App-Based Mindfulness Meditation Training and an Audiobook Intervention Reduce Symptom Severity but Do Not Modify Backward Inhibition in Adolescent Obsessive-Compulsive Disorder: Evidence from an EEG Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Intervention and Procedure
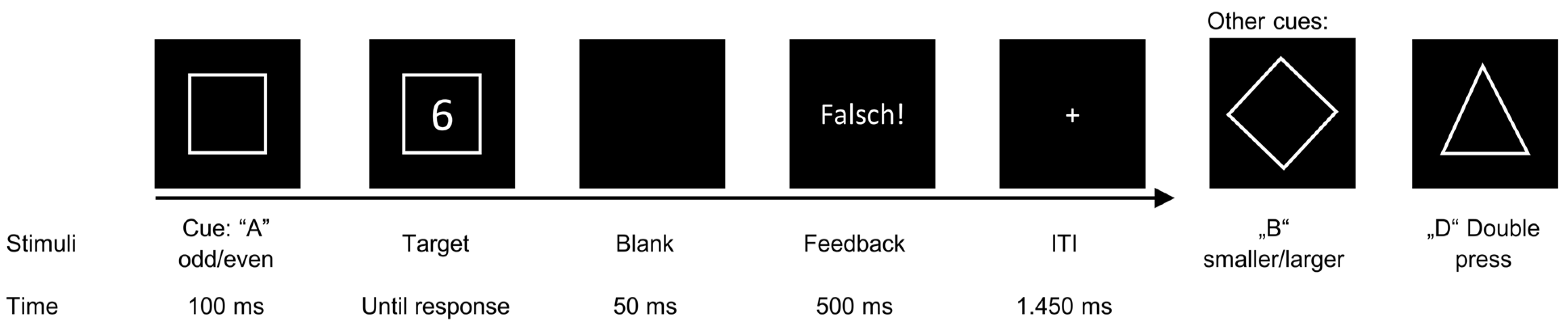
2.3. Backward Inhibition Task
2.4. EEG Recording and Analysis
2.5. Statistics
3. Results
3.1. Symptom Data
3.1.1. CY-BOCS
3.1.2. ZWIK-S
3.1.3. ZWIK-P
3.2. Backward Inhibition Paradigm
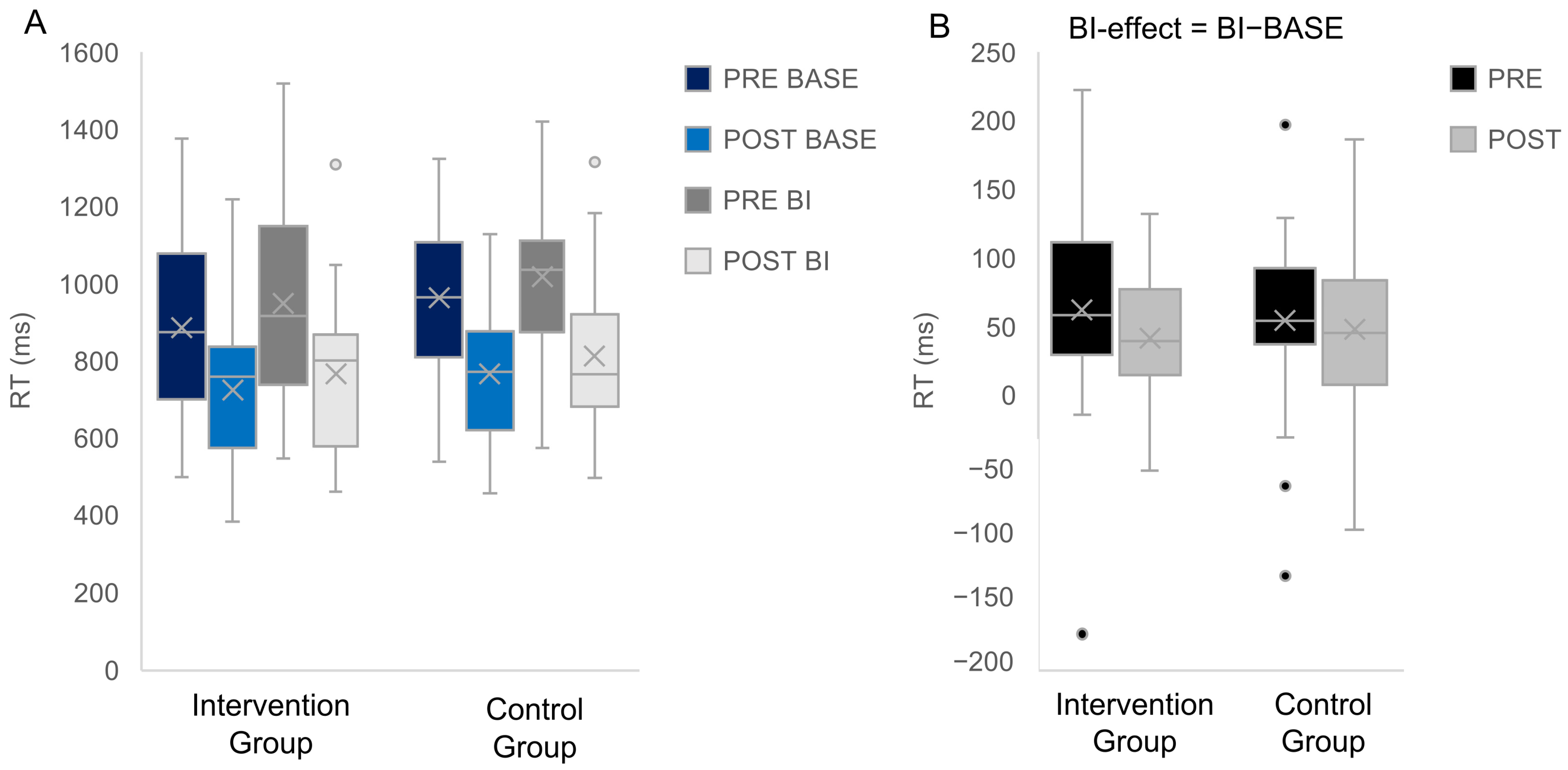
3.2.1. Behavioral Data
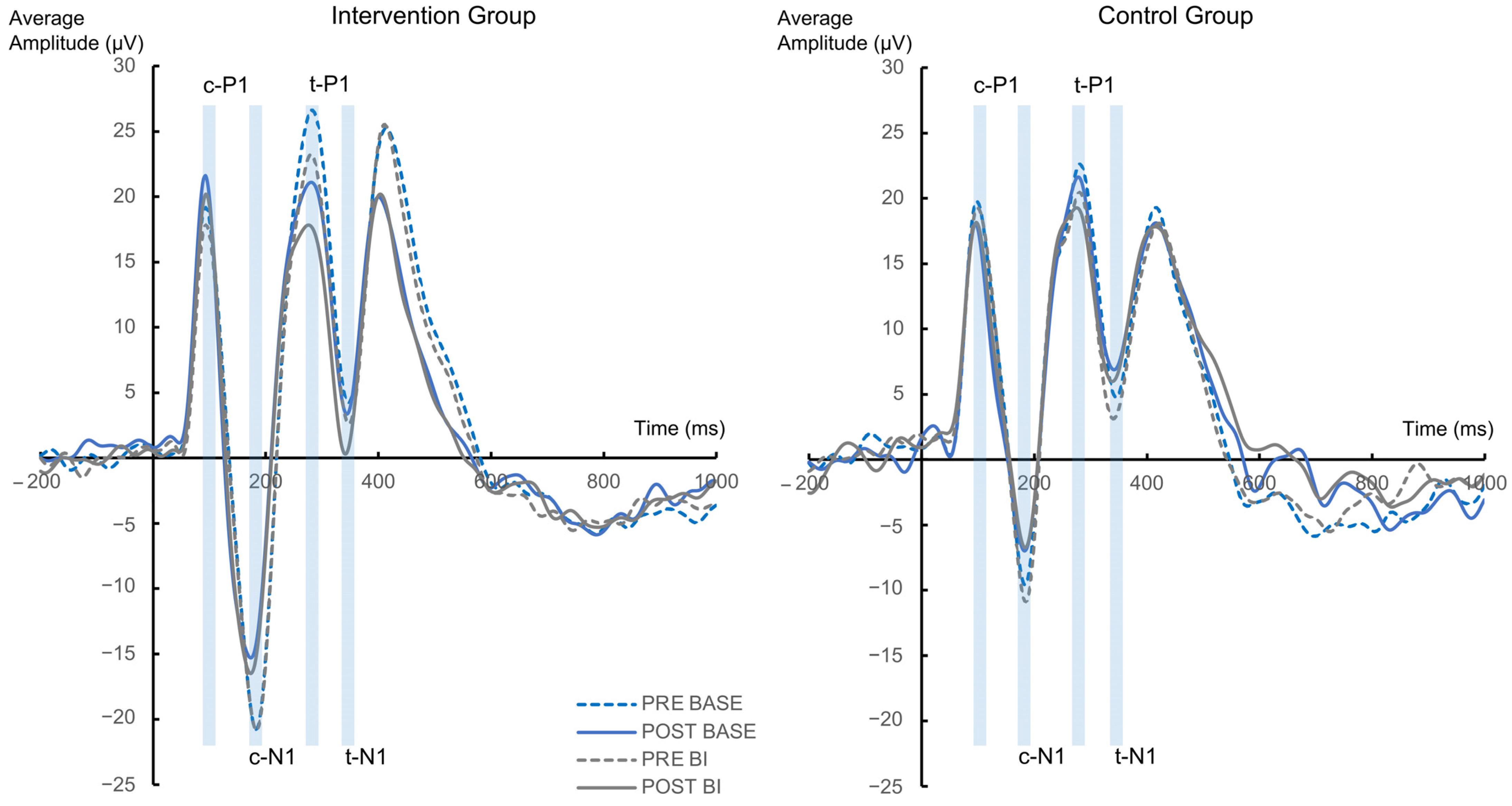
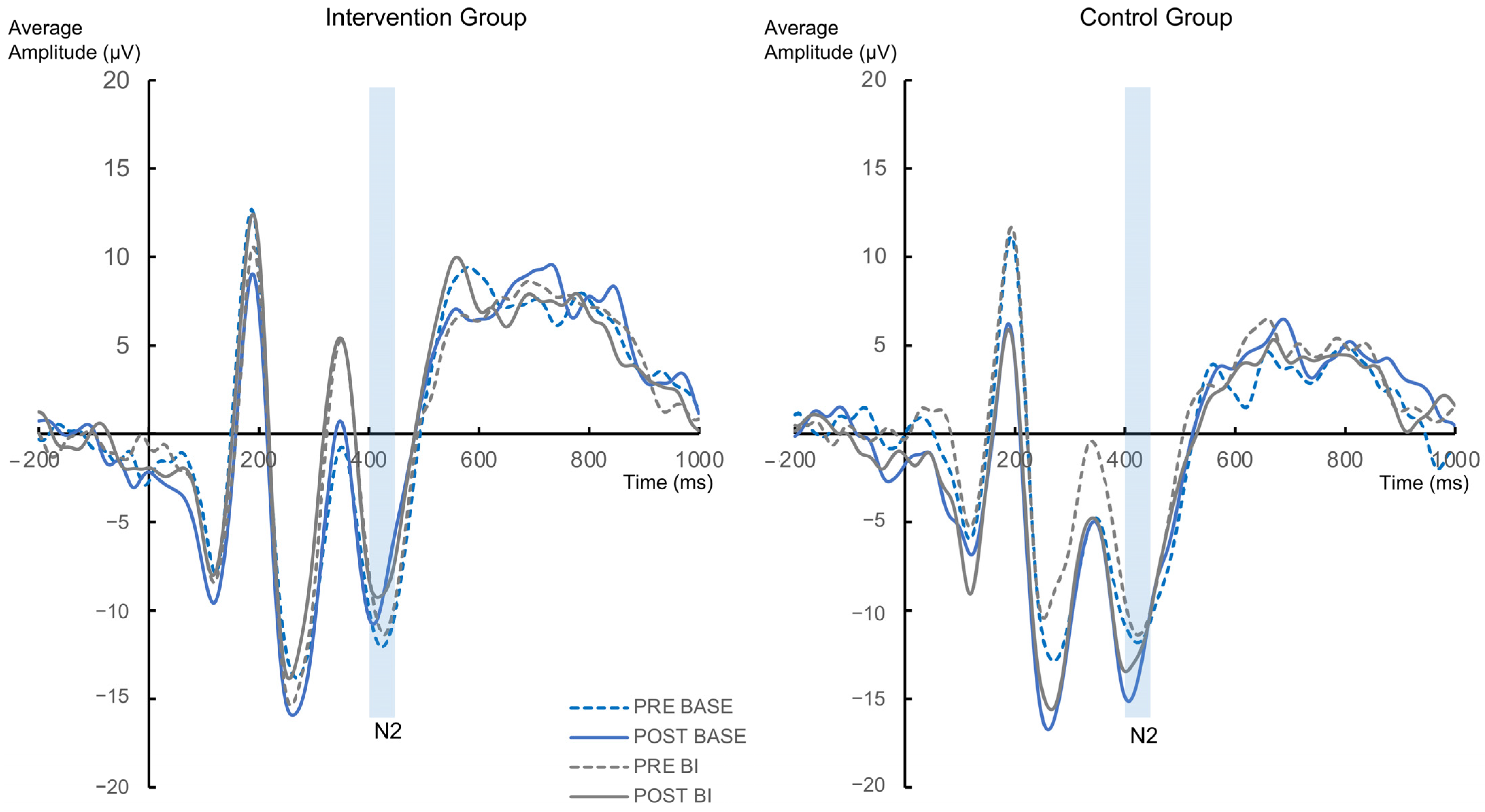
3.2.2. Neurophysiological Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013; ISBN 978-0-89042-554-1. [Google Scholar]
- Stewart, S.E.; Geller, D.A.; Jenike, M.; Pauls, D.; Shaw, D.; Mullin, B.; Faraone, S.V. Long-Term Outcome of Pediatric Obsessive-Compulsive Disorder: A Meta-Analysis and Qualitative Review of the Literature. Acta Psychiatr. Scand. 2004, 110, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, S.R.; Fineberg, N.A.; Menzies, L.A.; Blackwell, A.D.; Bullmore, E.T.; Robbins, T.W.; Sahakian, B.J. Impaired Cognitive Flexibility and Motor Inhibition in Unaffected First-Degree Relatives of Patients with Obsessive-Compulsive Disorder. AJP 2007, 164, 335–338. [Google Scholar] [CrossRef]
- Norman, L.J.; Taylor, S.F.; Liu, Y.; Radua, J.; Chye, Y.; De Wit, S.J.; Huyser, C.; Karahanoglu, F.I.; Luks, T.; Manoach, D.; et al. Error Processing and Inhibitory Control in Obsessive-Compulsive Disorder: A Meta-Analysis Using Statistical Parametric Maps. Biol. Psychiatry 2019, 85, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Bannon, S.; Gonsalvez, C.J.; Croft, R.J.; Boyce, P.M. Response Inhibition Deficits in Obsessive-Compulsive Disorder. Psychiatry Res. 2002, 110, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.-M.; Park, J.-Y.; Kang, D.-H.; Lee, S.J.; Yoo, S.Y.; Jo, H.J.; Choi, C.-H.; Lee, J.-M.; Kwon, J.S. Neural Correlates of Cognitive Inflexibility during Task-Switching in Obsessive-Compulsive Disorder. Brain 2007, 131, 155–164. [Google Scholar] [CrossRef]
- Gruner, P.; Pittenger, C. Cognitive Inflexibility in Obsessive-Compulsive Disorder. Neuroscience 2017, 345, 243–255. [Google Scholar] [CrossRef]
- Snyder, H.R.; Kaiser, R.H.; Warren, S.L.; Heller, W. Obsessive-Compulsive Disorder Is Associated with Broad Impairments in Executive Function: A Meta-Analysis. Clin. Psychol. Sci. 2015, 3, 301–330. [Google Scholar] [CrossRef]
- Diamond, A. Executive Functions. Annu. Rev. Psychol. 2013, 64, 135–168. [Google Scholar] [CrossRef]
