Sinus Augmentation for Implant Placement Utilizing a Novel Synthetic Graft Material with Delayed Immediate Socket Grafting: A 2-Year Case Study
Abstract
1. Introduction
2. Methods and Materials
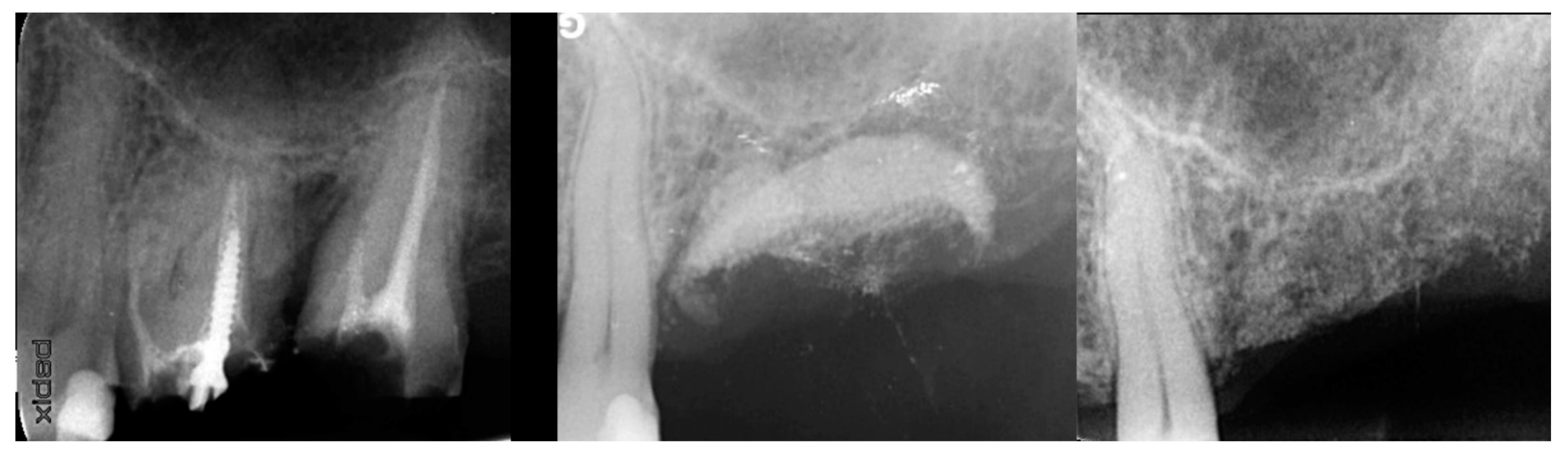
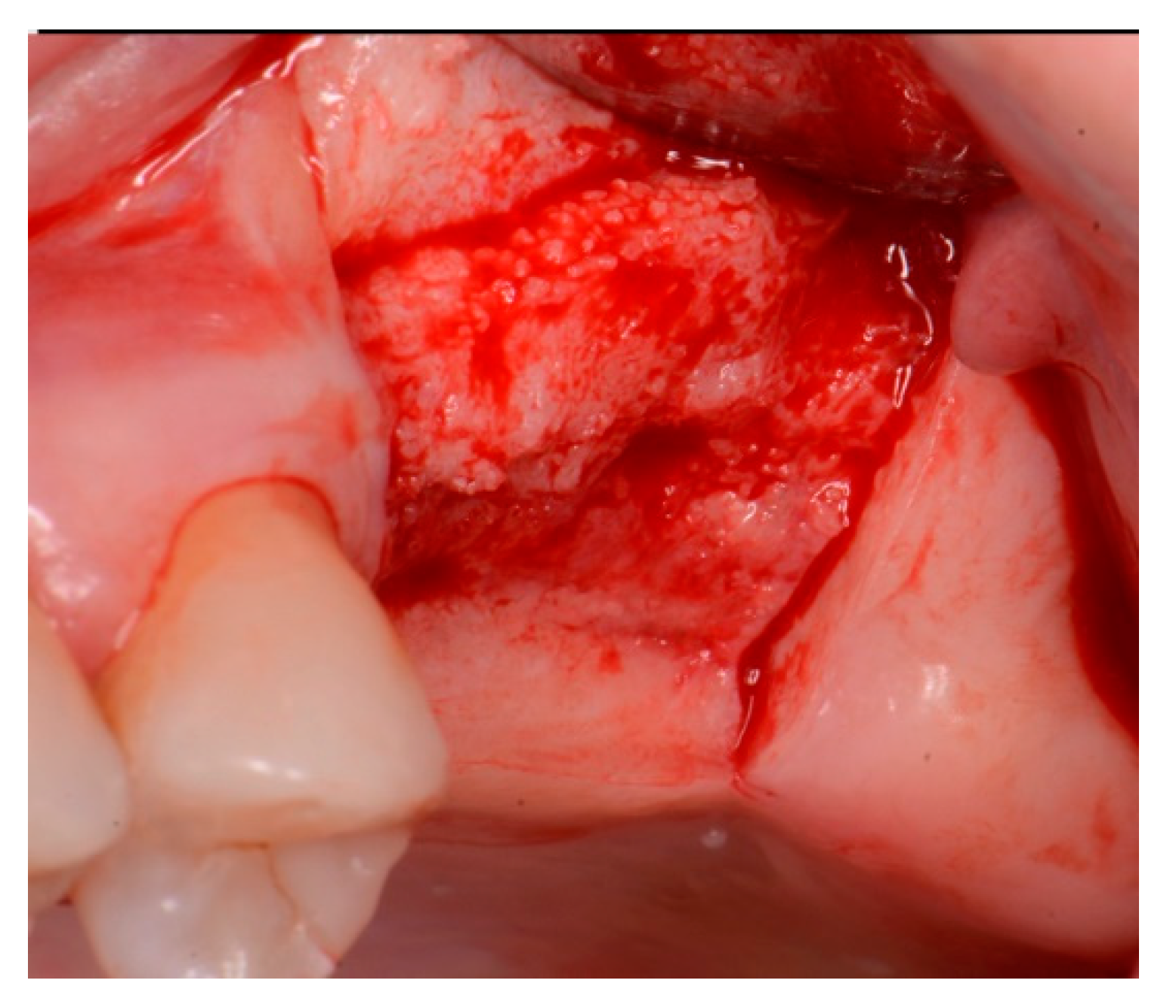
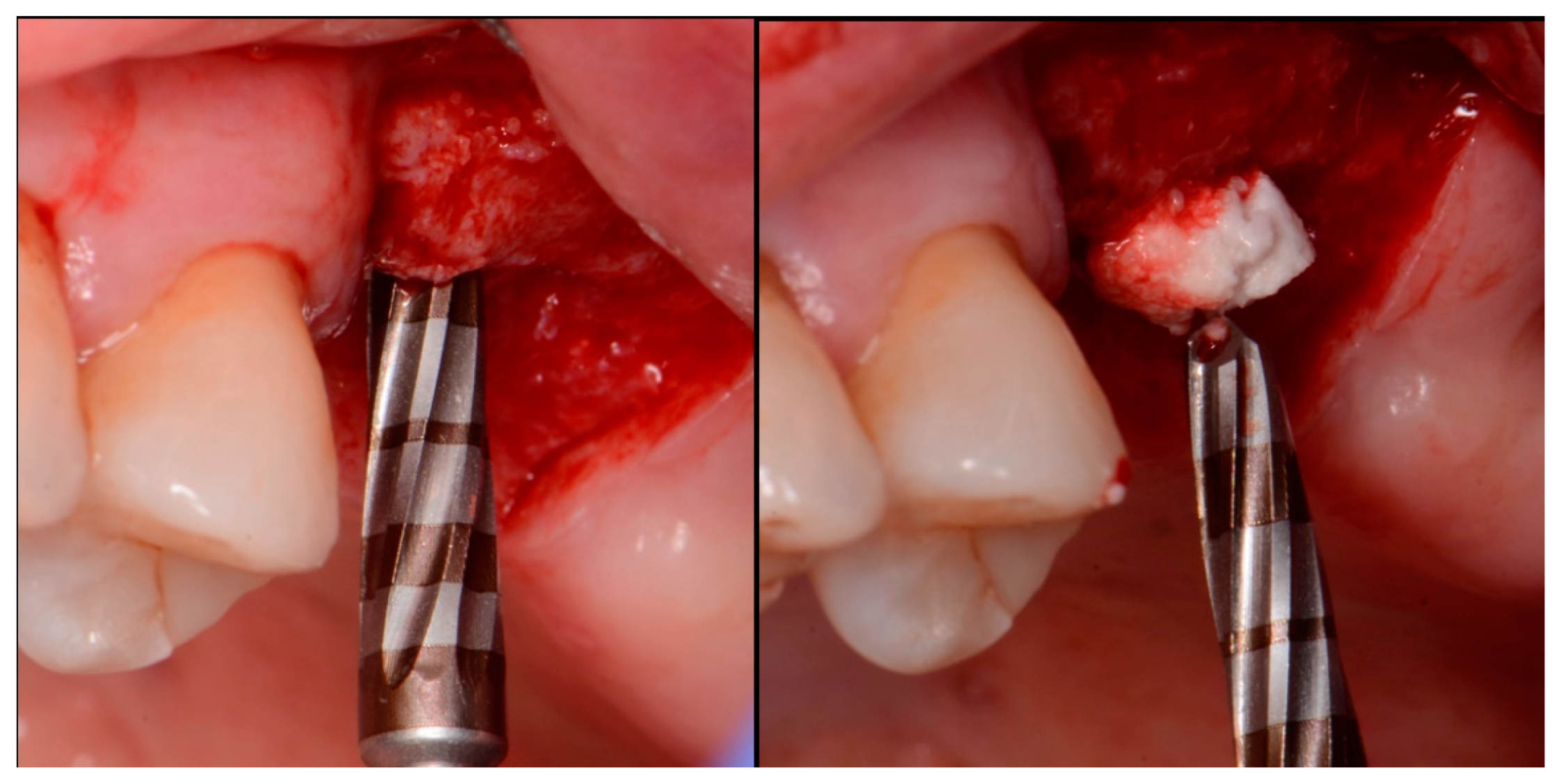
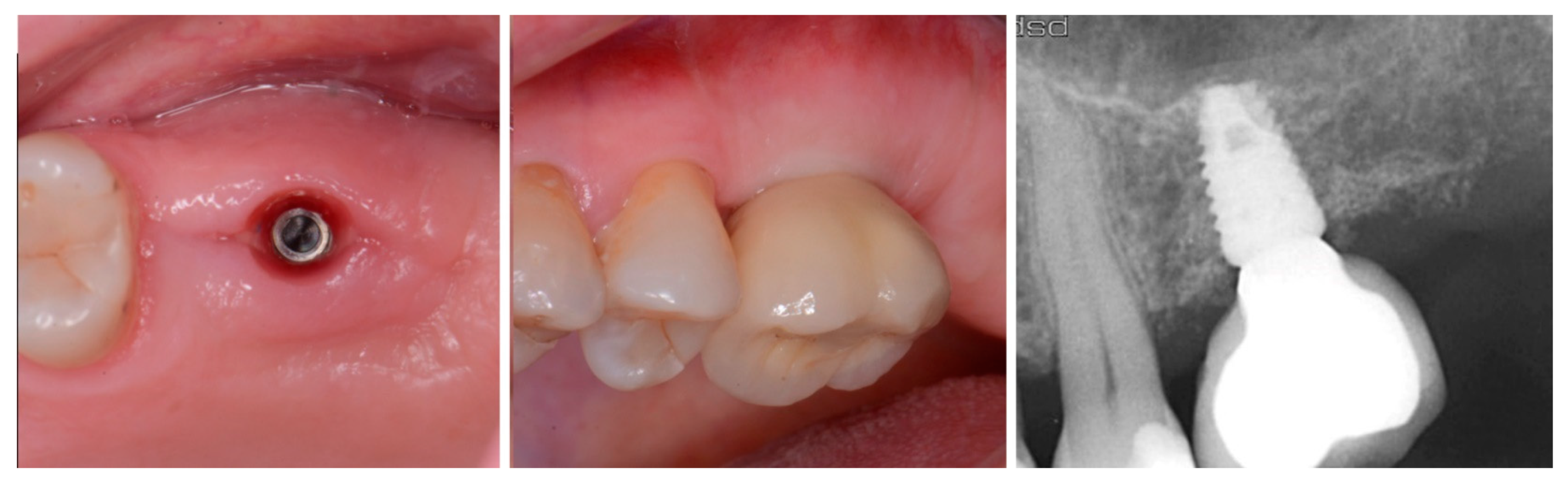
2.1. Case 1
2.2. Case 2
2.3. Case 3
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Moraissi, E.; Alhajj, W.A.; Al-Qadhi, G.; Christidis, N. Bone Graft Osseous Changes After Maxillary Sinus Floor Augmentation: A Systematic Review. J. Oral Implantol. 2022, 48, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Al-Moraissi, E.A.; Alkhutari, A.S.; Abotaleb, B.; Altairi, N.H.; Del Fabbro, M. Do osteoconductive bone substitutes result in similar bone regeneration for maxillary sinus augmentation when compared to osteogenic and osteoinductive bone grafts? A systematic review and frequentist network meta-analysis. Int. J. Oral Maxillofac. Surg. 2020, 49, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Coyac, B.R.; Wu, M.; Bahat, D.J.; Wolf, B.J.; Helms, J.A. Biology of sinus floor augmentation with an autograft versus a bone graft substitute in a preclinical in vivo experimental model. Clin. Oral Implant. Res. 2021, 32, 916–927. [Google Scholar] [CrossRef] [PubMed]
