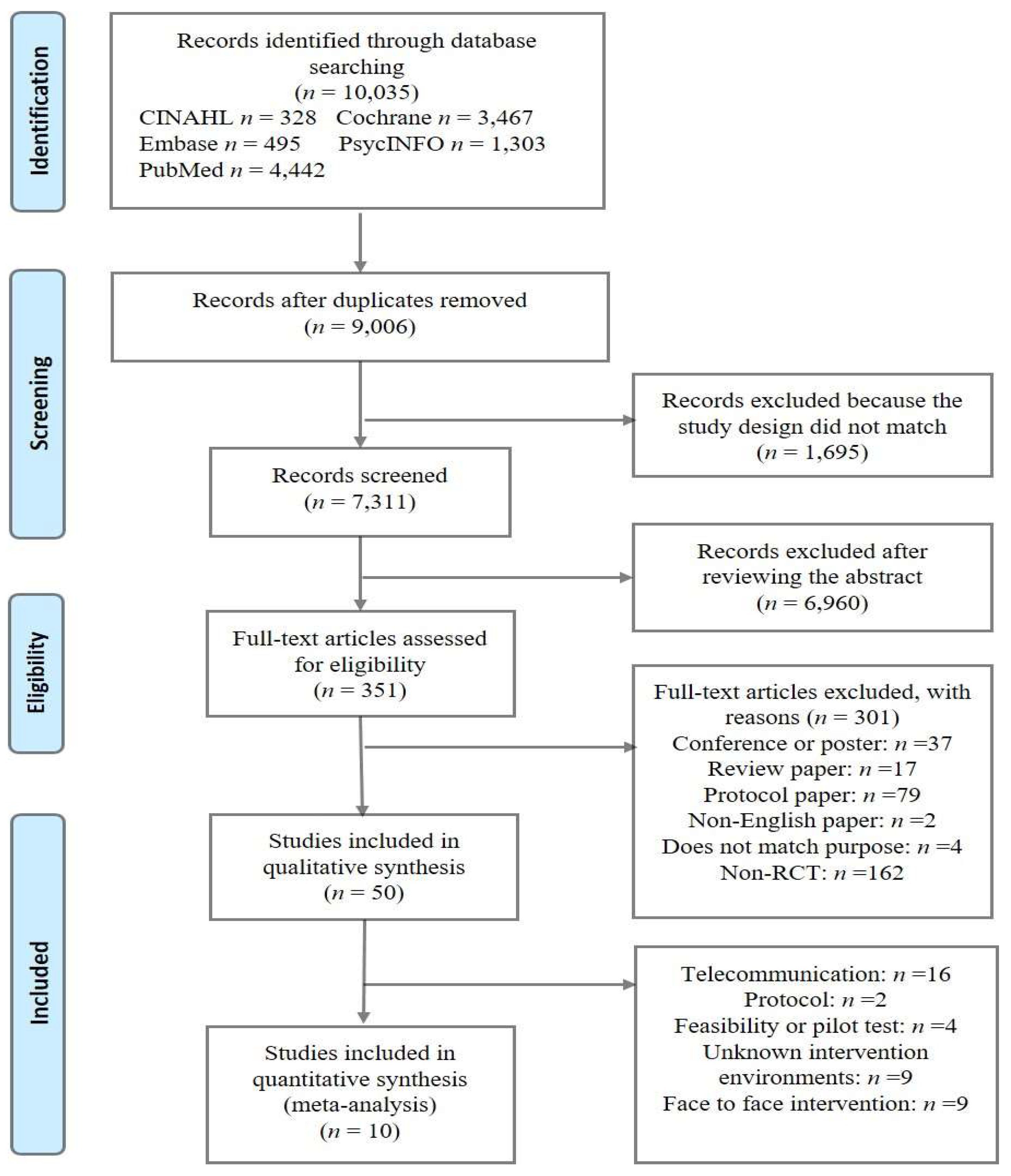
2. Materials and Methods
3. Results
3.1. Characteristics of the Included Studies
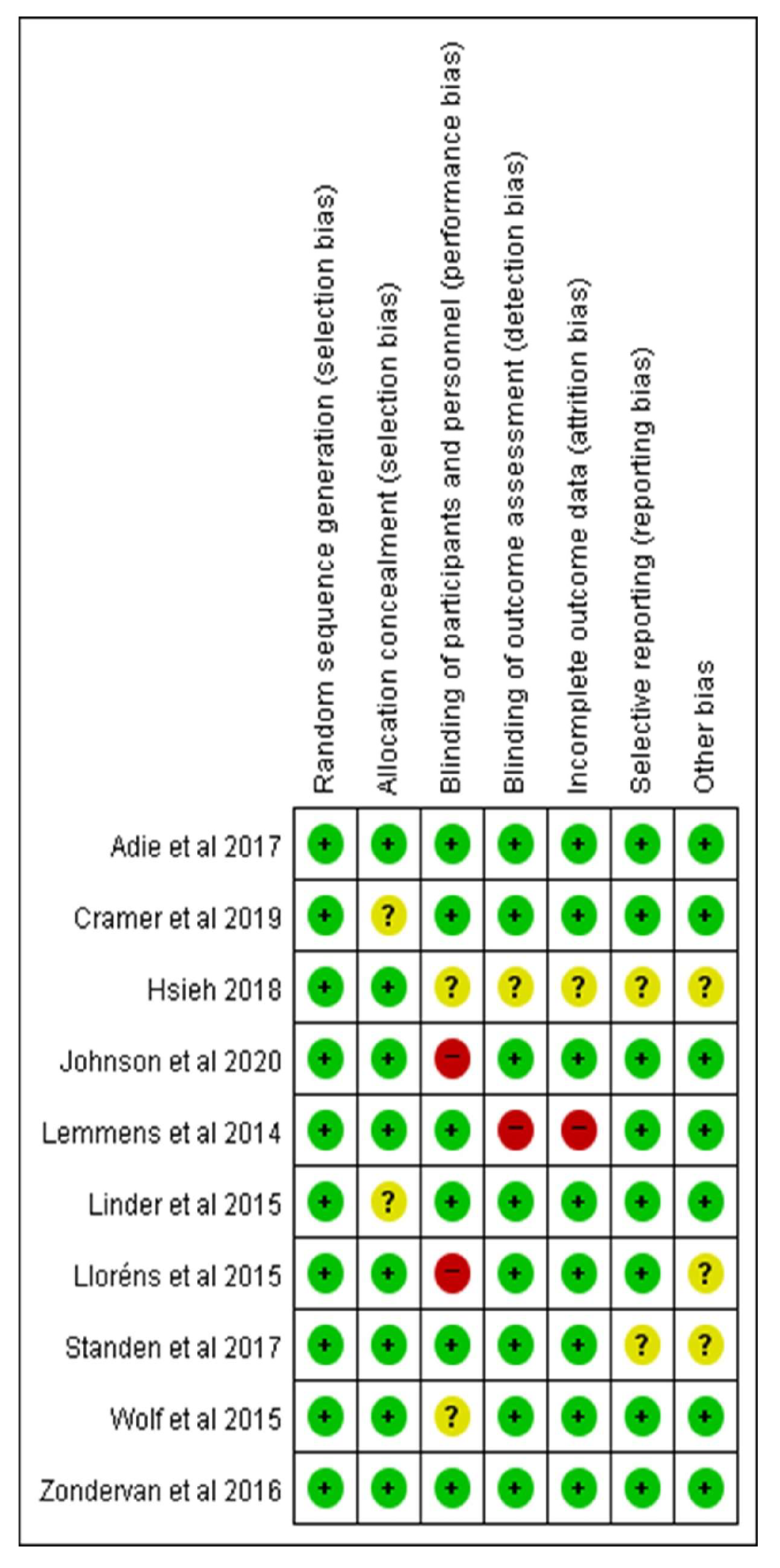
3.2. The Risk of Each Study
3.3. Publication Bias
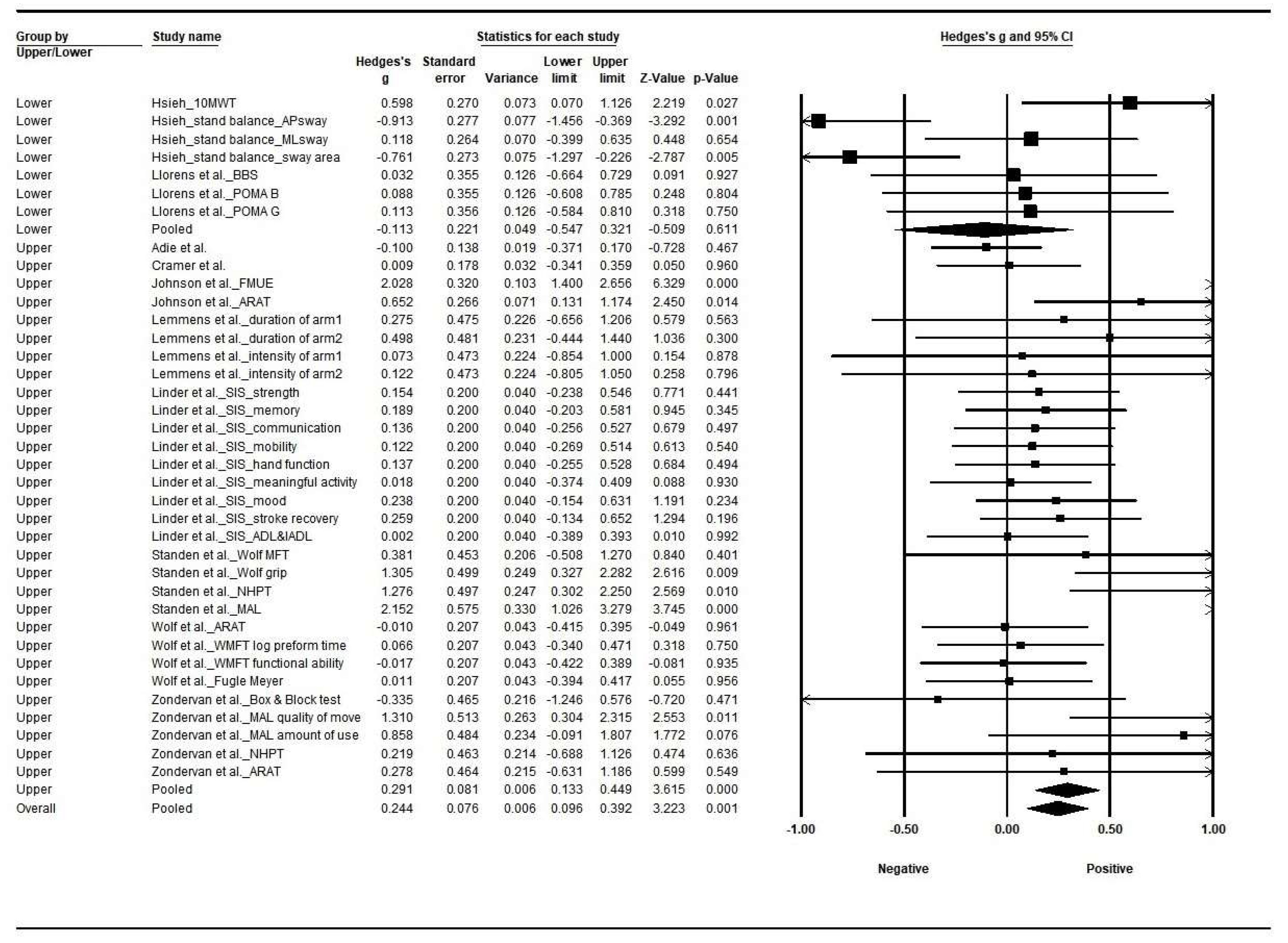
3.4. Effect Size by Types of Intervention
3.5. Effect Size by Applied Limb
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Askim, T.; Mørkved, S.; Engen, A.; Roos, K.; Aas, T.; Indredavik, B. Effects of a community-based intensive motor training program combined with early supported discharge after treatment in a comprehensive stroke unit: A randomized, controlled trial. Stroke 2010, 41, 1697–1703. [Google Scholar] [CrossRef]
- Dhamoon, M.S.; Moon, Y.P.; Paik, M.C.; Boden-Albala, B.; Rundek, T.; Sacco, R.L.; Elkind, M.S. Long-term functional recovery after first ischemic stroke: The Northern Manhattan Study. Stroke 2009, 40, 2805–2811. [Google Scholar] [CrossRef] [PubMed]
- Su, C.-J.; Chiang, C.-Y.; Huang, J.-Y. Kinect-enabled home-based rehabilitation system using Dynamic Time Warping and fuzzy logic. Appl. Soft Comput. 2014, 22, 652–666. [Google Scholar] [CrossRef]
- Tousignant, M.; Moffet, H.; Nadeau, S.; Mérette, C.; Boissy, P.; Corriveau, H.; Marquis, F.; Cabana, F.; Ranger, P.; Belzile, É.L.; et al. Cost analysis of in-home telerehabilitation for post-knee arthroplasty. J. Med. Internet Res. 2015, 17, e83. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Abel, K.T.; Janecek, J.T.; Chen, Y.; Zheng, K.; Cramer, S.C. Home-based technologies for stroke rehabilitation: A systematic review. Int. J. Med. Inform. 2019, 123, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Hatem, S.M.; Saussez, G.; Della Faille, M.; Prist, V.; Zhang, X.; Dispa, D.; Bleyenheuft, Y. Rehabilitation of motor function after stroke: A multiple systematic review focused on techniques to stimulate upper extremity recovery. Front. Hum. Neurosci. 2016, 10, 442. [Google Scholar] [CrossRef] [PubMed]