- Kiesel, A.; Steinhauser, M.; Wendt, M.; Falkenstein, M.; Jost, K.; Philipp, A.M.; Koch, I. Control and Interference in Task Switching—A Review. Psychol. Bull. 2010, 136, 849–874. [Google Scholar] [CrossRef]
- Wolff, N.; Giller, F.; Buse, J.; Roessner, V.; Beste, C. When Repetitive Mental Sets Increase Cognitive Flexibility in Adolescent Obsessive-Compulsive Disorder. J. Child Psychol. Psychiatr. 2018, 59, 1024–1032. [Google Scholar] [CrossRef]
- Koch, I.; Gade, M.; Schuch, S.; Philipp, A.M. The Role of Inhibition in Task Switching: A Review. Psychon. Bull. Rev. 2010, 17, 1–14. [Google Scholar] [CrossRef]
- Mayr, U.; Keele, S.W. Changing Internal Constraints on Action: The Role of Backward Inhibition. J. Exp. Psychol. Gen. 2000, 129, 4–26. [Google Scholar] [CrossRef] [PubMed]
- Koch, I.; Gade, M.; Philipp, A.M. Inhibition of Response Mode in Task Switching. Exp. Psychol. 2004, 51, 52–58. [Google Scholar] [CrossRef]
- Zhang, R.; Stock, A.-K.; Fischer, R.; Beste, C. The System Neurophysiological Basis of Backward Inhibition. Brain Struct. Funct. 2016, 221, 4575–4587. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W. Evoked Alpha and Early Access to the Knowledge System: The P1 Inhibition Timing Hypothesis. Brain Res. 2011, 1408, 52–71. [Google Scholar] [CrossRef]
- Keng, S.-L.; Smoski, M.J.; Robins, C.J. Effects of Mindfulness on Psychological Health: A Review of Empirical Studies. Clin. Psychol. Rev. 2011, 31, 1041–1056. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, J.; Reiner, K.; Meiran, N. “Off with the Old”: Mindfulness Practice Improves Backward Inhibition. Front. Psychol. 2013, 3, 618. [Google Scholar] [CrossRef]
- Wolff, N.; Beste, C. Short-Term Smartphone App-Based Focused Attention Meditation Diminishes Cognitive Flexibility. J. Cogn. Neurosci. 2020, 32, 1484–1496. [Google Scholar] [CrossRef]
- Ullrich, S.; Colzato, L.S.; Wolff, N.; Beste, C. Short-Term Focused Attention Meditation Restricts the Retrieval of Stimulus-Response Bindings to Relevant Information. Mindfulness 2021, 12, 1272–1281. [Google Scholar] [CrossRef]
- Fineberg, N.A.; Robbins, T.W. The Neurobiology and Treatment of OCD: Accelerating Progress. In Current Topics in Behavioral Neurosciences; Springer International Publishing: Cham, Switzerland, 2021; Volume 49, ISBN 978-3-030-75392-4. [Google Scholar]
- Patel, S.R.; Carmody, J.; Simpson, H.B. Adapting Mindfulness-Based Stress Reduction for the Treatment of Obsessive-Compulsive Disorder: A Case Report. Cogn. Behav. Pract. 2007, 14, 375–380. [Google Scholar] [CrossRef]