- Nappe, C.E.; Rezuc, A.B.; Montecinos, A.; Donoso, F.A.; Vergara, A.J.; Martinez, B. Histological comparison of an allograft, a xenograft and alloplastic graft as bone substitute materials. J. Osseointegr. 2016, 8, 20–26. [Google Scholar]
- Zampara, E.; Alshammari, M.; De Bortoli, J.; Mullings, O.; Gkisakis, I.G.; Benalcázar Jalkh, E.B.; Tovar, N.; Coelho, P.G.; Witek, L. A histological and histomorphometric evaluation of an allograft, xenograft, and alloplast graft for alveolar ridge preservation: Randomized clinical trial. J. Oral Implantol. 2022, 21, 541–549. [Google Scholar] [CrossRef]
- Rodriguez, A.E.; Nowzari, H. The long-term risks and complications of bovine-derived xenografts: A case series. J. Indian Soc. Periodontol. 2019, 23, 487–492. [Google Scholar] [CrossRef]
- Kim, Y.; Nowzari, H.; Rich, S.K. Risk of prion disease transmission through bovine-derived bone substitutes: A systematic review. Clin. Implant. Dent. Relat. Res. 2013, 15, 645–653. [Google Scholar] [CrossRef]
- Kim, Y.; Rodriguez, A.E.; Nowzari, H. The Risk of Prion Infection through Bovine Grafting Materials. Clin. Implant. Dent. Relat. Res. 2016, 18, 1095–1102. [Google Scholar] [CrossRef]
- Lutz, R.; Berger-Fink, S.; Stockmann, P.; Neukam, F.W.; Schlegel, K.A. Sinus floor augmentation with autogenous bone vs. A bovine-derived xenograft—A 5-year retrospective study. Clin. Oral Implant. Res. 2015, 26, 644–648. [Google Scholar] [CrossRef]