- Maier, M.; Rubio Ballester, B.; Duff, A.; Duarte Oller, E.; Verschure, P.F.M.J. Effect of specific over nonspecific VR-based rehabilitation on poststroke motor recovery: A systematic meta-analysis. Neurorehabil. Neural Repair 2019, 33, 112–129. [Google Scholar] [CrossRef]
- Rubin, M.N.; Wellik, K.E.; Channer, D.D.; Demaerschalk, B.M. Systematic review of telestroke for post-stroke care and rehabilitation. Curr. Atheroscler. Rep. 2013, 15, 343. [Google Scholar] [CrossRef]
- Borenstein, M.; Cooper, H.; Hedges, L.; Valentine, J. Effect sizes for continuous data. Handb. Res. Synth. Meta Anal. 2009, 2, 221–235. [Google Scholar]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.; Rothstein, H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods 2010, 1, 97–111. [Google Scholar] [CrossRef]
- Borenstein, M.; Higgins, J.P.; Hedges, L.V.; Rothstein, H.R. Basics of meta-analysis: I2 is not an absolute measure of heterogeneity. Res. Synth. Methods 2017, 8, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.; Bird, M.L.; Muthalib, M.; Teo, W.P. An Innovative STRoke Interactive Virtual thErapy (STRIVE) online platform for community-dwelling stroke survivors: A randomized controlled trial. Arch. Phys. Med. Rehabil. 2020, 101, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Cramer, S.C.; Dodakian, L.; Le, V.; See, J.; Augsburger, R.; McKenzie, A.; Zhou, R.J.; Chiu, N.L.; Heckhausen, J.; Cassidy, J.M.; et al. Efficacy of home-based telerehabilitation vs in-clinic therapy for adults after stroke: A randomized clinical trial. JAMA Neurol. 2019, 76, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.C. Training by using an adaptive foot switch and video games to improve balance and mobility following stroke: A randomised controlled trial. Brain Impair. 2019, 20, 16–23. [Google Scholar] [CrossRef]
- Adie, K.; Schofield, C.; Berrow, M.; Wingham, J.; Humfryes, J.; Pritchard, C.; James, M.; Allison, R. Does the use of Nintendo Wii SportsTM improve arm function? Trial of WiiTM in Stroke: A randomized controlled trial and economics analysis. Clin. Rehabil. 2017, 31, 173–185. [Google Scholar] [CrossRef]
