Neoadjuvant Therapy for Extrahepatic Biliary Tract Cancer: A Propensity Score-Matched Survival Analysis
Abstract
1. Introduction
2. Methods
2.1. Dataset and Study Population
2.2. Statistical Analysis
3. Results
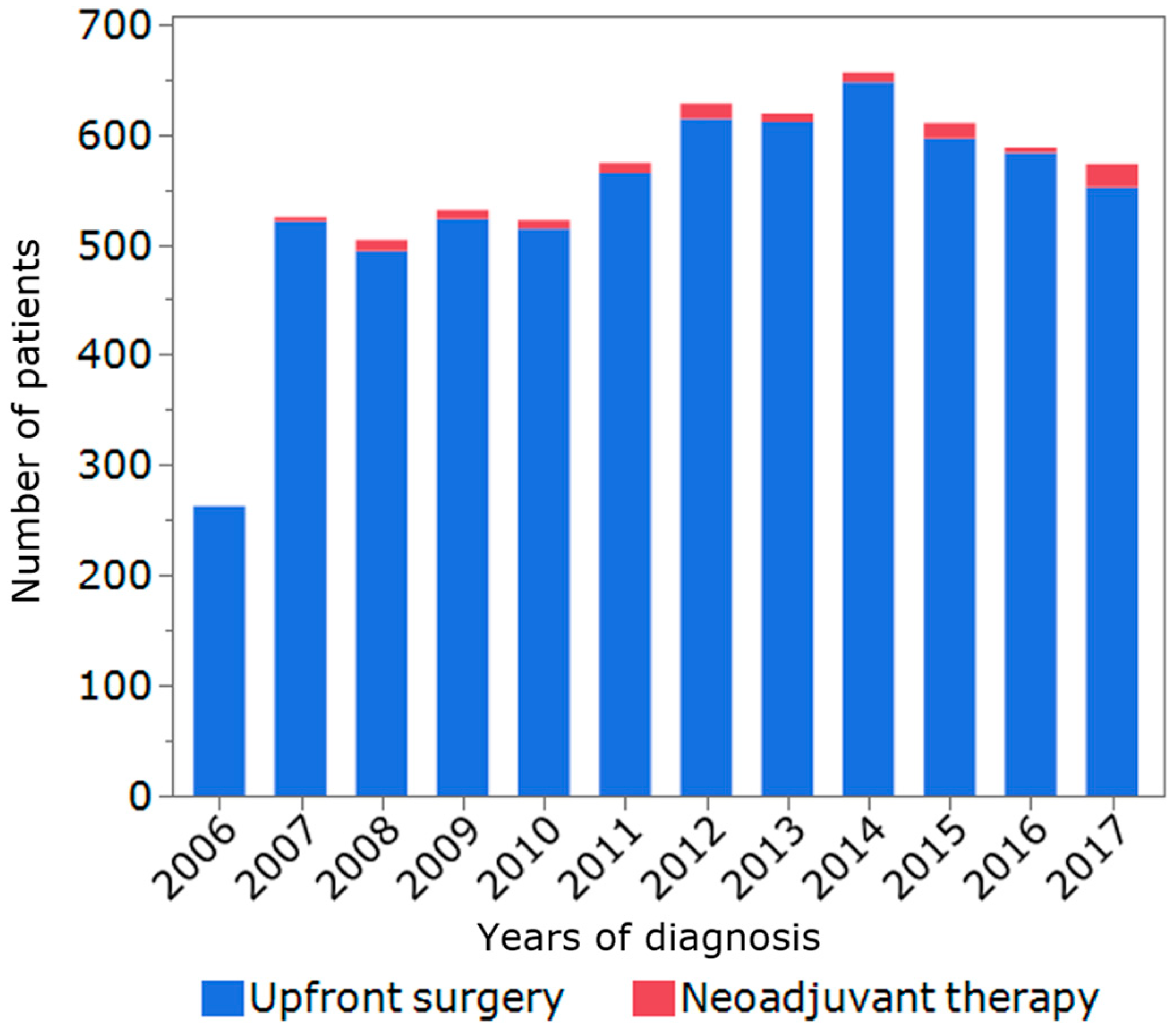
3.1. Patients, Tumor, and Treatment Characteristics
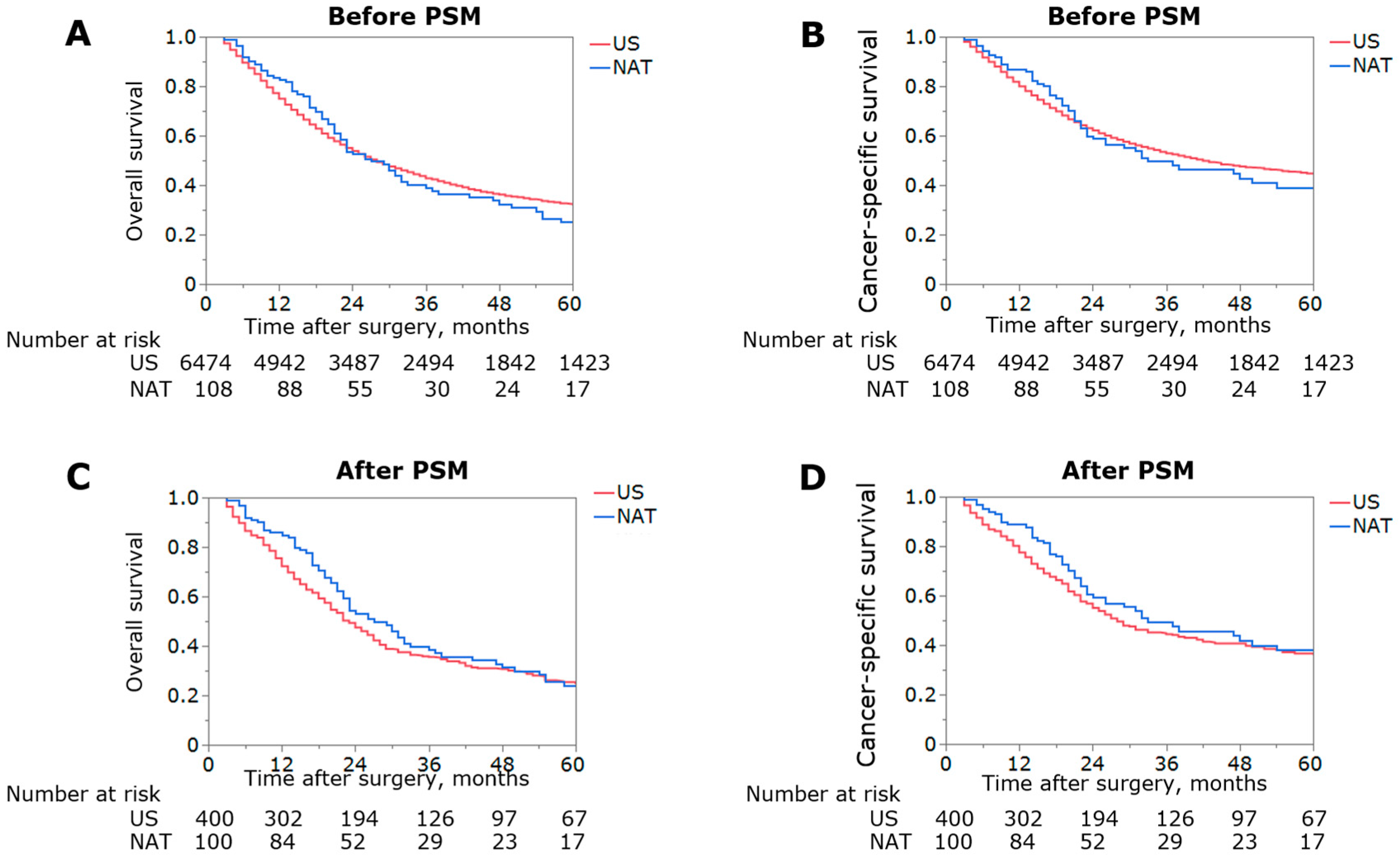
3.2. Impact of NAT on Overall Survival before and after PSM
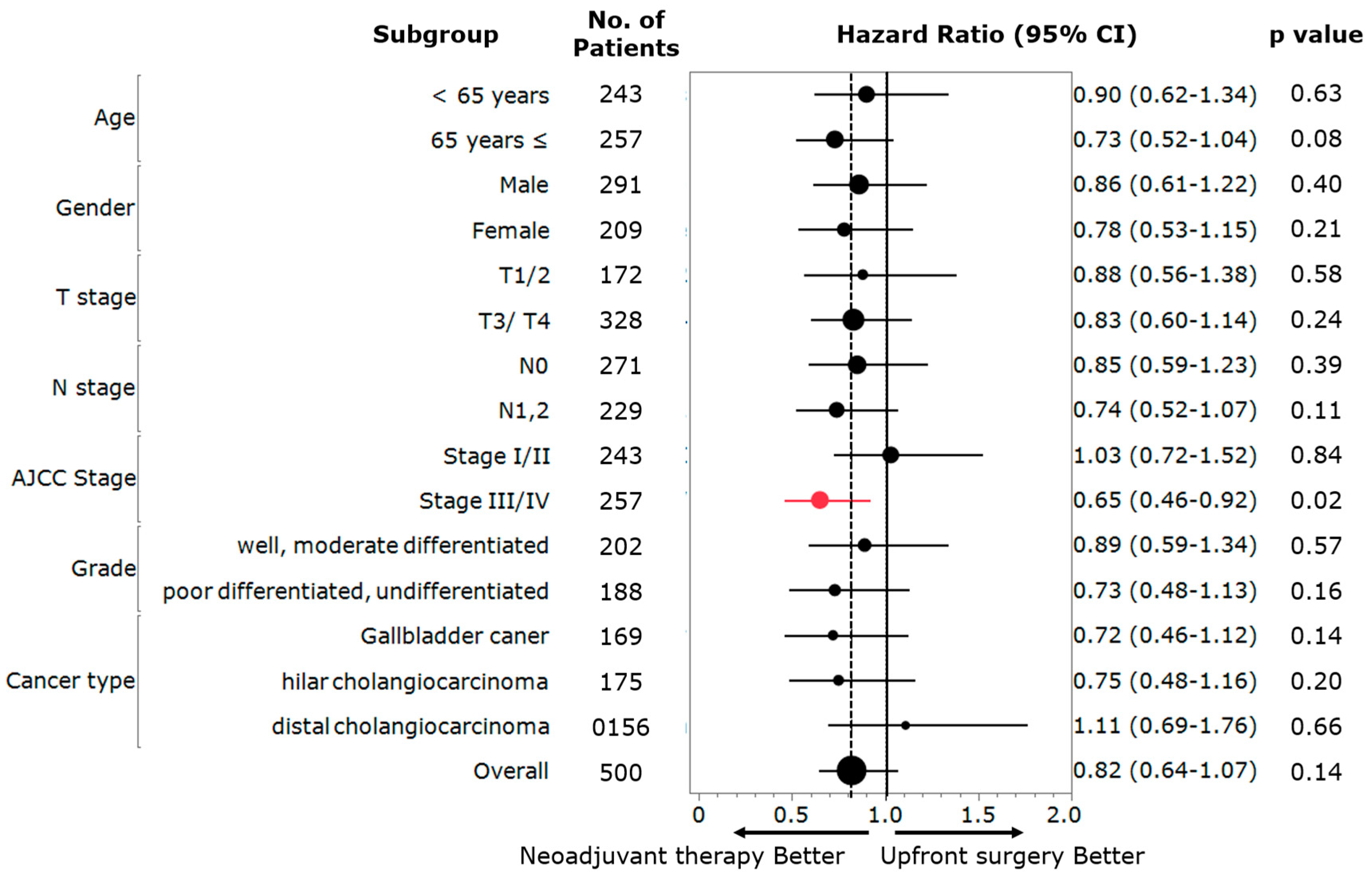
3.3. Subgroup Analysis of the Usefulness of NAT
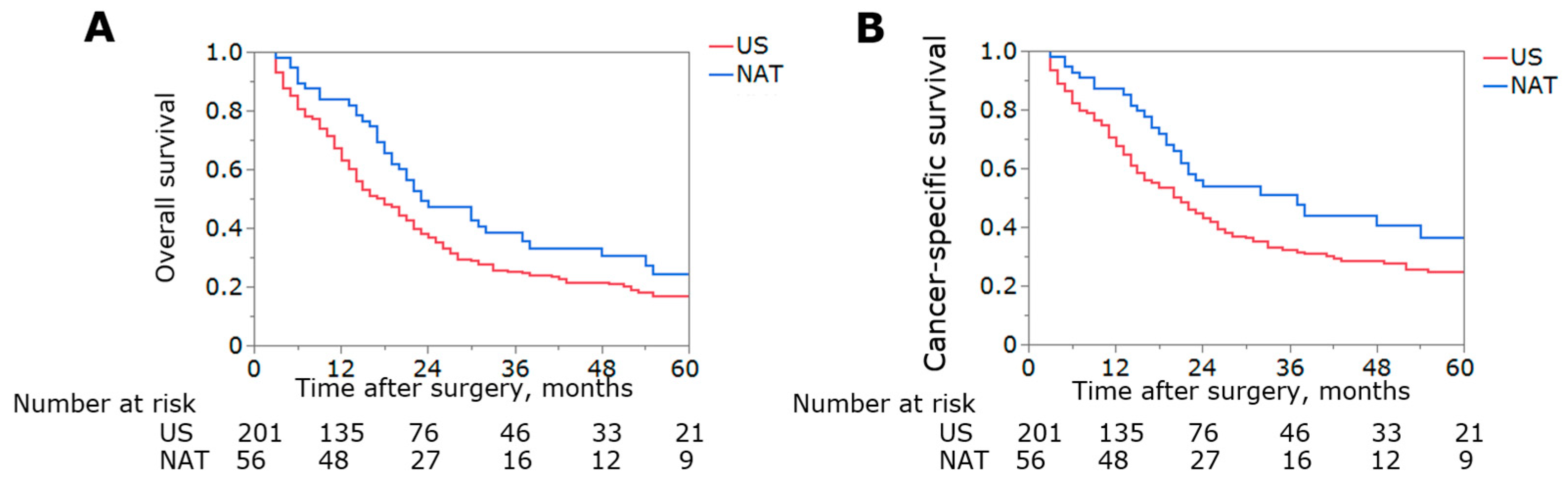
3.4. Sensitivity Analysis Excluding Patients with GBC
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Le, V.H.; O’Connor, V.V.; Li, D.; Melstrom, L.G.; Fong, Y.; DiFronzo, A.L. Outcomes of neoadjuvant therapy for cholangiocarcinoma: A review of existing evidence assessing treatment response and R0 resection rate. J. Surg. Oncol. 2021, 123, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Brandi, G. Neoadjuvant therapy for cholangiocarcinoma: A comprehensive literature review. Cancer Treat. Res. Commun. 2021, 27, 100354. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.W.; Kelley, R.K.; Nervi, B.; Oh, D.-Y.; Zhu, A.X. Biliary tract cancer. Lancet 2021, 397, 428–444. [Google Scholar] [CrossRef]
- Goetze, T.O.; Bechstein, W.O.; Bankstahl, U.S.; Keck, T.; Königsrainer, A.; Lang, S.A.; Pauligk, C.; Piso, P.; Vogel, A.; Al-Batran, S.-E. Neoadjuvant chemotherapy with gemcitabine plus cisplatin followed by radical liver resection versus immediate radical liver resection alone with or without adjuvant chemotherapy in incidentally detected gallbladder carcinoma after simple cholecystectomy or in front of radical resection of BTC (ICC/ECC)—A phase III study of the German registry of incidental gallbladder carcinoma platform (GR)—The AIO/CALGP/ACO-GAIN-trial–. BMC Cancer 2020, 20, 122. [Google Scholar] [CrossRef]
- Lamarca, A.; Edeline, J.; Goyal, L. How I treat biliary tract cancer. ESMO Open 2022, 7, 100378. [Google Scholar] [CrossRef] [PubMed]
- Krell, R.W.; Wei, A.C. Gallbladder cancer: Surgical management. Chin. Clin. Oncol. 2019, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Shroff, R.T.; Kennedy, E.B.; Bachini, M.; Bekaii-Saab, T.; Crane, C.; Edeline, J.; El-Khoueiry, A.; Feng, M.; Katz, M.H.; Primrose, J.; et al. Adjuvant Therapy for Resected Biliary Tract Cancer: ASCO Clinical Practice Guideline. J. Clin. Oncol. 2019, 37, 1015–1027. [Google Scholar] [CrossRef] [PubMed]
