CD177 Inhibits Neutrophil Extracellular Trap Formation and Protects against Acute Pancreatitis in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Human Peripheral Blood Samples Processing
2.3. Mice
2.4. Ethics Statement
2.5. AP Model Preparation and Sample Collection
2.6. Histological Examination and Immunohistochemistry (IHC)
2.7. Evaluation of Serum Enzymology and ELISA Determination
2.8. Flow Cytometry and ROS Determination
2.9. Confocal Microscope
2.10. Neutrophil Collection and Induction In Vitro
2.11. Detection of Related Indexes of NETs In Vivo
2.12. Detection of Related Indexes of NETs In Vitro
2.13. Western Blot Analysis
2.14. Statistical Analysis
3. Results
3.1. CD177+ Neutrophils Increase in AP Patients Associated with Disease Severity
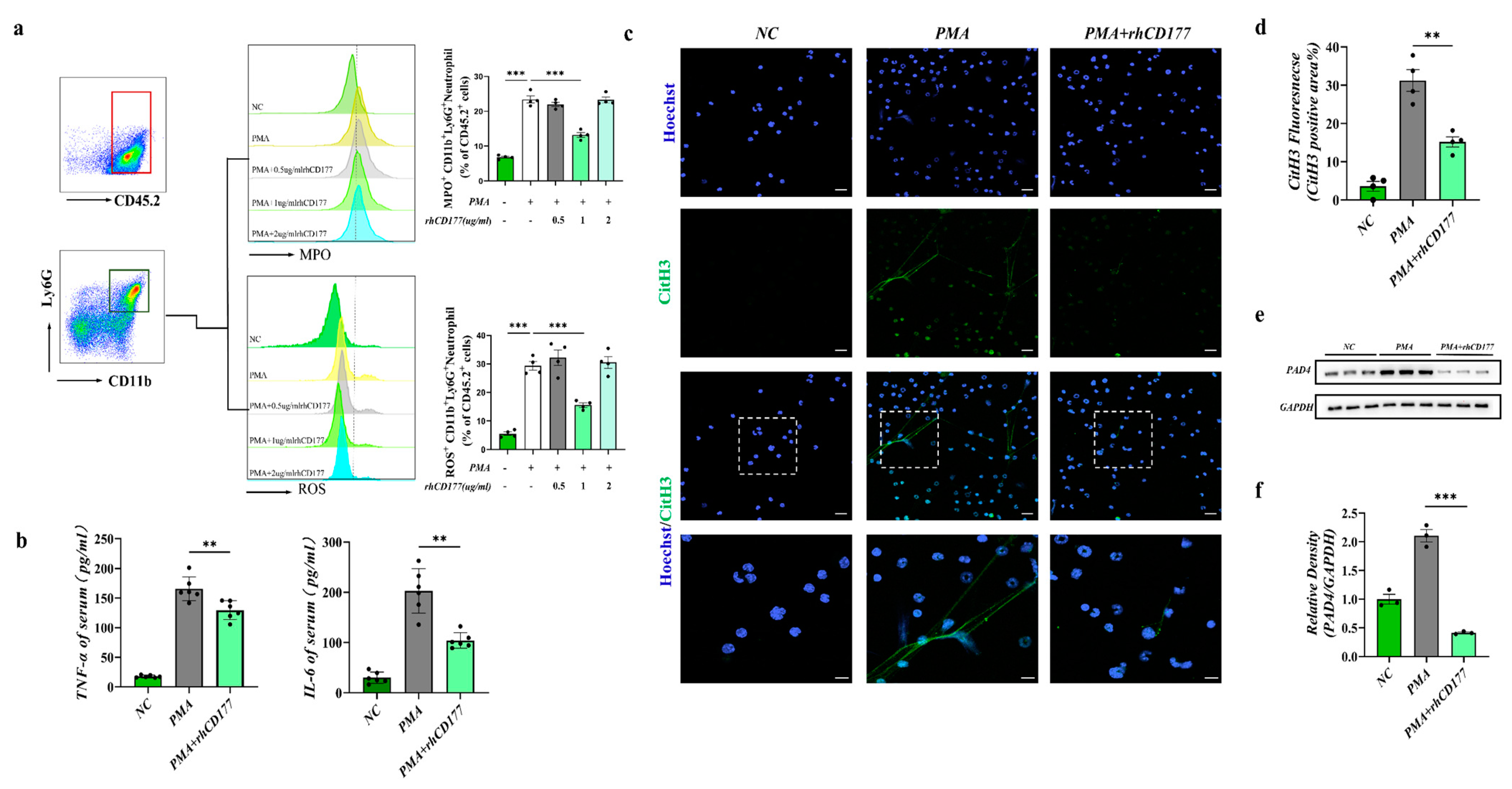
3.2. rhCD177 Successfully Inhibited NET Formation
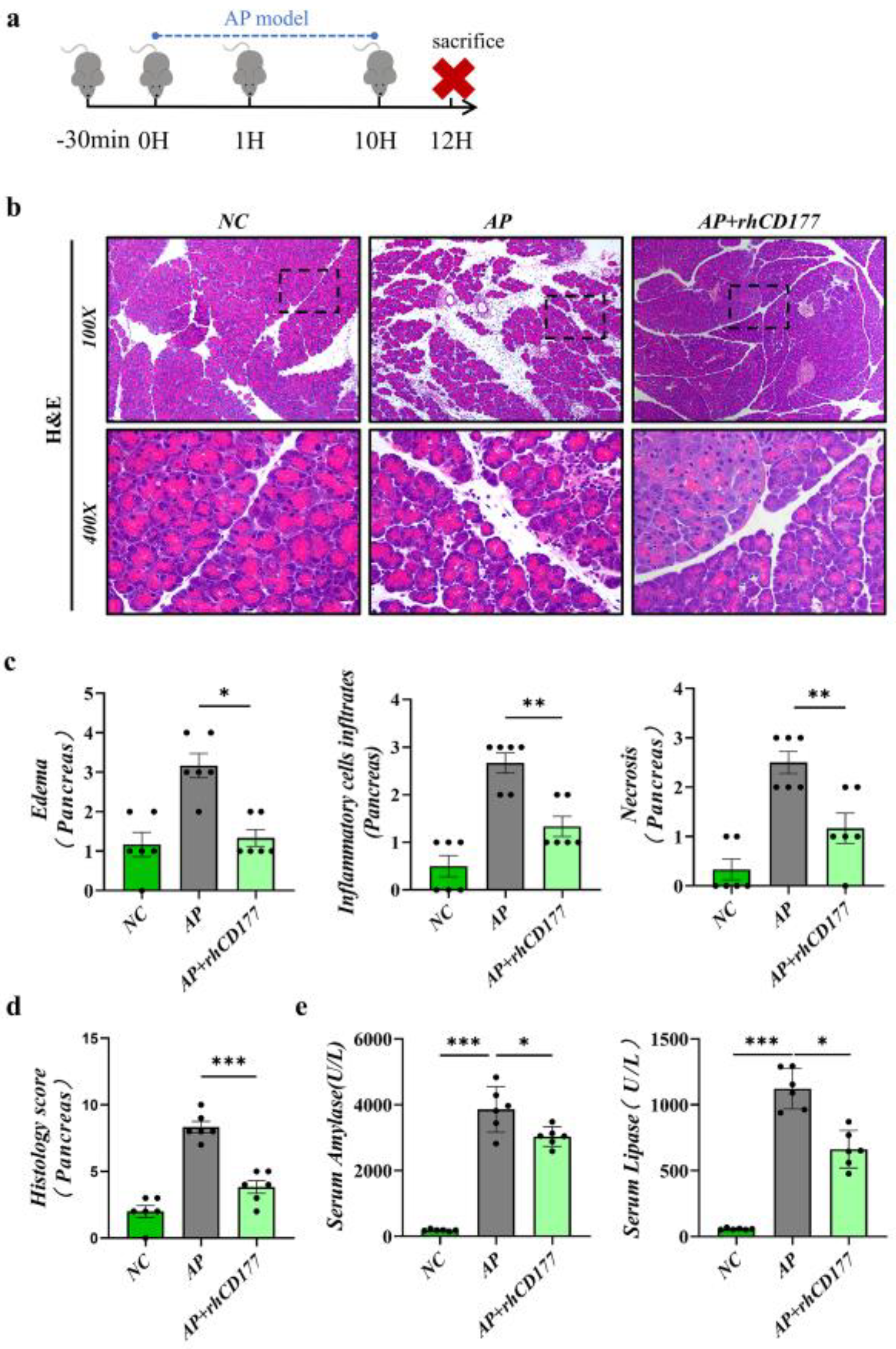
3.3. Severity of AP Is Alleviated by rhCD177 in Mice
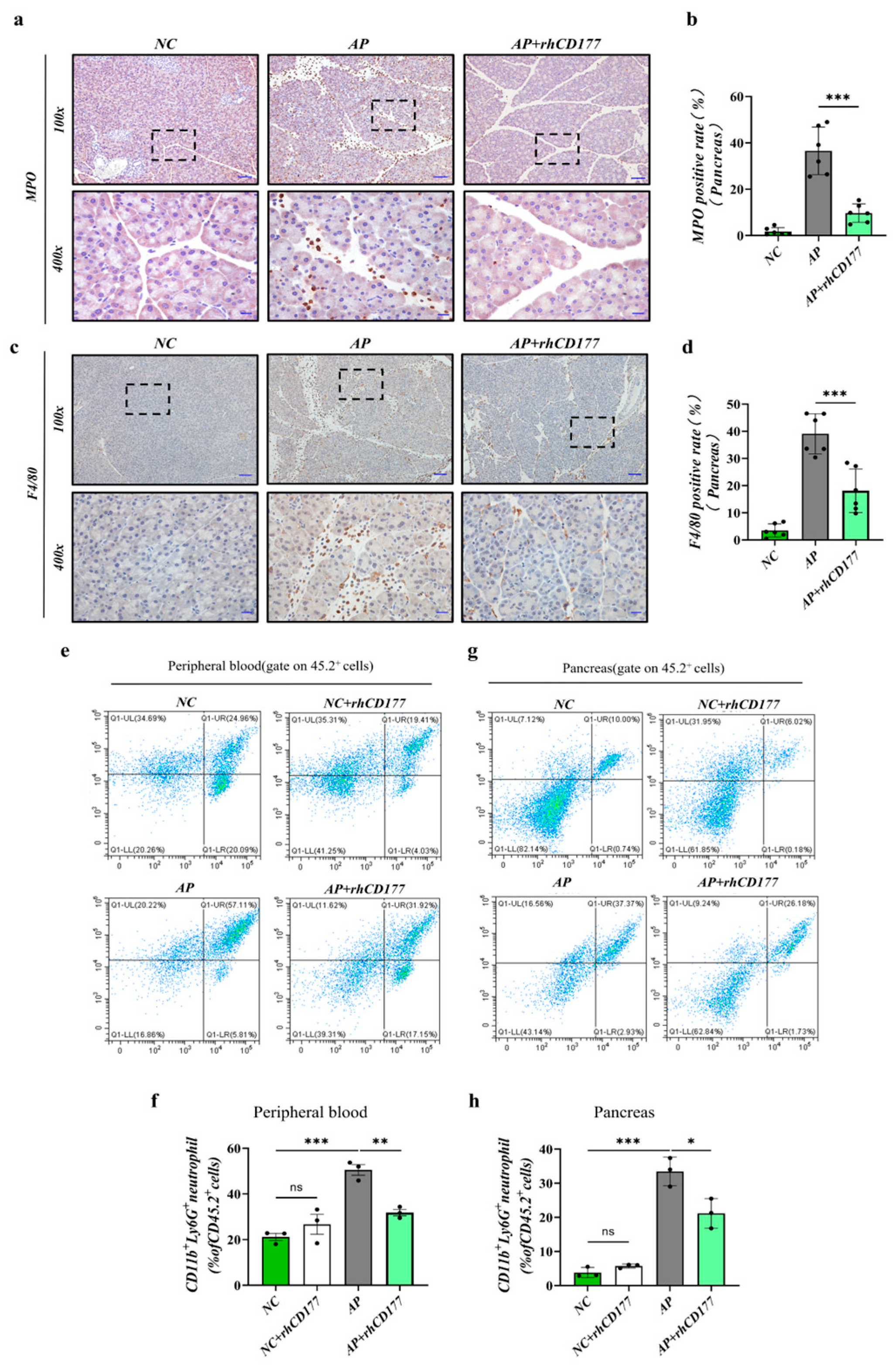
3.4. Infiltration of Immune Cells Is Prevented in Pancreatic Tissues of AP Mice
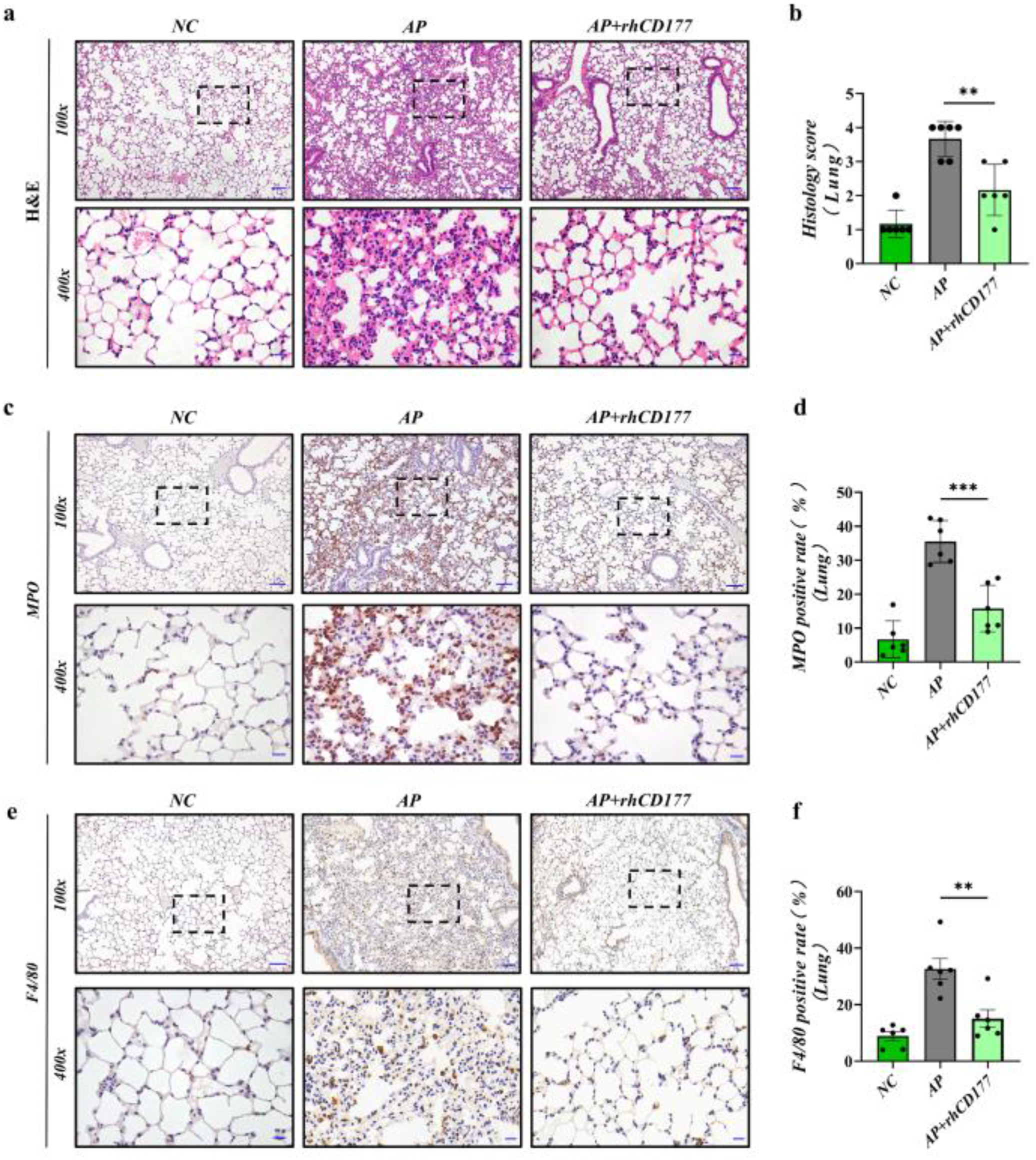
3.5. rhCD177 Can Protect against AP-Induced Acute Lung Injury and Attenuate Inflammatory Responses
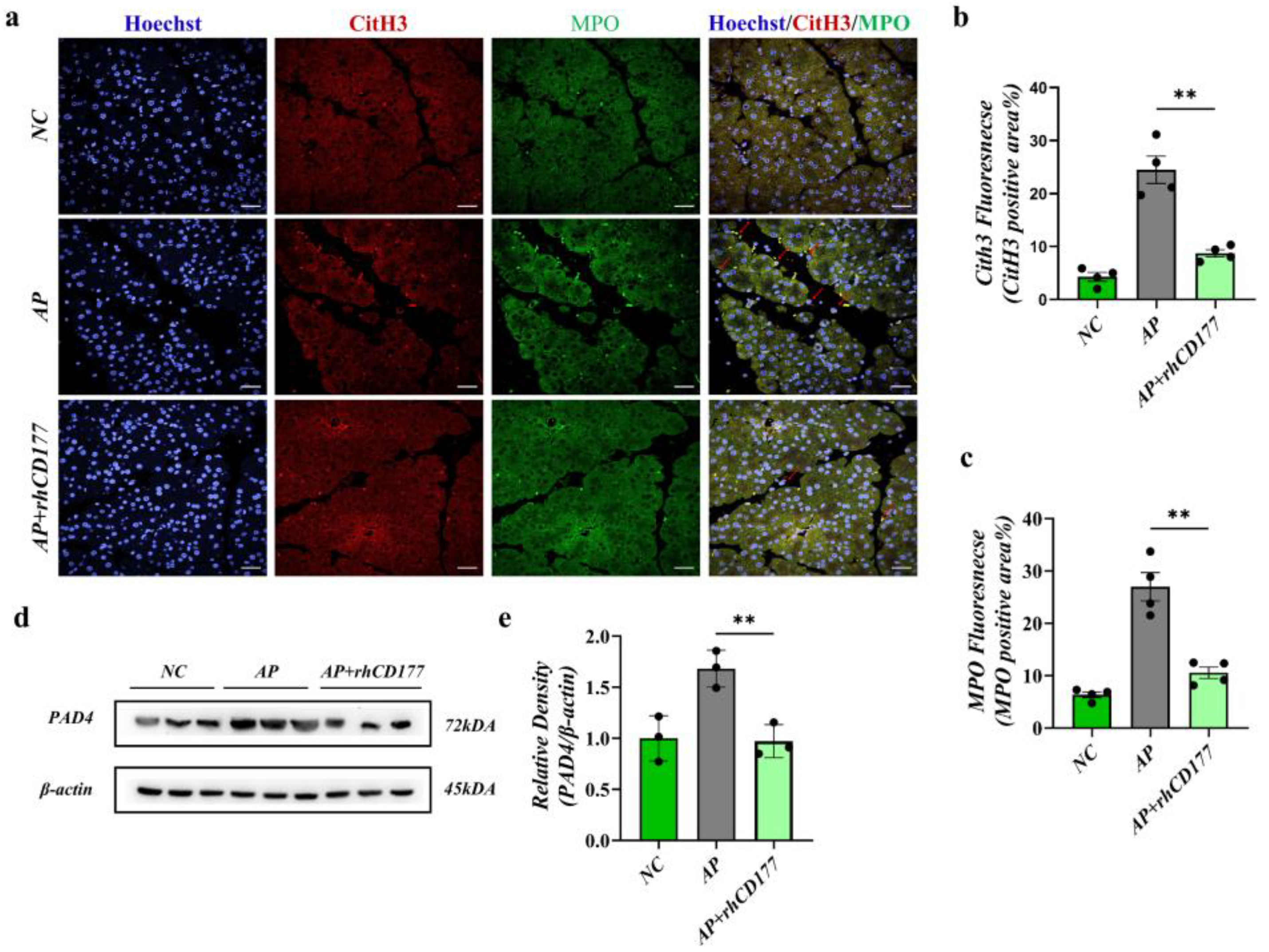
3.6. rhCD177 Alleviates NET Formation in Pancreatic Tissues of AP Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deng, Y.-Y.; Shamoon, M.; He, Y.; Bhatia, M.; Sun, J. Cathelicidin-related antimicrobial peptide modulates the severity of acute pancreatitis in mice. Mol. Med. Rep. 2016, 13, 3881–3885. [Google Scholar] [CrossRef] [Green Version]