- Standen, P.J.; Threapleton, K.; Richardson, A.; Connell, L.; Brown, D.J.; Battersby, S.; Platts, F.; Burton, A. A low cost virtual reality system for home based rehabilitation of the arm following stroke: A randomised controlled feasibility trial. Clin. Rehabil. 2017, 31, 340–350. [Google Scholar] [CrossRef]
- Zondervan, D.K.; Friedman, N.; Chang, E.; Zhao, X.; Augsburger, R.; Reinkensmeyer, D.J.; Cramer, S.C. Home-based hand rehabilitation after chronic stroke: Randomized, controlled single-blind trial comparing the MusicGlove with a conventional exercise program. J. Rehabil. Res. Dev. 2016, 53, 457–472. [Google Scholar] [CrossRef]
- Linder, S.M.; Rosenfeldt, A.B.; Bay, R.C.; Sahu, K.; Wolf, S.L.; Alberts, J.L. Improving quality of life and depression after stroke through telerehabilitation. Am. J. Occup. Ther. 2015, 69, 6902290020p1–6902290020p10. [Google Scholar] [CrossRef]
- Wolf, S.L.; Sahu, K.; Bay, R.C.; Buchanan, S.; Reiss, A.; Linder, S.; Rosenfeldt, A.; Alberts, J. The HAAPI (Home Arm Assistance Progression Initiative) Trial: A Novel Robotics Delivery Approach in Stroke Rehabilitation. Neurorehabil. Neural Repair 2015, 29, 958–968. [Google Scholar] [CrossRef]
- Lloréns, R.; Noé, E.; Colomer, C.; Alcañiz, M. Effectiveness, usability, and cost–benefit of a virtual reality–based telerehabilitation program for balance recovery after stroke: A randomized controlled trial. Arch. Phys. Med. Rehabil. 2015, 96, 418–425.e2.e412. [Google Scholar] [CrossRef]
- Lemmens, R.J.; Timmermans, A.A.; Janssen-Potten, Y.J.; Pulles, S.A.; Geers, R.P.; Bakx, W.G.; Smeets, R.J.; Seelen, H.A. Accelerometry measuring the outcome of robot-supported upper limb training in chronic stroke: A randomized controlled trial. PLoS ONE 2014, 9, e96414. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Xu, J.; Yue, C.; Li, Y.; Liang, Y. Collaborative care model based telerehabilitation exercise training program for acute stroke patients in China: A randomized controlled trial. J. Stroke Cerebrovasc. Dis. 2020, 29, 105328. [Google Scholar] [CrossRef] [PubMed]