- Primrose, J.N.; Fox, R.P.; Palmer, D.H.; Malik, H.Z.; Prasad, R.; Mirza, D.; Anthony, A.; Corrie, P.; Falk, S.; Finch-Jones, M.; et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): A randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019, 20, 663–673. [Google Scholar] [CrossRef]
- Ando, N.; Kato, H.; Igaki, H.; Shinoda, M.; Ozawa, S.; Shimizu, H.; Nakamura, T.; Yabusaki, H.; Aoyama, N.; Kurita, A.; et al. A Randomized Trial Comparing Postoperative Adjuvant Chemotherapy with Cisplatin and 5-Fluorouracil Versus Preoperative Chemotherapy for Localized Advanced Squamous Cell Carcinoma of the Thoracic Esophagus (JCOG9907). Ann. Surg. Oncol. 2012, 19, 68–74. [Google Scholar] [CrossRef]
- Shapiro, J.; van Lanschot, J.J.B.; Hulshof, M.C.C.M.; van Hagen, P.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.L.; van Laarhoven, H.W.M.; Nieuwenhuijzen, G.A.P.; Hospers, G.A.P.; Bonenkamp, J.J.; et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): Long-term results of a randomised controlled trial. Lancet Oncol. 2015, 16, 1090–1098. [Google Scholar] [CrossRef]
- Kang, Y.-K.; Yook, J.H.; Park, Y.-K.; Lee, J.S.; Kim, Y.-W.; Kim, J.Y.; Ryu, M.-H.; Rha, S.Y.; Chung, I.J.; Kim, I.-H.; et al. PRODIGY: A Phase III Study of Neoadjuvant Docetaxel, Oxaliplatin, and S-1 Plus Surgery and Adjuvant S-1 Versus Surgery and Adjuvant S-1 for Resectable Advanced Gastric Cancer. J. Clin. Oncol. 2021, 39, 2903–2913. [Google Scholar] [CrossRef] [PubMed]
- Motoi, F.; Kosuge, T.; Ueno, H.; Yamaue, H.; Satoi, S.; Sho, M.; Honda, G.; Matsumoto, I.; Wada, K.; Furuse, J.; et al. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP05). Jpn. J. Clin. Oncol. 2019, 49, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Versteijne, E.; van Dam, J.L.; Suker, M.; Janssen, Q.P.; Groothuis, K.; Akkermans-Vogelaar, J.M.; Besselink, M.G.; Bonsing, B.A.; Buijsen, J.; Busch, O.R.; et al. Neoadjuvant Chemoradiotherapy Versus Upfront Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Long-Term Results of the Dutch Randomized PREOPANC Trial. J. Clin. Oncol. 2022, 40, 1220–1230. [Google Scholar] [CrossRef] [PubMed]
- Sauer, R.; Liersch, T.; Merkel, S.; Fietkau, R.; Hohenberger, W.; Hess, C.; Becker, H.; Raab, H.-R.; Villanueva, M.-T.; Witzigmann, H.; et al. Preoperative Versus Postoperative Chemoradiotherapy for Locally Advanced Rectal Cancer: Results of the German CAO/ARO/AIO-94 Randomized Phase III Trial After a Median Follow-Up of 11 Years. J. Clin. Oncol. 2012, 30, 1926–1933. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Pendhakar, S.; Ali, R.; Chichra, A.; Pendharkar, D. Incidence and survival of gallbladder cancer over three decades: A SEER database study. J. Clin. Oncol. 2015, 33, e17651. [Google Scholar] [CrossRef]