- Easler, J.; Muddana, V.; Furlan, A.; Dasyam, A.; Vipperla, K.; Slivka, A.; Whitcomb, D.C.; Papachristou, G.I.; Yadav, D. Portosplenomesenteric venous thrombosis in patients with acute pancreatitis is associated with pancreatic necrosis and usually has a benign course. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2014, 12, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Tenner, S.; Baillie, J.; DeWitt, J.; Vege, S.S.; American College of Gastroenterology. American College of Gastroenterology Guideline: Management of Acute Pancreatitis. Am. J. Gastroenterol. 2013, 108, 1400–1415. [Google Scholar] [CrossRef] [PubMed]
- Leung, P.S.; Ip, S.P. Pancreatic acinar cell: Its role in acute pancreatitis. Int. J. Biochem. Cell Biol. 2006, 38, 1024–1030. [Google Scholar] [CrossRef]
- Banks, P.A.; Bollen, T.L.; Dervenis, C.; Gooszen, H.G.; Johnson, C.D.; Sarr, M.G.; Tsiotos, G.G.; Vege, S.S.; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis—2012: Revision of the Atlanta classification and definitions by international consensus. Gut 2013, 62, 102–111. [Google Scholar] [CrossRef]
- Ince, A.T.; Baysal, B. Pathophysiology, classification and available guidelines of acute pancreatitis. Turk. J. Gastroenterol. 2014, 25, 351–357. [Google Scholar] [CrossRef]
- Schepers, N.J.; Bakker, O.J.; Besselink, M.G.; Ali, U.A.; Bollen, T.L.; Gooszen, H.G.; Van Santvoort, H.C.; Bruno, M.J. Impact of characteristics of organ failure and infected necrosis on mortality in necrotising pancreatitis. Gut 2019, 68, 1044–1051. [Google Scholar] [CrossRef]