- Cannizzaro, G.; Felice, P.; Leone, M.; Viola, P.; Esposito, M. Early loading of implants in the atrophic posterior maxilla: Lateral sinus lift with autogenous bone and bio-oss versus crestal mini sinus lift and 8-mm hydroxyapatite-coated implants. A randomised controlled clinical trial. Eur. J. Oral Implantol. 2009, 2, 25–38. [Google Scholar]
- Bannister, S.R.; Powell, C.A. Foreign body reaction to anorganic bovine bone and autogenous bone with platelet-rich plasma in guided bone regeneration. J. Periodontol. 2008, 79, 1116–1120. [Google Scholar] [CrossRef] [PubMed]
- Scolozzi, P.; Perez, A.; Verdeja, R.; Courvoisier, D.S.; Lombardi, T. Association between maxillary sinus fungus ball and sinus bone grafting with deproteinized bovine bone substitutes: A case-control study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 121, e143–e147. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.; Schou, S.; Stavropoulos, A.; Terheyden, H.; Holmstrup, P. Maxillary sinus floor augmentation with bio-oss or bio-oss mixed with autogenous bone as graft: A systematic review. Clin. Oral Implant. Res. 2012, 23, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Bohner, M.; Santoni, B.L.G.; Döbelin, N. β-tricalcium phosphate for bone substitution: Synthesis and properties. Acta Biomater. 2020, 113, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Tebyanian, H.; Norahan, M.H.; Eyni, H.; Movahedin, M.; Mortazavi, S.J.; Karami, A.; Nourani, M.R.; Baheiraei, N. Effects of collagen/β-tricalcium phosphate bone graft to regenerate bone in critically sized rabbit calvarial defects. J. Appl. Biomater. Funct. Mater. 2019, 17, 2280800018820490. [Google Scholar] [CrossRef]