- Chen, Z.; Pu, L.; Gao, W.; Zhang, L.; Han, G.; Zhu, Q.; Li, X.; Wu, J.; Wang, X. Influence of marital status on the survival of adults with extrahepatic/intrahepatic cholangiocarcinoma. Oncotarget 2017, 8, 28959–28970. [Google Scholar] [CrossRef]
- Toyoda, J.; Sahara, K.; Tsilimigras, D.I.; Miyake, K.; Yabushita, Y.; Homma, Y.; Kumamoto, T.; Matsuyama, R.; Pawlik, T.M. Survival Benefit of Primary Tumor Resection Among Elderly Patients with Pancreatic Neuroendocrine Tumors. World J. Surg. 2021, 45, 3643–3651. [Google Scholar] [CrossRef]
- Utuama, O.; Permuth, J.B.; Dagne, G.; Sanchez-Anguiano, A.; Alman, A.; Kumar, A.; Denbo, J.; Kim, R.; Fleming, J.B.; Anaya, D.A. Neoadjuvant Chemotherapy for Intrahepatic Cholangiocarcinoma: A Propensity Score Survival Analysis Supporting Use in Patients with High-Risk Disease. Ann. Surg. Oncol. 2021, 28, 1939–1949. [Google Scholar] [CrossRef]
- Gkika, E.; Hawkins, M.A.; Grosu, A.-L.; Brunner, T.B. The Evolving Role of Radiation Therapy in the Treatment of Biliary Tract Cancer. Front. Oncol. 2020, 10, 604387. [Google Scholar] [CrossRef]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus Gemcitabine versus Gemcitabine for Biliary Tract Cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef]
- Yoo, C.; Shin, S.; Park, J.-O.; Kim, K.-P.; Jeong, J.; Ryoo, B.-Y.; Lee, W.; Song, K.-B.; Hwang, D.-W.; Park, J.-H.; et al. Current Status and Future Perspectives of Perioperative Therapy for Resectable Biliary Tract Cancer: A Multidisciplinary Review. Cancers 2021, 13, 1647. [Google Scholar] [CrossRef] [PubMed]
- Tsilimigras, D.I.; Sahara, K.; Wu, L.; Moris, D.; Bagante, F.; Guglielmi, A.; Aldrighetti, L.; Weiss, M.; Bauer, T.W.; Alexandrescu, S.; et al. Very Early Recurrence After Liver Resection for Intrahepatic Cholangiocarcinoma: Considering Alternative Treatment Approaches. JAMA Surg. 2020, 155, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Hakeem, A.R.; Papoulas, M.; Menon, K.V. The role of neoadjuvant chemotherapy or chemoradiotherapy for advanced gallbladder cancer—A systematic review. Eur. J. Surg. Oncol. (EJSO) 2019, 45, 83–91. [Google Scholar] [CrossRef]
- Guo, M.; Beal, E.W.; Miller, E.D.; Williams, T.M.; Tsung, A.; Dillhoff, M.; Ejaz, A.; Pawlik, T.M.; Cloyd, J.M. Neoadjuvant therapy versus surgery first for ampullary carcinoma: A propensity score-matched analysis of the NCDB. J. Surg. Oncol. 2021, 123, 1558–1567. [Google Scholar] [CrossRef] [PubMed]