- Abarca-Gómez, L.; Abdeen, Z.A.; Hamid, Z.A.; Abu-Rmeileh, M.A.; Acosta-Cazares, B.; Acuin, C.; Adams, R.J.; Aekplakorn, W.; Afsana, K.; Aguilar-Salinas, C.A.; et al. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef] [Green Version]
- Petrov, M.S.; Yadav, D. Global epidemiology and holistic prevention of pancreatitis. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 175–184. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil Extracellular Traps Kill Bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Madhi, R.; Rahman, M.; Taha, D.; Mörgelin, M.; Thorlacius, H. Targeting peptidylarginine deiminase reduces neutrophil extracellular trap formation and tissue injury in severe acute pancreatitis. J. Cell. Physiol. 2018, 234, 11850–11860. [Google Scholar] [CrossRef]
- Stroncek, D.F.; Caruccio, L.; Bettinotti, M. CD177: A member of the Ly-6 gene superfamily involved with neutrophil proliferation and polycythemia vera. J. Transl. Med. 2004, 2, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Göhring, K.; Wolff, J.; Doppl, W.; Schmidt, K.L.; Fenchel, K.; Pralle, H.; Sibelius, U.; Bux, J. Neutrophil CD177 (NB1 gp, HNA-2a) expression is increased in severe bacterial infections and polycythaemia vera. Br. J. Haematol. 2004, 126, 252–254. [Google Scholar] [CrossRef]
- Hu, N.; Westra, J.; Huitema, M.G.; Bijl, M.; Brouwer, E.; Stegeman, C.A.; Heeringa, P.; Limburg, P.C.; Kallenberg, C.G.M. Coexpression of CD177 and membrane proteinase 3 on neutrophils in antineutrophil cytoplasmic autoantibody-associated systemic vasculitis: Anti-proteinase 3-mediated neutrophil activation is independent of the role of CD177-expressing neutrophils. Arthritis Rheum. 