- Wilkinson-Tough, M.; Bocci, L.; Thorne, K.; Herlihy, J. Is Mindfulness-Based Therapy an Effective Intervention for Obsessive–Intrusive Thoughts: A Case Series. Clin. Psychol. Psychother. 2009, 17, 250–268. [Google Scholar] [CrossRef]
- Cludius, B.; Landmann, S.; Rose, N.; Heidenreich, T.; Hottenrott, B.; Schröder, J.; Jelinek, L.; Voderholzer, U.; Külz, A.K.; Moritz, S. Long-Term Effects of Mindfulness-Based Cognitive Therapy in Patients with Obsessive-Compulsive Disorder and Residual Symptoms after Cognitive Behavioral Therapy: Twelve-Month Follow-up of a Randomized Controlled Trial. Psychiatry Res. 2020, 291, 113119. [Google Scholar] [CrossRef] [PubMed]
- Külz, A.K.; Landmann, S.; Cludius, B.; Rose, N.; Heidenreich, T.; Jelinek, L.; Alsleben, H.; Wahl, K.; Philipsen, A.; Voderholzer, U.; et al. Mindfulness-Based Cognitive Therapy (MBCT) in Patients with Obsessive-Compulsive Disorder (OCD) and Residual Symptoms after Cognitive Behavioral Therapy (CBT): A Randomized Controlled Trial. Eur. Arch. Psychiatry Clin. Neurosci. 2019, 269, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Hawley, L.L.; Rector, N.A.; DaSilva, A.; Laposa, J.M.; Richter, M.A. Technology Supported Mindfulness for Obsessive Compulsive Disorder: Self-Reported Mindfulness and EEG Correlates of Mind Wandering. Behav. Res. Ther. 2021, 136, 103757. [Google Scholar] [CrossRef] [PubMed]
- Hawley, L.L.; Rector, N.A.; Richter, M.A. Technology Supported Mindfulness for Obsessive Compulsive Disorder: The Role of Obsessive Beliefs. J. Anxiety Disord. 2021, 81, 102405. [Google Scholar] [CrossRef]
- Hanstede, M.; Gidron, Y.; Nyklíček, I. The Effects of a Mindfulness Intervention on Obsessive-Compulsive Symptoms in a Non-Clinical Student Population. J. Nerv. Ment. Dis. 2008, 196, 776–779. [Google Scholar] [CrossRef]
- Strauss, C.; Lea, L.; Hayward, M.; Forrester, E.; Leeuwerik, T.; Jones, A.-M.; Rosten, C. Mindfulness-Based Exposure and Response Prevention for Obsessive Compulsive Disorder: Findings from a Pilot Randomised Controlled Trial. J. Anxiety Disord. 2018, 57, 39–47. [Google Scholar] [CrossRef]
- Rupp, C.; Jürgens, C.; Doebler, P.; Andor, F.; Buhlmann, U. A Randomized Waitlist-Controlled Trial Comparing Detached Mindfulness and Cognitive Restructuring in Obsessive-Compulsive Disorder. PLoS ONE 2019, 14, e0213895. [Google Scholar] [CrossRef]
- Mathur, S.; Sharma, M.P.; Balachander, S.; Kandavel, T.; Reddy, Y.J. A Randomized Controlled Trial of Mindfulness-Based Cognitive Therapy vs. Stress Management Training for Obsessive-Compulsive Disorder. J. Affect. Disord. 2021, 282, 58–68. [Google Scholar] [CrossRef]