- Engineer, R.; Goel, M.; Chopra, S.; Patil, P.; Purandare, N.; Rangarajan, V.; Ph, R.; Bal, M.; Shrikhande, S.; Shrivastava, S.K.; et al. Neoadjuvant Chemoradiation Followed by Surgery for Locally Advanced Gallbladder Cancers: A New Paradigm. Ann. Surg. Oncol. 2016, 23, 3009–3015. [Google Scholar] [CrossRef]
- Kato, A.; Shimizu, H.; Ohtsuka, M.; Yoshidome, H.; Yoshitomi, H.; Furukawa, K.; Takeuchi, D.; Takayashiki, T.; Kimura, F.; Miyazaki, M. Surgical Resection after Downsizing Chemotherapy for Initially Unresectable Locally Advanced Biliary Tract Cancer: A Retrospective Single-center Study. Ann. Surg. Oncol. 2013, 20, 318–324. [Google Scholar] [CrossRef]
- Sumiyoshi, T.; Shima, Y.; Okabayashi, T.; Negoro, Y.; Shimada, Y.; Iwata, J.; Matsumoto, M.; Hata, Y.; Noda, Y.; Sui, K.; et al. Chemoradiotherapy for Initially Unresectable Locally Advanced Cholangiocarcinoma. World J. Surg. 2018, 42, 2910–2918. [Google Scholar] [CrossRef]
- Katayose, Y.; Nakagawa, K.; Yoshida, H.; Morikawa, T.; Hayashi, H.; Okada, T.; Mizuma, M.; Sakata, N.; Ohtsuka, H.; Fukase, K.; et al. Neoadjuvant chemoradiation therapy for cholangiocarcinoma to improve R0 resection rate: The first report of phase II study. J. Clin. Oncol. 2015, 33, 402. [Google Scholar] [CrossRef]
- Katayose, Y.; Rikiyama, T.; Motoi, F.; Yamamoto, K.; Yoshida, H.; Morikawa, T.; Hayashi, H.; Kanno, A.; Hirota, M.; Satoh, K.; et al. Phase I trial of neoadjuvant chemoradiation with gemcitabine and surgical resection for cholangiocarcinoma patients (NACRAC study). Hepato-Gastroenterology 2011, 58, 1866–1872. [Google Scholar] [CrossRef]
- Patkar, S.; Patel, S.; Gupta, A.; Ramaswamy, A.; Ostwal, V.; Goel, M. Revision Surgery for Incidental Gallbladder Cancer—Challenging the Dogma: Ideal Timing and Real-World Applicability. Ann. Surg. Oncol. 2021, 28, 6758–6766. [Google Scholar] [CrossRef]
- Sahara, K.; Tsilimigras, D.I.; Maithel, S.K.; Abbott, D.E.; Poultsides, G.A.; Hatzaras, I.; Fields, R.C.; Weiss, M.; Scoggins, C.; Isom, C.A.; et al. Survival benefit of lymphadenectomy for gallbladder cancer based on the therapeutic index: An analysis of the US extrahepatic biliary malignancy consortium. J. Surg. Oncol. 2020, 121, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Gani, F.; Buettner, S.; Margonis, G.A.; Ethun, C.G.; Poultsides, G.; Tran, T.; Idrees, K.; Isom, C.A.; Fields, R.C.; Krasnick, B.; et al. Assessing the impact of common bile duct resection in the surgical management of gallbladder cancer. J. Surg. Oncol. 