- Winstein, C.J.; Stein, J.; Arena, R.; Bates, B.; Cherney, L.R.; Cramer, S.C.; Deruyter, F.; Eng, J.J.; Fisher, B.; Harvey, R.L.; et al. Guidelines for adult stroke rehabilitation and recovery: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2016, 47, e98–e169. [Google Scholar] [CrossRef]
- Dąbrowski, J.; Czajka, A.; Zielińska-Turek, J.; Jaroszyński, J.; Furtak-Niczyporuk, M.; Mela, A.; Poniatowski, Ł.A.; Drop, B.; Dorobek, M.; Barcikowska-Kotowicz, M.; et al. Brain functional reserve in the context of neuroplasticity after stroke. Neural Plast. 2019, 2019, 9708905. [Google Scholar] [CrossRef]
- Steinle, B.; Corbaley, J. Rehabilitation of stroke: A new horizon. Mo. Med. 2011, 108, 284–288. [Google Scholar] [PubMed]
- Mela, A.; Poniatowski, Ł.A.; Drop, B.; Furtak-Niczyporuk, M.; Jaroszyński, J.; Wrona, W.; Staniszewska, A.; Dąbrowski, J.; Czajka, A.; Jagielska, B.; et al. Overview and analysis of the cost of drug programs in Poland: Public payer expenditures and coverage of cancer and non-neoplastic diseases related drug therapies from 2015–2018 years. Front. Pharmacol. 2020, 11, 1123. [Google Scholar] [CrossRef]
- Hsieh, Y.W.; Chang, K.C.; Hung, J.W.; Wu, C.Y.; Fu, M.H.; Chen, C.C. Effects of home-based versus clinic-based rehabilitation combining mirror therapy and task-specific training for patients with stroke: A randomized crossover trial. Arch. Phys. Med. Rehabil. 2018, 99, 2399–2407. [Google Scholar] [CrossRef]
- Ardern, C.L.; Büttner, F.; Andrade, R.; Weir, A.; Ashe, M.C.; Holden, S.; Impellizzeri, F.M.; Delahunt, E.; Dijkstra, H.P.; Mathieson, S.; et al. Implementing the 27 PRISMA 2020 statement items for systematic reviews in the sport and exercise medicine, musculoskeletal rehabilitation and sports science fields: The persist (implementing Prisma in exercise, rehabilitation, sport medicine and sports science) guidance. Br. J. Sport. Med. 2022, 56, 175–195. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]