2009, 60, 1548–1557. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Wang, L.; Schoeb, T.R.; Elson, C.O.; Cong, Y. Microbiota innate stimulation is a prerequisite for T cell spontaneous proliferation and induction of experimental colitis. J. Exp. Med. 2010, 207, 1321–1332. [Google Scholar] [CrossRef] [Green Version]
- Scheibe, K.; Backert, I.; Wirtz, S.; Hueber, A.; Schett, G.; Vieth, M.; Probst, H.C.; Bopp, T.; Neurath, M.F.; Neufert, C. IL-36R signalling activates intestinal epithelial cells and fibroblasts and promotes mucosal healing in vivo. Gut 2017, 66, 823–838. [Google Scholar] [CrossRef]
- Wu, W.; Liu, H.-P.; Chen, F.; Liu, H.; Cao, A.T.; Yao, S.; Sun, M.; Evans-Marin, H.L.; Zhao, Y.; Zhao, Q.; et al. Commensal A4 bacteria inhibit intestinal Th2-cell responses through induction of dendritic cell TGF-β production. Eur. J. Immunol. 2016, 46, 1162–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.-B.; Liao, D.-H.; Nissen, T.D. Animal models of pancreatitis: Can it be translated to human pain study? World J. Gastroenterol. 2013, 19, 7222–7230. [Google Scholar] [CrossRef]
- Zhu, Q.; Hao, L.; Shen, Q.; Pan, J.; Liu, W.; Gong, W.; Hu, L.; Xiao, W.; Wang, M.; Liu, X.; et al. CaMK II Inhibition Attenuates ROS Dependent Necroptosis in Acinar Cells and Protects against Acute Pancreatitis in Mice. Oxidative Med. Cell. Longev. 2021, 2021, 4187398. [Google Scholar] [CrossRef] [PubMed]