2016, 114, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Komaya, K.; Ebata, T.; Shirai, K.; Ohira, S.; Morofuji, N.; Akutagawa, A.; Yamaguchi, R.; Nagino, M.; Aoba, T.; Kaneoka, Y.; et al. Recurrence after resection with curative intent for distal cholangiocarcinoma. Br. J. Surg. 2017, 104, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Shirai, Y.; Sakata, J.; Wakai, T.; Ohashi, T.; Ajioka, Y.; Hatakeyama, K. Assessment of lymph node status in gallbladder cancer: Location, number, or ratio of positive nodes. World J. Surg. Oncol. 2012, 10, 87. [Google Scholar] [CrossRef]
- Oshiro, Y.; Sasaki, R.; Kobayashi, A.; Murata, S.; Fukunaga, K.; Kondo, T.; Oda, T.; Ohkohchi, N. Prognostic relevance of the lymph node ratio in surgical patients with extrahepatic cholangiocarcinoma. Eur. J. Surg. Oncol. (EJSO) 2011, 37, 60–64. [Google Scholar] [CrossRef]
- Sakata, J.; Wakai, T.; Matsuda, Y.; Ohashi, T.; Hirose, Y.; Ichikawa, H.; Kobayashi, T.; Minagawa, M.; Kosugi, S.-I.; Koyama, Y.; et al. Comparison of Number Versus Ratio of Positive Lymph Nodes in the Assessment of Lymph Node Status in Extrahepatic Cholangiocarcinoma. Ann. Surg. Oncol. 2016, 23, 225–234. [Google Scholar] [CrossRef]
- Hao, Y.; Liu, Y.; Ishibashi, H.; Wakama, S.; Nishino, E.; Yonemura, Y. Downstaging of lymph node metastasis after neoadjuvant intraperitoneal and systemic chemotherapy in gastric carcinoma with peritoneal metastasis. Eur. J. Surg. Oncol. (EJSO) 2019, 45, 1493–1497. [Google Scholar] [CrossRef]
- Caricato, M.; Ausania, F.; De Dominicis, E.; Vincenzi, B.; Rabitti, C.; Tonini, G.; Cellini, F.; Coppola, R. Tumor regression in mesorectal lymphnodes after neoadjuvant chemoradiation for rectal cancer. Eur. J. Surg. Oncol. (EJSO) 2007, 33, 724–728. [Google Scholar] [CrossRef]
- Dominici, L.S.; Gonzalez, V.M.N.; Buzdar, A.U.; Lucci, A.; Mittendorf, E.A.; Le-Petross, H.T.; Babiera, G.V.; Meric-Bernstam, F.; Hunt, K.K.; Kuerer, H.M. Cytologically proven axillary lymph node metastases are eradicated in patients receiving preoperative chemotherapy with concurrent trastuzumab for HER2-positive breast cancer. Cancer 2010, 116, 2884–2889. [Google Scholar] [CrossRef]
- Hagens, E.; Tukanova, K.; Jamel, S.; Henegouwen, M.V.B.; Hanna, G.B.; Gisbertz, S.; Markar, S.R. Prognostic relevance of lymph node regression on survival in esophageal cancer: A systematic review and meta-analysis. Dis. Esophagus 2021, 35, doab021. [Google Scholar] [CrossRef]