| Author | Intervention | Interventions | Duration | Participants n = 761 (Mean Age) | Inclusion Criteria | Primary Outcome Measures | Main Findings |
|---|---|---|---|---|---|---|---|
| 1. Johnson et al., 2020 [12] | VR | Virtual therapy: 45 min, 2 times/week | 2 m | Exp = 28 (64.7 yr) Cont = 30 (59.3 yr) | ≥18 yr, FMA (upper extremity) Score 25–45, MMSE > 24, at least 3 months poststroke | FMA (upper extremity), ARAT | The FMA (upper extremity) score was improved |
| 2. Cramer et al. [13] | Games | Computer game (36 sessions) for TR group Cont: printed homework (contents the same as the TR group) | Exp = 62 (62 yr) Cont = 62 (60 yr) | 18 yr old, Stroke 4 to 36 wks prior, mild to severe arm motor deficit | Arm motor (FMA, BBT) | No difference in the FMA score | |
| 3. Hsieh [14] | Games | VG intervention (foot switch): 30 min (once a week) | 2.5 m | Exp = 28 (58.3 yr) Cont = 28 (59.3 yr) | Stroke onset over 3 m, ankle dorsiflexion (>10°), ability to walk | Walking performance (10MWT, CoP sway, AP sway, ML sway) and standing balance (CoP sway) | Walking performance was improved (except CoP and ML sway) |
| 4. Adie et al. [15] | Games | Wii exercise: 45 min/daily × 6 wks | 1.5 m | Exp = 117 (66.8 yr) Cont = 118 (68 yr) | Stroke within 6 m, Medical Research Council Scale ≤ 5 | ARAT | No differences in all variables |
| 5. Standen et al. [16] | VR | VR Nintendo Wii remote): 20 min × 3 times/day | 2 m | Exp = 17 (59 yr) Cont = 10 (63 yr) | Residual arm dysfunction | WMFT, Nine-Hole Peg test, MAL, Nottingham Extended ADL, Frequency of use | The WMFT and MAL score were improved |
| 6. Zondervan et al. [17] | RD | MusicGlove device and laptop: 3 wks | 3 wks | Exp = 9 (60 yr) Cont = 8 (59 yr) | BBT ˃ 1, age ˂ 75 | BBT | No difference in BBT score |