- Madani, N.A.M.; Kananifar, N.; Atashpour, S.H.; Habil, M.H.B. The Effects of Mindfulness Group Training on the Rate of Obsessive-Compulsive Disorder Symptoms on the Women in Isfahan City (Iran). Int. Med. J. 2013, 20, 13–17. [Google Scholar]
- Cludius, B.; Hottenrott, B.; Alsleben, H.; Peter, U.; Schröder, J.; Moritz, S. Mindfulness for OCD? No Evidence for a Direct Effect of a Self-Help Treatment Approach. J. Obs. Compuls. Relat. Disord. 2015, 6, 59–65. [Google Scholar] [CrossRef]
- Fairfax, H. The Use of Mindfulness in Obsessive Compulsive Disorder: Suggestions for Its Application and Integration in Existing Treatment. Clin. Psychol. Psychother. 2008, 15, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Hertenstein, E.; Rose, N.; Voderholzer, U.; Heidenreich, T.; Nissen, C.; Thiel, N.; Herbst, N.; Külz, A.K. Mindfulness-Based Cognitive Therapy in Obsessive-Compulsive Disorder—A Qualitative Study on Patients’ Experiences. BMC Psychiatry 2012, 12, 185. [Google Scholar] [CrossRef] [PubMed]
- Goodman, W.K.; Scahill, L.; Price, L.H.; Rasmussen, S.A.; Riddle, M.A.; Rapoport, J.L. Children’s Yale-Brown Obsessive Compulsive Scale (Cy-Bocs). J. Am. Acad. Child Adolesc. Psychiatry 1997, 36, 844–852. [Google Scholar]
- Chen, J.; Wu, S.; Li, F. Cognitive Neural Mechanism of Backward Inhibition and Deinhibition: A Review. Front. Behav. Neurosci. 2022, 16, 14. [Google Scholar] [CrossRef]
- Remschmidt, H. Multiaxiales Klassifikationsschema für Psychische Störungen des Kindes—Und Jugendalters Nach ICD-10 der WHO: Mit Einem Synoptischen Vergleich von ICD-10 mit DSM-IV; Korrigierte Auflage: Bern, Switzerland, 2012; Volume 6, ISBN 978-3-456-85102-0. [Google Scholar]
- Oswald, W.D.; Roth, E. Der Zahlen-Verbindungs-Test (ZVT); Hogrefe Verlag fuer Psychologie: Gottingen, Germany, 1987. [Google Scholar]
- Zaudig, M.; Hauke, W.; Hegerl, U. Die Zwangsstörung: Diagnostik und Therapie. In Aktualisierte und Erweiterte Auflage; Schattauer: Stuttgart, Germany, 2002; Volume 2, ISBN 978-3-7945-2145-6. [Google Scholar]
- Goletz, H.; Döpfner, M. ZWIK, Zwangsinventar Für Kinder Und Jugendliche. Klinisch-psychiatrische Ratingskalen für das Kindes-und Jugendalter. Hogrefe Göttingen 2011, 489–493. [Google Scholar]
- Wolff, N.; Buse, J.; Tost, J.; Roessner, V.; Beste, C. Modulations of Cognitive Flexibility in Obsessive Compulsive Disorder Reflect Dysfunctions of Perceptual Categorization. J. Child Psychol. Psychiatr. 2017, 58, 939–949. [Google Scholar] [CrossRef]