- Yoo, C.; Lee, S.S.; Song, K.B.; Jeong, J.H.; Hyung, J.; Park, D.H.; Song, T.J.; Seo, D.W.; Lee, S.K.; Kim, M.-H.; et al. Neoadjuvant modified FOLFIRINOX followed by postoperative gemcitabine in borderline resectable pancreatic adenocarcinoma: A Phase 2 study for clinical and biomarker analysis. Br. J. Cancer 2020, 123, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Shroff, R.T.; Javle, M.M.; Xiao, L.; Kaseb, A.O.; Varadhachary, G.R.; Wolff, R.A.; Raghav, K.P.S.; Iwasaki, M.; Masci, P.; Ramanathan, R.K.; et al. Gemcitabine, Cisplatin, and nab-Paclitaxel for the Treatment of Advanced Biliary Tract Cancers. JAMA Oncol. 2019, 5, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.; Han, B.; Kim, H.S.; Kim, K.-P.; Kim, D.; Jeong, J.H.; Lee, J.-L.; Kim, T.W.; Kim, J.H.; Choi, D.R.; et al. Multicenter Phase II Study of Oxaliplatin, Irinotecan, and S-1 as First-line Treatment for Patients with Recurrent or Metastatic Biliary Tract Cancer. Cancer Res. Treat. 2018, 50, 1324–1330. [Google Scholar] [CrossRef] [PubMed]
- Ioka, T.; Kanai, M.; Kobayashi, S.; Sakai, D.; Eguchi, H.; Baba, H.; Seo, S.; Taketomi, A.; Takayama, T.; Yamaue, H.; et al. Randomized phase III study of gemcitabine, cisplatin plus S-1 versus gemcitabine, cisplatin for advanced biliary tract cancer (KHBO1401 MITSUBA). J. Hepato-Biliary-Pancreatic Sci. 2022, 30, 102–110. [Google Scholar] [CrossRef]
- Oh, D.-Y.; Lee, K.-H.; Lee, D.-W.; Kim, T.Y.; Bang, J.-H.; Nam, A.-R.; Lee, Y.; Zhang, Q.; Rebelatto, M.; Li, W.; et al. Phase II study assessing tolerability, efficacy, and biomarkers for durvalumab (D) ± tremelimumab (T) and gemcitabine/cisplatin (GemCis) in chemo-naïve advanced biliary tract cancer (aBTC). J. Clin. Oncol. 2020, 38, 4520. [Google Scholar] [CrossRef]
- Oh, D.-Y.; He, A.R.; Qin, S.; Chen, L.-T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Lee, M.A.; Kitano, M.; et al. Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer. NEJM Evid. 2022, 1, EVIDoa2200015. [Google Scholar] [CrossRef]
| All | Upfront Surgery | Neoadjuvant Therapy | |||||
|---|---|---|---|---|---|---|---|
| Variable | n = 6582 | %, IQR | n = 6474 | %, IQR | n = 108 | %, IQR | p Value |
| Age, mean (IQR) | 70 | 61–78 | 70 | 61–78 | 65 | 57–71 | <0.01 |
| Gender, n (%) | <0.01 | ||||||
| Male | 2753 | 41.8 | 2694 | 41.6 | 59 | 54.6 | |
| Female | 3829 | 58.2 | 3780 | 58.4 | 49 | 45.4 | |
| Race, n (%) | 0.06 | ||||||
| White | 5046 | 76.7 | 4954 | 76.5 | 92 | 85.2 | |