| 7. Linder et al. [18] | RD | Home-based robot (Hand Mentor Pro) assisted rehab: 8 wks, 5 days a week, 3 h | 2 m | Exp = 48 (55.5 yr) Cont = 51 (59.4 yr) | Stroke within 6 m, FMA 11–55 | SIS, CESD | No difference in SIS and CESD scores |
| 8. Wolf et al. [19] | RD | Home-based robot (Hand Mentor Pro) assisted rehab: 5 days/week, 3 h (total 120 h) | 2 m | Exp = 51 (59.1 yr) Cont = 48 (54.7 yr) | Stroke within 6 m, FMA 11–55 | ARAT | No difference in variable |
| 9. Lloréns et al. [20] | VR | Home-based VR for balance: 45 min, 3 times/week | 2 m | Exp = 15 (55.5 yr) Cont = 15 (55.6 yr) | ≥40 aged ≤75, >6 m, BBA 7–12, MMSE > 23 | BBS, POMA-B, POMA-G, BBA, SIS, IMI | The scores of BBS, POMA-B, POMA-G, and BBA were improved |
| 10. Lemmens et al. [21] | RD | Robot-supported task-oriented arm-hand training: 8 wks, 4 times/week, 2 × 30 min/day | 2 m | Exp = 8 (63.5 yr) Cont = 8 (55.0 yr) | 18–85 aged, first-ever stroke, MRC grade 2–4, post-stroke time ≥ 12 m, MMSE ≥ 26 | FMA, ARAT, MAL | No differences in both groups |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bok, S.-K.; Song, Y.; Lim, A.; Jin, S.; Kim, N.; Ko, G. High-Tech Home-Based Rehabilitation after Stroke: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 2668. https://doi.org/10.3390/jcm12072668
Bok S-K, Song Y, Lim A, Jin S, Kim N, Ko G. High-Tech Home-Based Rehabilitation after Stroke: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2023; 12(7):2668. https://doi.org/10.3390/jcm12072668
Chicago/Turabian StyleBok, Soo-Kyung, Youngshin Song, Ancho Lim, Sohyun Jin, Nagyeong Kim, and Geumbo Ko. 2023. "High-Tech Home-Based Rehabilitation after Stroke: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 12, no. 7: 2668. https://doi.org/10.3390/jcm12072668
APA StyleBok, S.-K., Song, Y., Lim, A., Jin, S., Kim, N., & Ko, G. (2023). High-Tech Home-Based Rehabilitation after Stroke: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 12(7), 2668. https://doi.org/10.3390/jcm12072668