- Sekimoto, M.; Takada, T.; Kawarada, Y.; Hirata, K.; Mayumi, T.; Yoshida, M.; Hirota, M.; Kimura, Y.; Takeda, K.; Isaji, S.; et al. JPN Guidelines for the management of acute pancreatitis: Epidemiology, etiology, natural history, and outcome predictors in acute pancreatitis. J. Hepato-Biliary-Pancreat. Surg. 2006, 13, 10–24. [Google Scholar] [CrossRef] [Green Version]
- Flint, R.; Windsor, J.; Bonham, M. Trends in the management of severe acute pancreatitis: Interventions and outcome. ANZ J. Surg. 2004, 74, 335–342. [Google Scholar] [CrossRef]
- Guan, X.; Lu, Y.; Zhu, H.; Yu, S.; Zhao, W.; Chi, X.; Xie, C.; Yin, Z. The Crosstalk between Cancer Cells and Neutrophils Enhances Hepatocellular Carcinoma Metastasis via Neutrophil Extracellular Traps-Associated Cathepsin G Component: A Potential Therapeutic Target. J. Hepatocell. Carcinoma 2021, 8, 451–465. [Google Scholar] [CrossRef]
- Shields, C.J.; Winter, D.C.; Redmond, H.P. Lung injury in acute pancreatitis: Mechanisms, prevention, and therapy. Curr. Opin. Crit. Care 2002, 8, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Merza, M.; Hartman, H.; Rahman, M.; Hwaiz, R.; Zhang, E.; Renström, E.; Luo, L.; Mörgelin, M.; Regner, S.; Thorlacius, H. Neutrophil Extracellular Traps Induce Trypsin Activation, Inflammation, and Tissue Damage in Mice with Severe Acute Pancreatitis. Gastroenterology 2015, 149, 1920–1931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.; Zeng, Y.; Fan, Y.; Wu, J.; Mulatibieke, T.; Ni, J.; Yu, G.; Wan, R.; Wang, X.; Hu, G. Reverse-migrated neutrophils regulated by JAM-C are involved in acute pancreatitis-associated lung injury. Sci. Rep. 2016, 6, 20545. [Google Scholar] [CrossRef] [Green Version]