- Giller, F.; Zhang, R.; Roessner, V.; Beste, C. The Neurophysiological Basis of Developmental Changes during Sequential Cognitive Flexibility between Adolescents and Adults. Hum. Brain Mapp. 2019, 40, 552–565. [Google Scholar] [CrossRef]
- Giller, F.; Beste, C. Effects of Aging on Sequential Cognitive Flexibility Are Associated with Fronto-Parietal Processing Deficits. Brain Struct. Funct. 2019, 224, 2343–2355. [Google Scholar] [CrossRef]
- Zhang, R.; Stock, A.-K.; Beste, C. The Neurophysiological Basis of Reward Effects on Backward Inhibition Processes. NeuroImage 2016, 142, 163–171. [Google Scholar] [CrossRef]
- Masson, M.E.J. A Tutorial on a Practical Bayesian Alternative to Null-Hypothesis Significance Testing. Behav. Res. 2011, 43, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 20th ed.; Lawrence Earlbaum Associates: New York, NY, USA, 1988. [Google Scholar]
- Jacobson, N.S.; Truax, P. Clinical Significance: A Statistical Approach to Defining Meaningful Change in Psychotherapy Research. J. Consult. Clin. Psychol. 1991, 59, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Raftery, A.E. Bayesian Model Selection in Social Research. Sociol. Methodol. 1995, 1, 111–163. [Google Scholar] [CrossRef]
- Öst, L.-G.; Riise, E.N.; Wergeland, G.J.; Hansen, B.; Kvale, G. Cognitive Behavioral and Pharmacological Treatments of OCD in Children: A Systematic Review and Meta-Analysis. J. Anxiety Disord. 2016, 43, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Feierabend, S.; Rathgeb, T.; Kheredmand, H.; Glöckler, S. JIM-Studie 2020: Jugend, Information, Medien: Basisuntersuchung Zum Medienumgang 12-Bis 19-Jähriger. In Medienpädagogischer Forschungsverbund Südwest; Schattauer: Stuttgart, Germany, 2020. [Google Scholar]
- Helbig, S.; Hoyer, J. Hilft wenig viel? Eine Minimalintervention für Patienten während der Wartezeit auf ambulante Verhaltenstherapie. Verhaltenstherapie 2007, 17, 109–115. [Google Scholar] [CrossRef]
- Grawe, K.; Grawe-Gerber, M. Ressourcenaktivierung. Psychotherapeut 1999, 44, 63–73. [Google Scholar] [CrossRef]
- Heyman, I.; Mataix-Cols, D.; Fineberg, N.A. Obsessive-Compulsive Disorder. BMJ 2006, 333, 424–429. [Google Scholar] [CrossRef]
- Ribeiro, L. Adherence to Practice of Mindfulness in Novice Meditators: Practices Chosen, Amount of Time Practiced, and Long-Term Effects Following a Mindfulness-Based Intervention. Mindfulness 2018, 9, 401–411. [Google Scholar] [CrossRef]
- Segal, Z.V.; Williams, J.M.G.; Teasdale, J.D.; Kabat-Zinn, J. Mindfulness-Based Cognitive Therapy for Depression, 2nd ed.; The Guilford Press: New York, NY, USA, 2018; ISBN 978-1-4625-3703-7. [Google Scholar]