- Ariizumi, T.; Kawashima, H.; Hatano, H.; Yamagishi, T.; Oike, N.; Sasaki, T.; Umezu, H.; Xu, Y.; Endo, N.; Ogose, A. Osteoinduction and Osteoconduction with Porous Beta-Tricalcium Phosphate Implanted after Fibular Resection in Humans. J. Biomater. Nanobiotechnol. 2019, 10, 159–173. [Google Scholar] [CrossRef]
- Ishikawa, K.; Miyamoto, Y.; Tsuchiya, A.; Hayashi, K.; Tsuru, K.; Ohe, G. Physical and Histological Comparison of Hydroxyapatite, Carbonate Apatite, and β-Tricalcium Phosphate Bone Substitutes. Materials 2018, 11, 1993. [Google Scholar] [CrossRef]
- Coetzee, A.S. Regeneration of bone in the presence of calcium sulfate. Arch. Otolaryngol. 1980, 106, 405–409. [Google Scholar] [CrossRef]
- Aquino-Martínez, R.; Angelo, A.P.; Pujol, F.V. Calcium-containing scaffolds induce bone regeneration by regulating mesenchymal stem cell differentiation and migration. Stem Cell Res. Ther. 2017, 8, 265. [Google Scholar] [CrossRef]
- Ricci, J.; Alexander, H.; Nadkarni, P.; Hawkins, M.; Turner, J.; Rosenblum, S.; Brezenoff, L.; DeLeonardis, D.; Pecora, G. Biological mechanisms of calcium sulfate replacement by bone. In Bone Engineering; Davies, J.E., Ed.; Em2 Inc.: Toronto, ON, Canada, 2000; pp. 332–344. [Google Scholar]