- Fuchs, T.A.; Abed, U.; Goosmann, C.; Hurwitz, R.; Schulze, I.; Wahn, V.; Weinrauch, Y.; Brinkmann, V.; Zychlinsky, A. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007, 176, 231–241. [Google Scholar] [CrossRef]
- Yipp, B.G.; Petri, B.; Salina, D.; Jenne, C.N.; Scott, B.N.V.; Zbytnuik, L.D.; Pittman, K.; Asaduzzaman, M.; Wu, K.; Meijndert, H.C.; et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat. Med. 2012, 18, 1386–1393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.; Xiao, Y.; Du, Y.; Chen, J.; Ni, Q.; Guo, X.; Xue, G.; Xie, X. Diagnostic and Prognostic Value of Neutrophil Extracellular Trap Levels in Patients with Acute Aortic Dissection. Front. Cardiovasc. Med. 2022, 8, 683445. [Google Scholar] [CrossRef]
- Mócsai, A. Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J. Exp. Med. 2013, 210, 1283–1299. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Meng, X.; Xu, P. Central role of neutrophil in the pathogenesis of severe acute pancreatitis. J. Cell. Mol. Med. 2015, 19, 2513–2520. [Google Scholar] [CrossRef]
- Fusco, R.; Cordaro, M.; Siracusa, R.; D’Amico, R.; Genovese, T.; Gugliandolo, E.; Peritore, A.F.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; et al. Biochemical Evaluation of the Antioxidant Effects of Hydroxytyrosol on Pancreatitis-Associated Gut Injury. Antioxidants 2020, 9, 781. [Google Scholar] [CrossRef] [PubMed]