| Black | 638 | 9.7 | 633 | 9.8 | 5 | 4.6 | |
| Others | 882 | 13.4 | 872 | 13.5 | 10 | 9.3 | |
| Unknown | 16 | 0.2 | 15 | 0.2 | 1 | 0.9 | |
| Type of cancer, n (%) | <0.01 | ||||||
| Gallbladder cancer | 4467 | 67.9 | 4429 | 68.4 | 38 | 35.2 | |
| Hilar cholangiocarcinoma | 1135 | 17.2 | 1095 | 16.9 | 40 | 37.0 | |
| Distal cholangiocarcinoma | 980 | 14.9 | 950 | 14.7 | 30 | 27.8 | |
| Year of diagnosis, n (%) | 0.18 | ||||||
| 2006–2009 | 1820 | 27.6 | 1798 | 27.8 | 22 | 20.4 | |
| 2010–2013 | 2342 | 35.6 | 2303 | 35.6 | 39 | 36.1 | |
| 2014–2017 | 2220 | 36.8 | 2373 | 36.6 | 47 | 43.5 | |
| AJCC T stage, n (%) | <0.01 | ||||||
| T1 | 1223 | 18.6 | 1209 | 18.7 | 14 | 13.0 | |
| T2 | 2838 | 43.1 | 2813 | 43.4 | 25 | 23.1 | |
| T3 | 2260 | 34.3 | 2205 | 34.1 | 55 | 50.9 | |
| T4 | 261 | 4.0 | 247 | 3.8 | 14 | 13.0 | |
| AJCC N stage, n (%) | <0.01 | ||||||
| N0 | 4488 | 68.2 | 4435 | 68.5 | 53 | 19.1 | |
| N1 | 2038 | 31.0 | 1986 | 30.7 | 52 | 48.1 | |
| N2 | 56 | 0.8 | 53 | 0.8 | 3 | 2.8 | |
| AJCC Stage, n (%) | <0.01 | ||||||
| Stage I | 1281 | 19.4 | 1265 | 19.5 | 16 | 14.8 | |
| Stage II | 2586 | 39.3 | 2556 | 39.5 | 30 | 27.8 | |
| Stage III | 2440 | 37.1 | 2391 | 36.9 | 49 | 45.4 | |
| Stage IVA | 275 | 4.2 | 262 | 4.1 | 13 | 12.0 | |
| Tumor grade, n (%) | <0.01 | ||||||
| Well | 1028 | 15.6 | 1020 | 15.8 | 8 | 7.4 | |
| Moderate | 3010 | 45.7 | 2978 | 46.0 | 32 | 29.6 | |
| Poor | 2010 | 30.5 | 1977 | 30.5 | 33 | 30.6 | |
| Undifferentiated | 62 | 1.0 | 61 | 0.9 | 1 | 0.9 | |
| Unknown | 472 | 7.2 | 438 | 6.8 | 34 | 31.5 | |
| Number of lymph nodes examined, median (IQR) | 4 | 1–11 | 4 | 1–11 | 7 | 3–15 | <0.01 |
| Number of positive lymph nodes, N1 median (IQR) | 1 | 1–3 | 1 | 1–3 | 1 | 1–2 | 0.15 |
| Lymph node ratio, N1 median (IQR) | 0.40 | 0.17–1.00 | 0.40 | 0.17–1.00 | 0.18 | 0.10–0.50 | < 0.01 |
| Neoadjuvant radiation, n (%) | 28 | 0.4 | - | - | 28 | 25.9 | |
| Adjuvant therapy, n (%) | 2840 | 43.1 | 2794 | 43.2 | 46 | 42.6 | 0.91 |
| Univariate Analysis | Multivariate Analysis | ||||||
|---|---|---|---|---|---|---|---|
| Variables | Reference | HR | 95% CI | p | HR | 95% CI | p |
| OS | |||||||
| Age ≥ 65 | <65 | 1.31 | 1.07–1.60 | <0.01 | 1.30 | 1.07–1.60 | <0.01 |
| Male | Female | 0.95 | 0.77–1.28 | 0.64 | 1.00 | 0.81–1.23 | 0.99 |
| White | Other | 0.96 | 0.74–1.25 | 0.76 | 0.91 | 0.70–1.20 | 0.52 |
| GBC | EHCC | 1.13 | 0.92–1.40 | 0.24 | 1.18 | 0.94–1.47 | 0.14 |
| Grade poor, undifferentiated | Well, moderate | 1.25 | 1.00–1.57 | 0.04 | 1.15 | 0.92–1.45 | 0.21 |