- Lee, S.M.; Suh, H.-W.; Kwak, H.-Y.; Kim, J.W.; Chung, S.-Y. Meditation-Based Intervention for Obsessive-Compulsive Disorder: A PRISMA-Compliant Systematic Review and Meta-Analysis. Medicine 2022, 101, e29147. [Google Scholar] [CrossRef]
- Bergman, R.L.; Rozenman, M. New Wave Therapies for Pediatric OCD. In Innovations in CBT for Childhood Anxiety, OCD, and PTSD; Farrell, L.J., Ollendick, T.H., Muris, P., Eds.; Cambridge University Press: Cambridge, MA, USA, 2019; pp. 506–522. ISBN 978-1-108-23565-5. [Google Scholar]
- Leopold, R.; Backenstrass, M. Neuropsychological Differences between Obsessive-Compulsive Washers and Checkers: A Systematic Review and Meta-Analysis. J. Anxiety Disord. 2015, 30, 48–58. [Google Scholar] [CrossRef]
- Moritz, S.; Hübner, M.; Kluwe, R. Task Switching and Backward Inhibition in Obsessive-Compulsive Disorder. J. Clin. Exp. Neuropsychol. 2004, 26, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Vakili, Y.; Gharaee, B.; Habibi, M. Acceptance and Commitment Therapy, Selective Serotonin Reuptake Inhibitors and Their Combination in the Improvement of Obsessive-Compulsive Symptoms and Experiential Avoidance in Patients with Obsessive-Compulsive Disorder. Iran J. Psychiatry Behav. Sci. 2015, 9, e845. [Google Scholar] [CrossRef] [PubMed]
- Cásedas, L.; Pirruccio, V.; Vadillo, M.A.; Lupiáñez, J. Does Mindfulness Meditation Training Enhance Executive Control? A Systematic Review and Meta-Analysis of Randomized Controlled Trials in Adults. Mindfulness 2020, 11, 411–424. [Google Scholar] [CrossRef]
- Nielen, M.M.A.; Den Boer, J.A. Neuropsychological Performance of OCD Patients before and after Treatment with Fluoxetine: Evidence for Persistent Cognitive Deficits. Psychol. Med. 2003, 33, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, S.R.; Blackwell, A.D.; Fineberg, N.A.; Robbins, T.W.; Sahakian, B.J. The Neuropsychology of Obsessive Compulsive Disorder: The Importance of Failures in Cognitive and Behavioural Inhibition as Candidate Endophenotypic Markers. Neurosci. Biobehav. Rev. 2005, 29, 399–419. [Google Scholar] [CrossRef] [PubMed]

| Intervention Group (N = 31) | Control Group (N = 27) | Comparison between Groups | ||||||
|---|---|---|---|---|---|---|---|---|
| M (SD) | Range | M (SD) | Range | t | df | p | 95% CI | |
| Age in years | 15.75 (2.17) | 11.66–19.59 | 15.29 (1.99) | 10.73–18.97 | 0.85 | 56 | 0.399 | [−0.64–1.57] |
| IQ a | 106.34 (14.09) | 85–139 | 99.39 (13.7) | 79–136 | 1.898 | 56 | 0.063 | [−0.39–14.29] |
| CY-BOCS b | ||||||||
| Obsessions PRE | 9.19 (4.22) | 0–19 | 7.93 (3.58) | 1–17 | 1.223 | 56 | 0.017 | [−0.81–3.35] |