- Kvedaraite, E. Neutrophil-T cell crosstalk in inflammatory bowel disease. Immunology 2021, 164, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Mortaz, E.; Alipoor, S.D.; Adcock, I.M.; Mumby, S.; Koenderman, L. Update on Neutrophil Function in Severe Inflammation. Front Immunol. 2018, 9, 2171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, G.; Yu, L.; Fang, L.; Yang, W.; Yu, T.; Miao, Y.; Chen, M.; Wu, K.; Chen, F.; Cong, Y.; et al. CD177+ neutrophils as functionally activated neutrophils negatively regulate IBD. Gut 2018, 67, 1052–1063. [Google Scholar] [CrossRef]
- Bauer, S.; Abdgawad, M.; Gunnarsson, L.; Segelmark, M.; Tapper, H.; Hellmark, T. Proteinase 3 and CD177 are expressed on the plasma membrane of the same subset of neutrophils. J. Leukoc. Biol. 2007, 81, 458–464. [Google Scholar] [CrossRef]
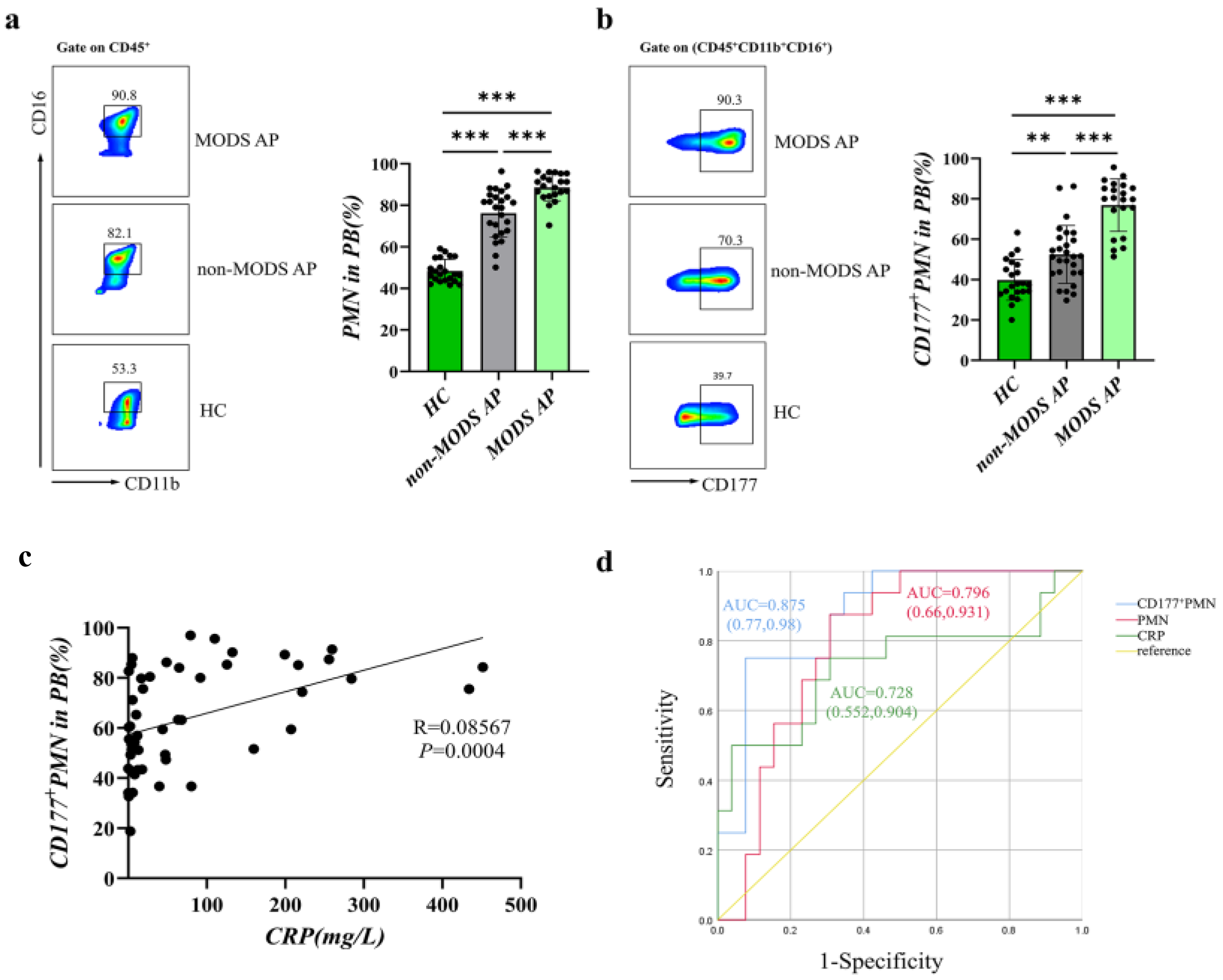

| e | Non-MODS AP | MODS AP | p Value |
|---|---|---|---|
| e | 29 | 21 | |
| Sex (M/F) | 15/14 | 15/6 | 0.165 |
| Age (y, mean ± SEM) | 47.00 (36.50, 57.50) | 44.00 (32.75, 62.5) | 0.316 |
| Total leukocyte count (109/L) | 9.89 (7.10, 12.76) | 11.18 (9.42, 13.91) | 0.047 * |
| Neutropill (%) | 81.29 (67.22, 86.88) | 88.87 (84.60, 92.01) | <0.001 *** |
| Lymphocyte count (109/L) | 1.32 (0.90, 1.79) | 0.96 (0.71, 1.11) | 0.166 |
| CRP (mg/L) | 6.95 (2.95, 43.29) | 76.94 (10.36, 212.10) | 0.014 ** |
| Etiology | |||
| BAP | 13 | 9 | |
| HLAP | 13 | 11 | |
| AAP | 3 | 0 | |
| Others | 0 | 1 | |
| Length of hospitalization (d, mediam, 25–75%) | 8.00 (5.00, 10.50) | 11.50 (6.00, 18.00) | |
| CTSI | 1.00 (1.00–2.00) | 4.00 (2.25, 5.75) | |