- Pförringer, D.; Harrasser, N.; Mühlhofer, H.; Kiokekli, M.; Stemberger, A.; van Griensven, M.; Lucke, M.; Burgkart, R.; Obermeier, A. Osteoinduction and -conduction through absorbable bone substitute materials based on calcium sulfate: In vivo biological behavior in a rabbit model. J. Mater. Sci. Mater. Med. 2018, 29, 17. [Google Scholar] [CrossRef]
- Yuan, H.; Fernandes, H.; Habibovic, P.; de Boer, J.; Barradas, A.; de Ruiter, A.; Walsh, W.; van Blitterswijk, C.; de Bruijn, J.D. Osteoinductive ceramics as a synthetic alternative to autologous bone grafting. Proc. Natl. Acad. Sci. USA 2010, 107, 13614–13619. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Zhang, Q.; Sculean, A.; Buser, D.; Pippenger, B.; Dard, M.; Shirakata, Y.; Chandad, F.; Zhang, Y. Osteoinductive potential of 4 commonly employed bone grafts. Clin. Oral Investig. 2016, 20, 2259–2265. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Zhu, X.; Tang, Z.; Yang, X.; Tan, Y.; Fan, Y.; Zhang, X. Enhanced effect of β-tricalcium phosphate phase on neovascularization of porous calcium phosphate ceramics: In vitro and in vivo evidence. Acta Biomater. 2015, 11, 435–448. [Google Scholar] [CrossRef] [PubMed]
- Flifl, M.A.S.; Marzook, H.; Denewar, M.; Elsheikh, H.A. Biological Impact of Alloplastic Bone Graft vs Bovine Xenograft and Allograft Materials in Bone Healing: An Experimental Study. J. Contemp. Dent. Pract. 2022, 23, 482–491. [Google Scholar]