| Obsessions POST | 7.32 (3.54) | 0–13 | 6.00 (3.14) | 0–12 | 1.495 | 56 | 0.141 | [−0.45–3.1] |
| t(30) = 2.862; p = 0.008 | t(26) = 3.366; p = 0.001 | |||||||
| Compulsions PRE | 9.97 (3.29) | 5–18 | 7.78 (3.49) | 3–16 | 2.458 | 56 | 0.018 | [0.41–3.98] |
| Compulsions POST | 7.84 (3.23) | 0–13 | 5.48 (3.67) | 0–12 | 2.603 | 56 | 0.012 | [0.543–4.17] |
| t(30) = 3.298; p = 0.003 | t(26) = 4.395; p < 0.001 | |||||||
| Total score PRE | 19.48 (7.28) | 8–37 | 15.33 (5.84) | 7–24 | 2.369 | 56 | 0.021 | [0.64–7.66] |
| Total score POST | 15.16 (6.36) | 0–25 | 11.48 (6.51) | 0–23 | 2.175 | 56 | 0.034 | [0.291–7.07] |
| t(30) = 3.776; p < 0.001 | t(26) = 4.386; p < 0.011 | |||||||
| ZWIK-S c | ||||||||
| PRE | 43.65 (29.82) | 2–123 | 39.52 (26.95) | 4–101 | 0.55 | 56 | 0.585 | [−19.92–19.17] |
| POST | 37.28 (27.16) | 5–113 | 26.48 (18.53) | 5–77 | 1.725 | 54 | 0.09 | [−1.75–23.34] |
| t(28) = 2.255; p = 0.016 | t(26) = 3.237; p = 0.003 | |||||||
| ZWIK-P d | ||||||||
| PRE | 30.1 (20.04) | 4–84 | 34.52 (16.52) | 8–72 | −1.018 | 56 | 0.313 | [−14.65–4.77] |
| POST | 23.77 (15.75) | 3–59 | 26.37 (18.81) | 7–88 | −0.569 | 55 | 0.572 | [−11.78–6.57] |
| t(29) = 2.129; p = 0.04 | t(26) = 2.09; p = 0.047 | |||||||
| Total minutes App used | 813.16 (278.62) | 268–1231 | 1081.48 (283.57) | 166–1436 | −3.628 | 56 | <0.001 | [−416.46–−120.18] |
| N (%) | N (%) | X2 | df | p | ||||
| Male | 13 e (42) | 12 (44) | 0.037 | 1 | 0.847 | |||
| Comorbidity | 12 (39) | 9 (33) | 0.181 | 1 | 0.671 | |||
| Medication | 12 (39) | 8 (30) | 0.527 | 1 | 0.468 | |||
| BF | PBIC(H0|D) | PBIC(H1|D) | |
|---|---|---|---|
| Symptom data | |||
| CY-BOCS | |||
| Main effect Group | 0.34 | 0.25 | 0.75 |
| Interaction Group × Appointment | 7.23 | 0.88 | 0.12 |
| ZWIK-S | |||
| Main effect Group | 3.66 | 0.79 | 0.21 |
| Interaction Group × Appointment | 3.56 | 0.78 | 0.22 |
| ZWIK-P | |||
| Main effect Group | 5.16 | 0.84 | 0.16 |
| Interaction Group × Appointment | 7.02 | 0.88 | 0.12 |
| Behavioral data | |||
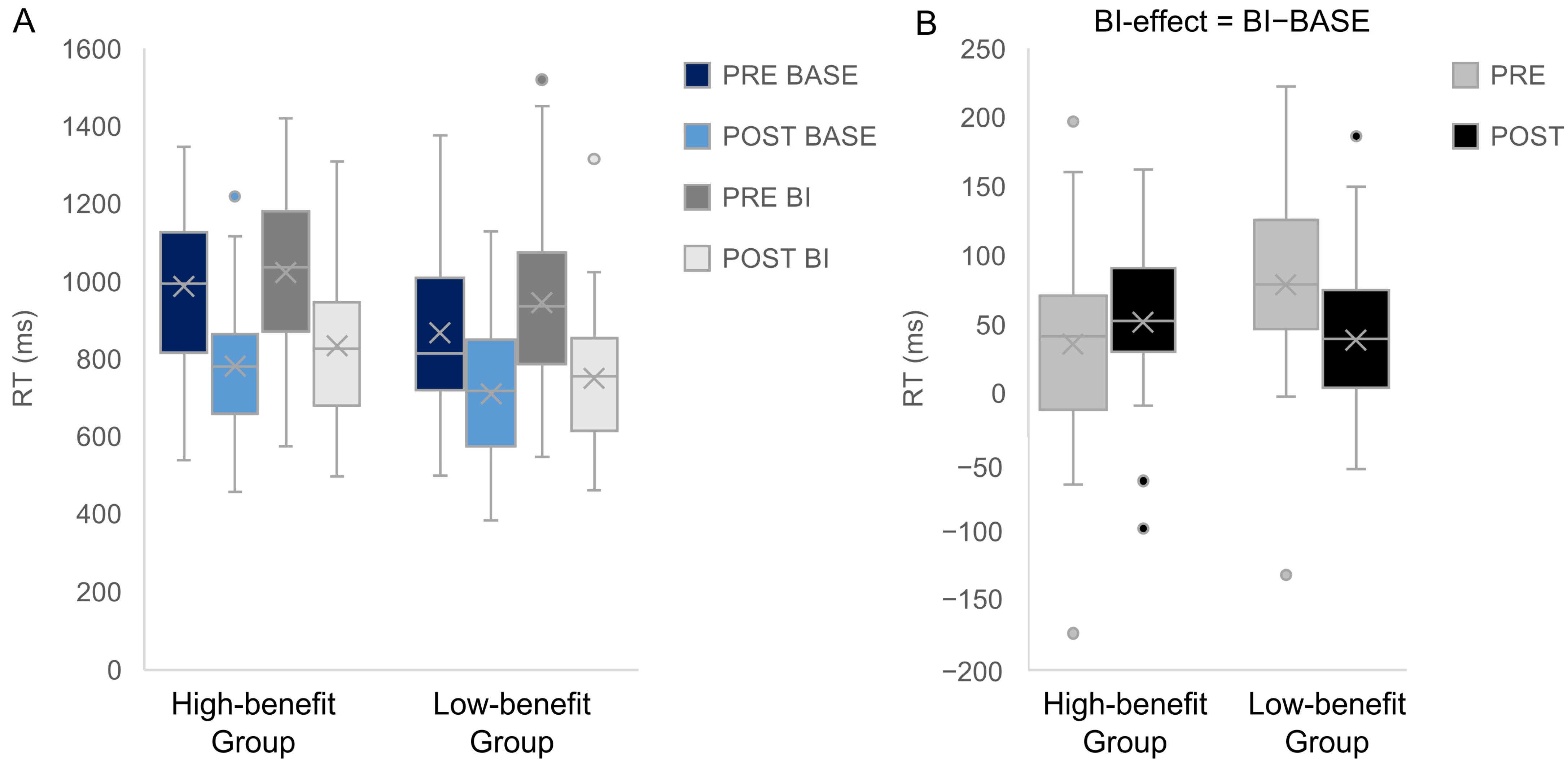
| Hit RT | |||
| Main effect Group | 4.07 | 0.80 | 0.20 |
| Interaction Group × Appointment | 5.38 | 0.84 | 0.16 |
| Interaction Group × Appointment × Condition | 8.57 | 0.90 | 0.10 |
| Hit Accuracy | |||
| Main effect Group | 7.5 | 0.88 | 0.12 |
| Interaction Group × Appointment | 6.37 | 0.86 | 0.14 |
| Interaction Group × Appointment × Condition | 2.05 | 0.67 | 0.33 |
| Neurophysiological data | |||
| c-P1 | |||
| Main effect Group | 7.11 | 0.88 | 0.12 |
| Interaction Group × Appointment | 4.83 | 0.83 | 0.17 |
| Interaction Group × Appointment × Condition | 12.02 | 0.92 | 0.08 |