| Improved Marshall scoring system | 0.00 (0.00, 0.00) | 2.00 (2.00, 2.75) |
| Parameter | Cutoff | Sensitivity (%) | Specificity (%) | AUC |
|---|---|---|---|---|
| Non-MODS AP vs. MODS AP | ||||
| CD177+ PMN | 72.810 | 75.000 | 92.300 | 0.875 (0.770, 0.980) |
| PMN | 83.880 | 87.500 | 69.200 | 0.796 (0.660, 0.931) |
| CRP | 89.060 | 50.000 | 96.200 | 0.728 (0.552, 0.904) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Yang, X.; Xu, X.; Shen, Q.; Han, F.; Zhu, Q.; Wu, K.; Gu, A.; Wu, D.; Xiao, W. CD177 Inhibits Neutrophil Extracellular Trap Formation and Protects against Acute Pancreatitis in Mice. J. Clin. Med. 2023, 12, 2533. https://doi.org/10.3390/jcm12072533
Zhang J, Yang X, Xu X, Shen Q, Han F, Zhu Q, Wu K, Gu A, Wu D, Xiao W. CD177 Inhibits Neutrophil Extracellular Trap Formation and Protects against Acute Pancreatitis in Mice. Journal of Clinical Medicine. 2023; 12(7):2533. https://doi.org/10.3390/jcm12072533
Chicago/Turabian StyleZhang, Junxian, Xin Yang, Xingmeng Xu, Qinhao Shen, Fei Han, Qingtian Zhu, Keyan Wu, Aidong Gu, Dong Wu, and Weiming Xiao. 2023. "CD177 Inhibits Neutrophil Extracellular Trap Formation and Protects against Acute Pancreatitis in Mice" Journal of Clinical Medicine 12, no. 7: 2533. https://doi.org/10.3390/jcm12072533
APA StyleZhang, J., Yang, X., Xu, X., Shen, Q., Han, F., Zhu, Q., Wu, K., Gu, A., Wu, D., & Xiao, W. (2023). CD177 Inhibits Neutrophil Extracellular Trap Formation and Protects against Acute Pancreatitis in Mice. Journal of Clinical Medicine, 12(7), 2533. https://doi.org/10.3390/jcm12072533