- Artzi, Z.; Weinreb, M.; Givol, N.; Rohrer, M.D.; Nemcovsky, C.E.; Prasad, H.S.; Tal, H. Biomaterial resorption rate and healing site morphology of inorganic bovine bone and beta-tricalcium phosphate in the canine: A 24-month longitudinal histologic study and morphometric analysis. Int. J. Oral Maxillofac. Implant. 2004, 19, 357–368. [Google Scholar]
- Ramalingam, S.; Al-Rasheed, A.; ArRejaie, A.; Nooh, N.; Al-Kindi, M.; Al-Hezaimi, K. Guided bone regeneration in standardized calvarial defects using beta-tricalcium phosphate and collagen membrane: A real-time in vivo micro-computed tomographic experiment in rats. Odontology 2016, 104, 199–210. [Google Scholar] [CrossRef]
- Fairbairn, P.; Leventis, M. Protocol for bone augmentation with simultaneous early implant placement: A retrospective multicenter clinical study. Int. J. Dent. 2015, 2015, 589135. [Google Scholar] [CrossRef] [PubMed]
- Leventis, M.D.; Fairbairn, P.; Dontas, I.; Faratzis, G.; Valavanis, K.D.; Khaldi, L.; Kostakis, G.; Eleftheriadis, E. Biological response to β-tricalcium phosphate/calcium sulfate synthetic graft material: An experimental study. Implant. Dent. 2014, 23, 37–43. [Google Scholar] [CrossRef]
- Ruga, E.; Gallesio, C.; Chiusa, L.; Boffano, P. Clinical and histologic outcomes of calcium sulfate in the treatment of postextraction sockets. J. Craniofacial Surg. 2011, 22, 494–498. [Google Scholar] [CrossRef]