| t-P1 | |||
| Main effect Group | 7.47 | 0.88 | 0.12 |
| Interaction Group × Appointment | 5.77 | 0.85 | 0.15 |
| Interaction Group × Appointment × Condition | 12.91 | 0.93 | 0.07 |
| c-N1 | |||
| Main effect Group | 1.27 | 0.56 | 0.44 |
| Interaction Group × Appointment | 7.24 | 0.88 | 0.12 |
| Interaction Group × Appointment × Condition | 1.65 | 0.62 | 0.38 |
| t-N1 | |||
| Main effect Group | 6.32 | 0.86 | 0.14 |
| Interaction Group × Appointment | 4.96 | 0.83 | 0.17 |
| Interaction Group × Appointment × Condition | 3.96 | 0.8 | 0.2 |
| N2 | |||
| Main effect Group | 5.41 | 0.84 | 0.16 |
| Interaction Group × Appointment | 3.33 | 0.77 | 0.23 |
| Interaction Group × Appointment × Condition | 11.82 | 0.92 | 0.08 |
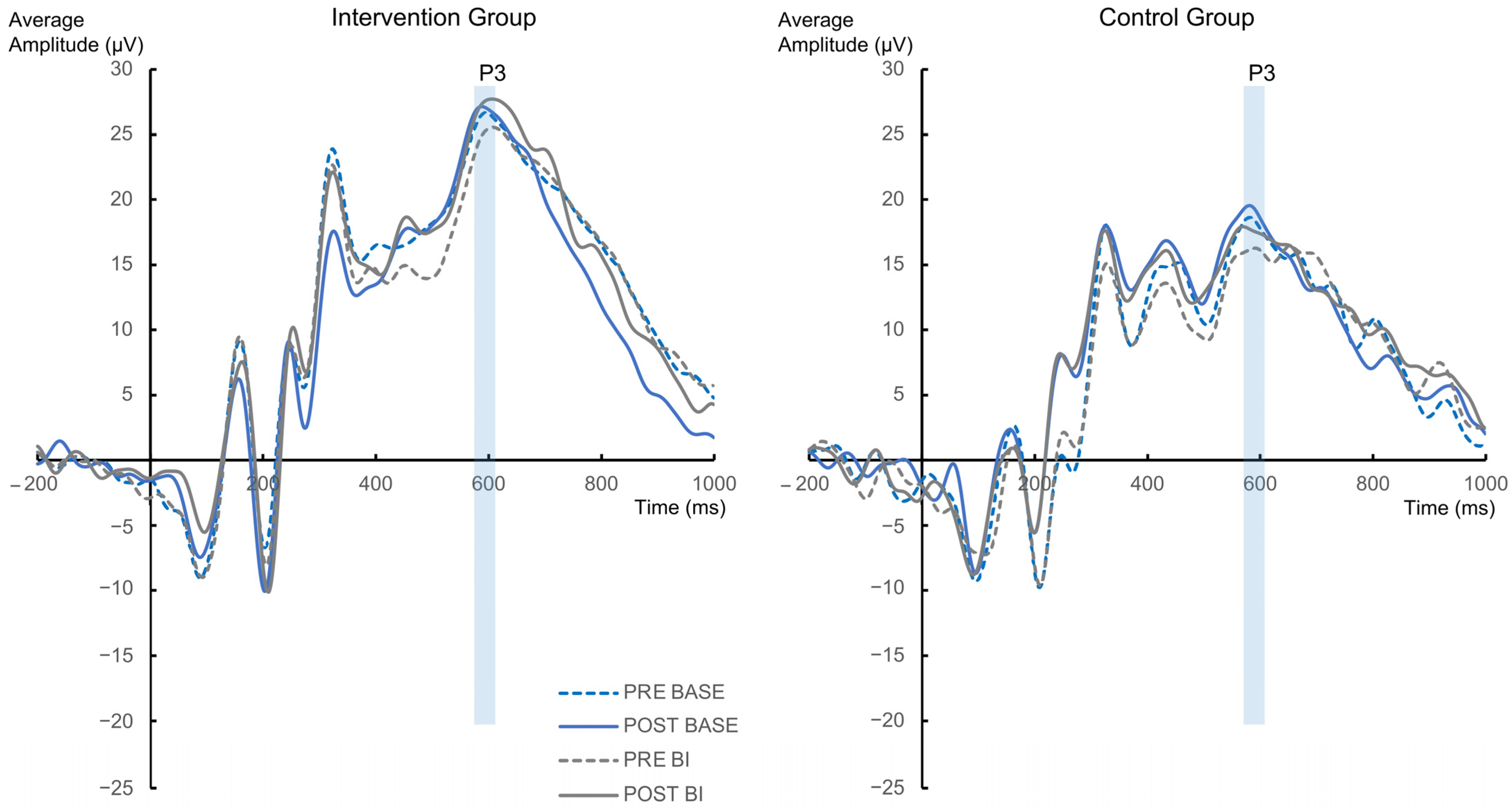
| P3 | |||
| Main effect Group | 0.5 | 0.33 | 0.67 |
| Interaction Group × Appointment | 7.55 | 0.88 | 0.12 |
| Interaction Group × Appointment × Condition | 9.36 | 0.9 | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rempel, S.; Backhausen, L.L.; McDonald, M.; Roessner, V.; Vetter, N.C.; Beste, C.; Wolff, N. App-Based Mindfulness Meditation Training and an Audiobook Intervention Reduce Symptom Severity but Do Not Modify Backward Inhibition in Adolescent Obsessive-Compulsive Disorder: Evidence from an EEG Study. J. Clin. Med. 2023, 12, 2486. https://doi.org/10.3390/jcm12072486
Rempel S, Backhausen LL, McDonald M, Roessner V, Vetter NC, Beste C, Wolff N. App-Based Mindfulness Meditation Training and an Audiobook Intervention Reduce Symptom Severity but Do Not Modify Backward Inhibition in Adolescent Obsessive-Compulsive Disorder: Evidence from an EEG Study. Journal of Clinical Medicine. 2023; 12(7):2486. https://doi.org/10.3390/jcm12072486
Chicago/Turabian StyleRempel, Sarah, Lea L. Backhausen, Maria McDonald, Veit Roessner, Nora C. Vetter, Christian Beste, and Nicole Wolff. 2023. "App-Based Mindfulness Meditation Training and an Audiobook Intervention Reduce Symptom Severity but Do Not Modify Backward Inhibition in Adolescent Obsessive-Compulsive Disorder: Evidence from an EEG Study" Journal of Clinical Medicine 12, no. 7: 2486. https://doi.org/10.3390/jcm12072486
APA StyleRempel, S., Backhausen, L. L., McDonald, M., Roessner, V., Vetter, N. C., Beste, C., & Wolff, N. (2023). App-Based Mindfulness Meditation Training and an Audiobook Intervention Reduce Symptom Severity but Do Not Modify Backward Inhibition in Adolescent Obsessive-Compulsive Disorder: Evidence from an EEG Study. Journal of Clinical Medicine, 12(7), 2486. https://doi.org/10.3390/jcm12072486