| T3/4 | T1/2 | 1.84 | 1.47–2.28 | <0.01 | 1.68 | 1.33–2.13 | <0.01 |
| N1/2 | N0 | 1.54 | 1.26–1.88 | <0.01 | 1.38 | 1.11–1.70 | <0.01 |
| Stages III/IVA | Stages I/II | 1.52 | 1.24–1.86 | <0.01 | - | - | - |
| Neoadjuvant therapy | No | 0.82 | 0.63–1.07 | 0.14 | 0.79 | 0.61–1.03 | 0.08 |
| Adjuvant therapy | No | 1.11 | 0.91–1.36 | 0.28 | 0.93 | 0.75–1.16 | 0.55 |
| CSS | |||||||
| Age ≥ 65 | <65 | 1.06 | 0.83–1.34 | 0.63 | 1.05 | 0.83–1.33 | 0.66 |
| Male | Female | 0.86 | 0.91–1.46 | 0.22 | 0.92 | 0.72–1.18 | 0.54 |
| White | Other | 0.91 | 0.68–1.24 | 0.57 | 0.83 | 0.62–1.13 | 0.25 |
| GBC | EHCC | 1.23 | 0.96–1.57 | 0.10 | 1.29 | 1.00–1.67 | 0.04 |
| Grade poor, undifferenced | Well, moderate | 1.30 | 1.00–1.69 | 0.04 | 1.17 | 0.89–1.53 | 0.24 |
| T3/4 | T1/2 | 2.24 | 1.71–2.95 | <0.01 | 2.05 | 1.53–2.74 | <0.01 |
| N1/2 | N0 | 1.69 | 1.33–2.14 | <0.01 | 1.45 | 1.13–1.85 | <0.01 |
| Stages III/IVA | Stages I/II | 1.84 | 1.45–2.34 | <0.01 | - | - | - |
| Neoadjuvant therapy | No | 0.82 | 0.61–1.12 | 0.22 | 0.78 | 0.58–1.07 | 0.13 |
| Adjuvant therapy | No | 1.21 | 0.95–1.53 | 0.11 | 0.97 | 0.76–1.25 | 0.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toyoda, J.; Sahara, K.; Takahashi, T.; Miyake, K.; Yabushita, Y.; Sawada, Y.; Homma, Y.; Matsuyama, R.; Endo, I.; Pawlik, T.M. Neoadjuvant Therapy for Extrahepatic Biliary Tract Cancer: A Propensity Score-Matched Survival Analysis. J. Clin. Med. 2023, 12, 2654. https://doi.org/10.3390/jcm12072654
Toyoda J, Sahara K, Takahashi T, Miyake K, Yabushita Y, Sawada Y, Homma Y, Matsuyama R, Endo I, Pawlik TM. Neoadjuvant Therapy for Extrahepatic Biliary Tract Cancer: A Propensity Score-Matched Survival Analysis. Journal of Clinical Medicine. 2023; 12(7):2654. https://doi.org/10.3390/jcm12072654
Chicago/Turabian StyleToyoda, Junya, Kota Sahara, Tomoaki Takahashi, Kentaro Miyake, Yasuhiro Yabushita, Yu Sawada, Yuki Homma, Ryusei Matsuyama, Itaru Endo, and Timothy M. Pawlik. 2023. "Neoadjuvant Therapy for Extrahepatic Biliary Tract Cancer: A Propensity Score-Matched Survival Analysis" Journal of Clinical Medicine 12, no. 7: 2654. https://doi.org/10.3390/jcm12072654
APA StyleToyoda, J., Sahara, K., Takahashi, T., Miyake, K., Yabushita, Y., Sawada, Y., Homma, Y., Matsuyama, R., Endo, I., & Pawlik, T. M. (2023). Neoadjuvant Therapy for Extrahepatic Biliary Tract Cancer: A Propensity Score-Matched Survival Analysis. Journal of Clinical Medicine, 12(7), 2654. https://doi.org/10.3390/jcm12072654