- Eleftheriadis, E.; Leventis, M.D.; Tosios, K.I.; Faratzis, G.; Titsinidis, S.; Eleftheriadi, I.; Dontas, I. Osteogenic activity of betatricalcium phosphate in a hydroxyl sulphate matrix and demineralized bone matrix: A histological study in rabbit mandible. J. Oral Sci. 2010, 52, 377–384. [Google Scholar] [CrossRef]
- Podaropoulos, L.; Veis, A.A.; Papadimitriou, S.; Alexandridis, C.; Kalyvas, D. Bone regeneration using beta-tricalcium phosphate in a calcium sulfate matrix. J. Oral Implantol. 2009, 35, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Anson, D. Using calcium sulfate in guided tissue regeneration: A recipe for success. Compend. Contin. Educ. Dent. 2000, 21, 365–370. [Google Scholar] [PubMed]
- Liu, C.C.; Solderer, A.; Heumann, C.; Attin, T.; Schmidlin, P.R. Tricalcium phosphate (-containing) biomaterials in the treatment of periodontal infra-bony defects: A systematic review and meta-analysis. J. Dent. 2021, 114, 103812. [Google Scholar] [CrossRef]
- Sukumar, S.; Drizhal, I.; Bukac, J.; Paulusova, V.; Pilathadka, S. Surgical treatment of periodontal intrabony defects with calcium sulphate in combination with beta tricalcium phosphate—A 12-month retrospective clinical evaluation. Acta Med. 2010, 53, 229–234. [Google Scholar] [CrossRef]
- Leventis, M.; Fairbairn, P.; Horowitz, R.A.; Kalyvas, D. Synthetic bone grafts in contemporary Oral Surgery and Implantology. Newest developments and perspectives. Odontostomatol. Prog. 2017, 71, 222–235. [Google Scholar]
- Fairbairn, P.; Leventis, M.; Mangham, C.; Horowitz, R. Alveolar ridge preservation using a novel synthetic grafting material: A case with two-year follow-up. Case Rep. Dent. 2018, 2018, 6412806. [Google Scholar] [CrossRef]
- Cheah, C.W.; Al-Namnam, N.M.; Lau, M.N.; Lim, G.S.; Raman, R.; Fairbairn, P.; Ngeow, W.C. Synthetic Material for Bone, Periodontal, and Dental Tissue Regeneration: Where Are We Now, and Where Are We Heading Next? Materials 2021, 14, 6123. [Google Scholar] [CrossRef]
- Sasaki, H.; Koyama, S.; Yokoyama, M.; Yamaguchi, K.; Itoh, M.; Sasaki, K. Bone metabolic activity around dental implants under loading observed using bone scintigraphy. Int. J. Oral Maxillofac. Implant. 2008, 23, 827–834. [Google Scholar]
- Fairbairn, P. Bone Augmentation using Alloplastic Particulate Graft Material with Simultaneous early Implant placement: A case report. J. Parodontol. Implantol. Orale 2016, 35, 291–298. [Google Scholar]
- Fairbairn, P. Clinical case of delayed immediate prosthetics: Regeneration of bones with use new stable synthetic material. EthOss. Perio. IQ 2018, 31, 147–155. [Google Scholar]


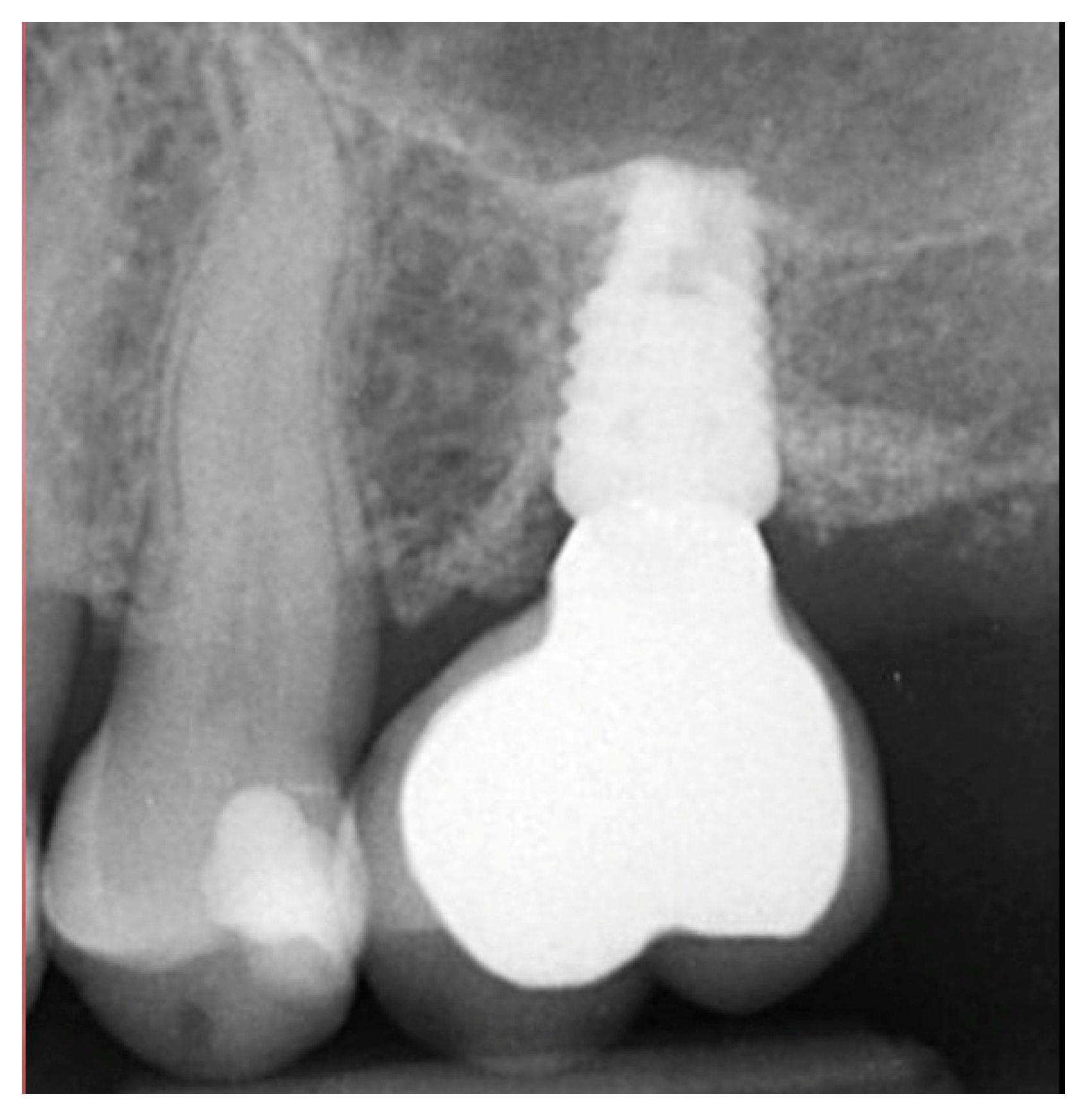
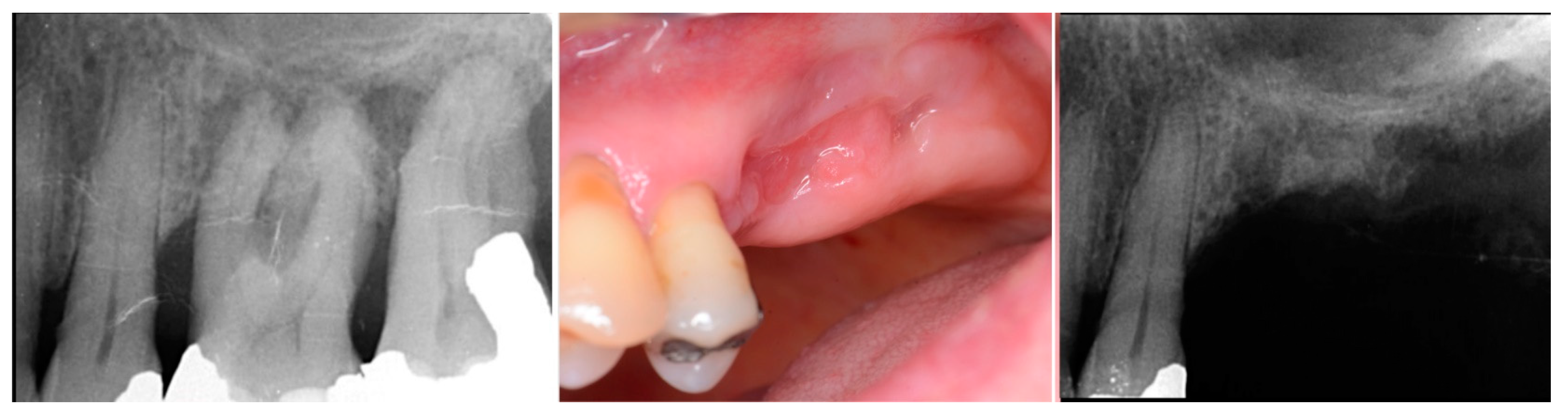




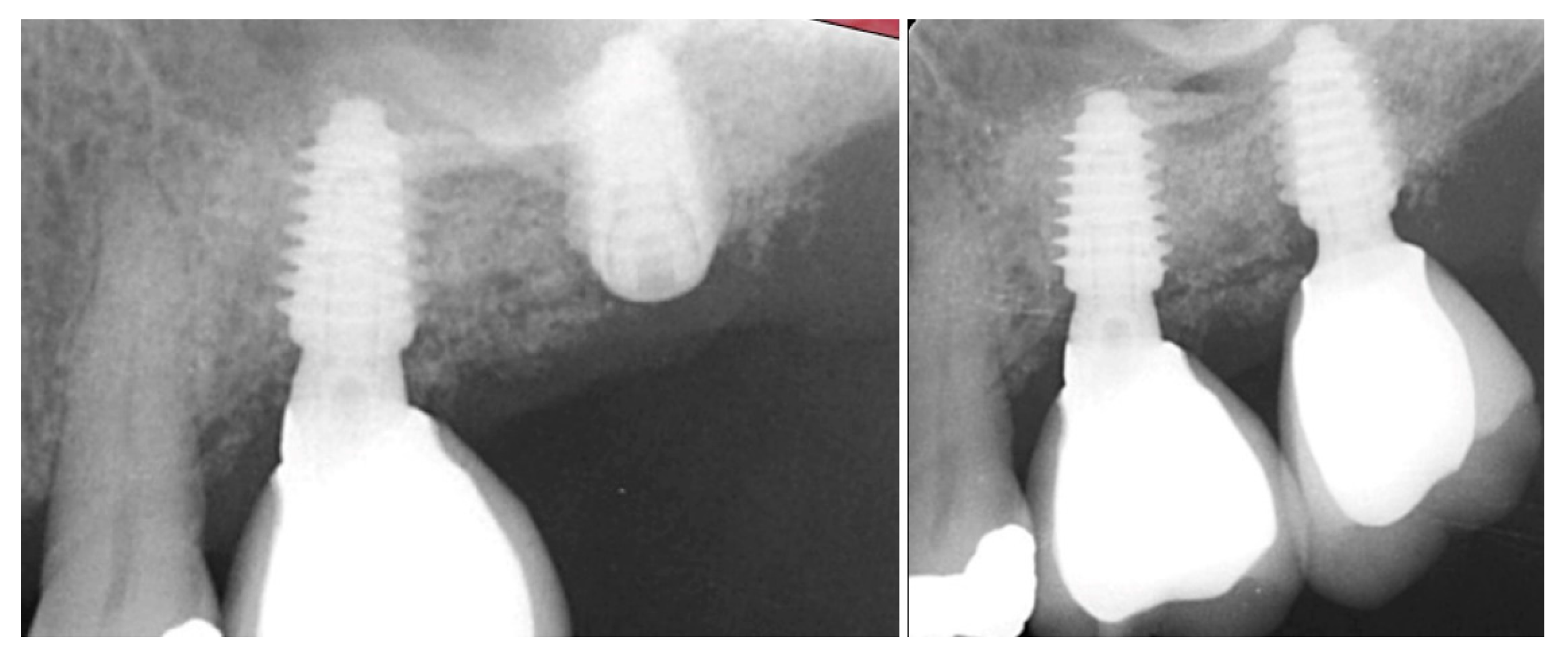
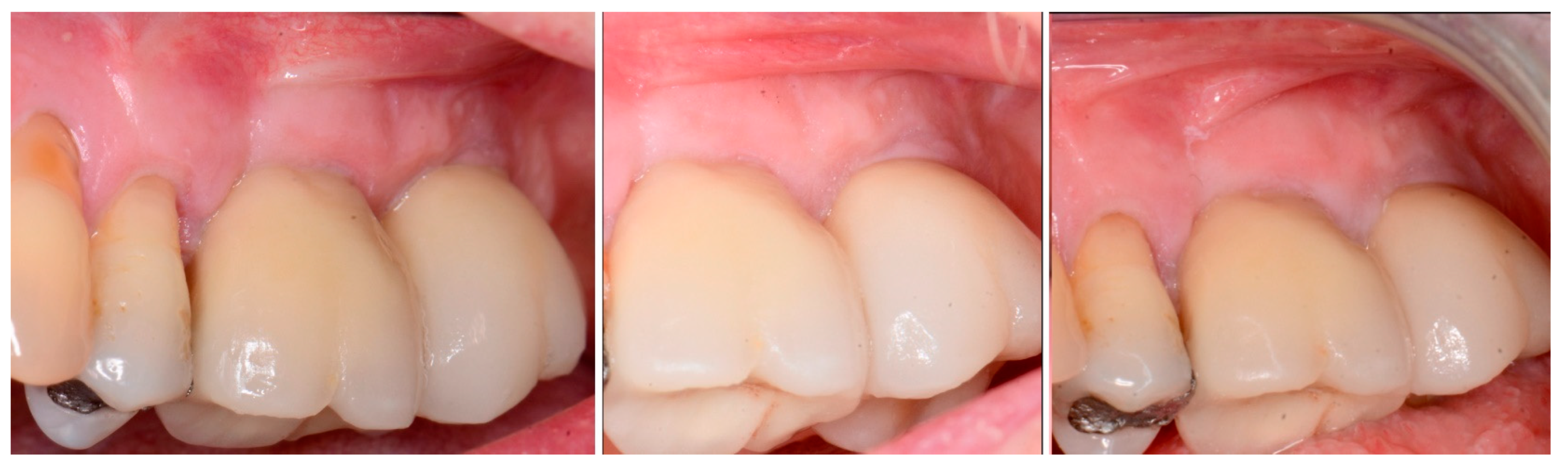
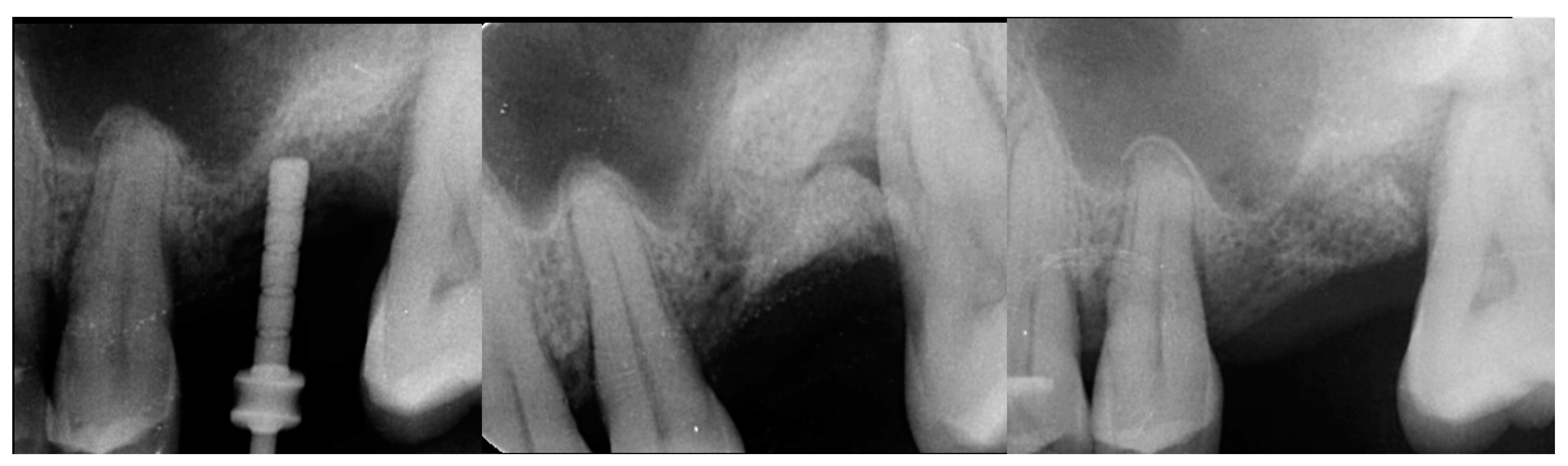
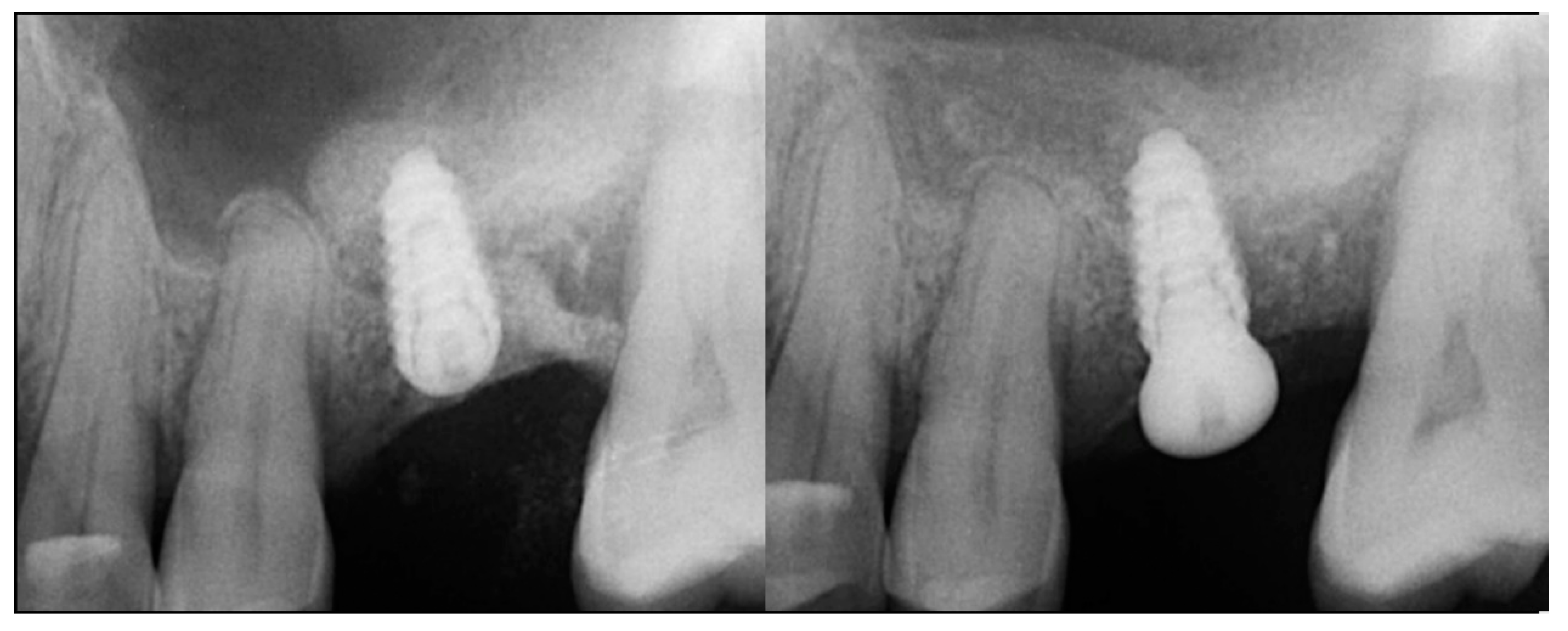

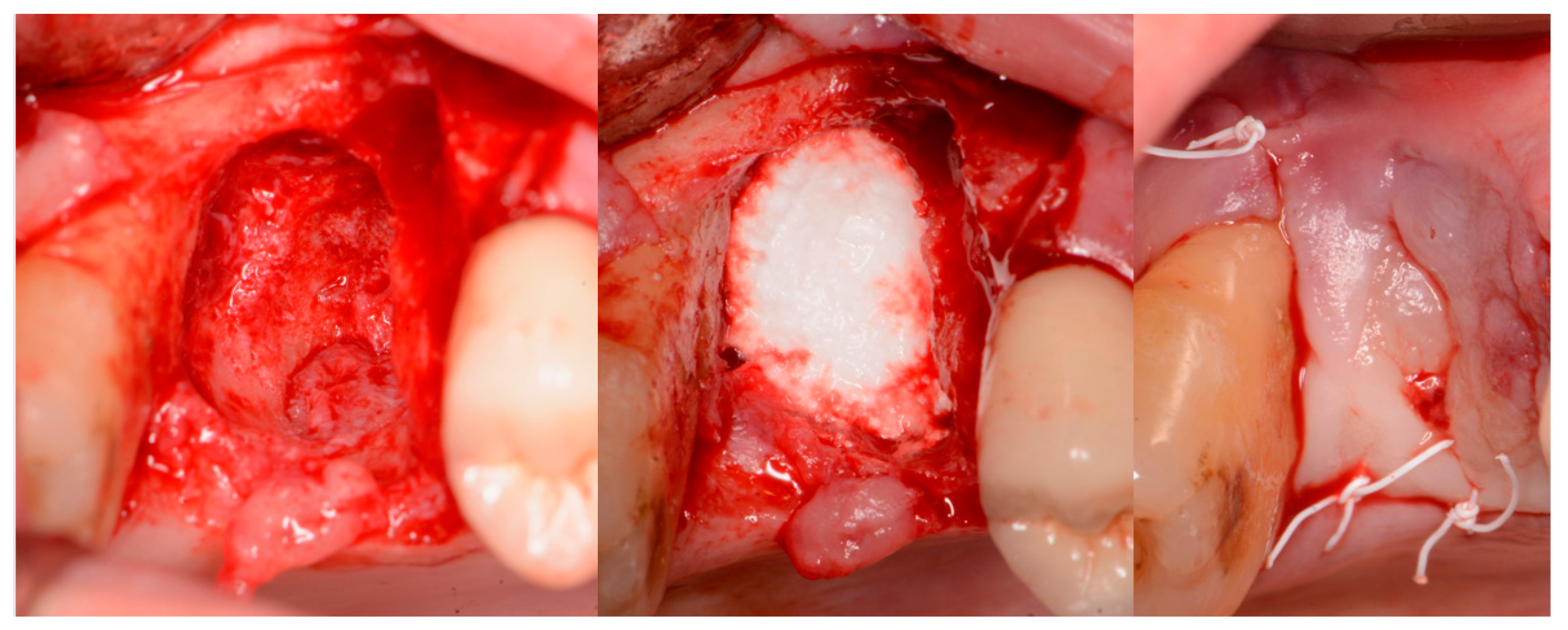
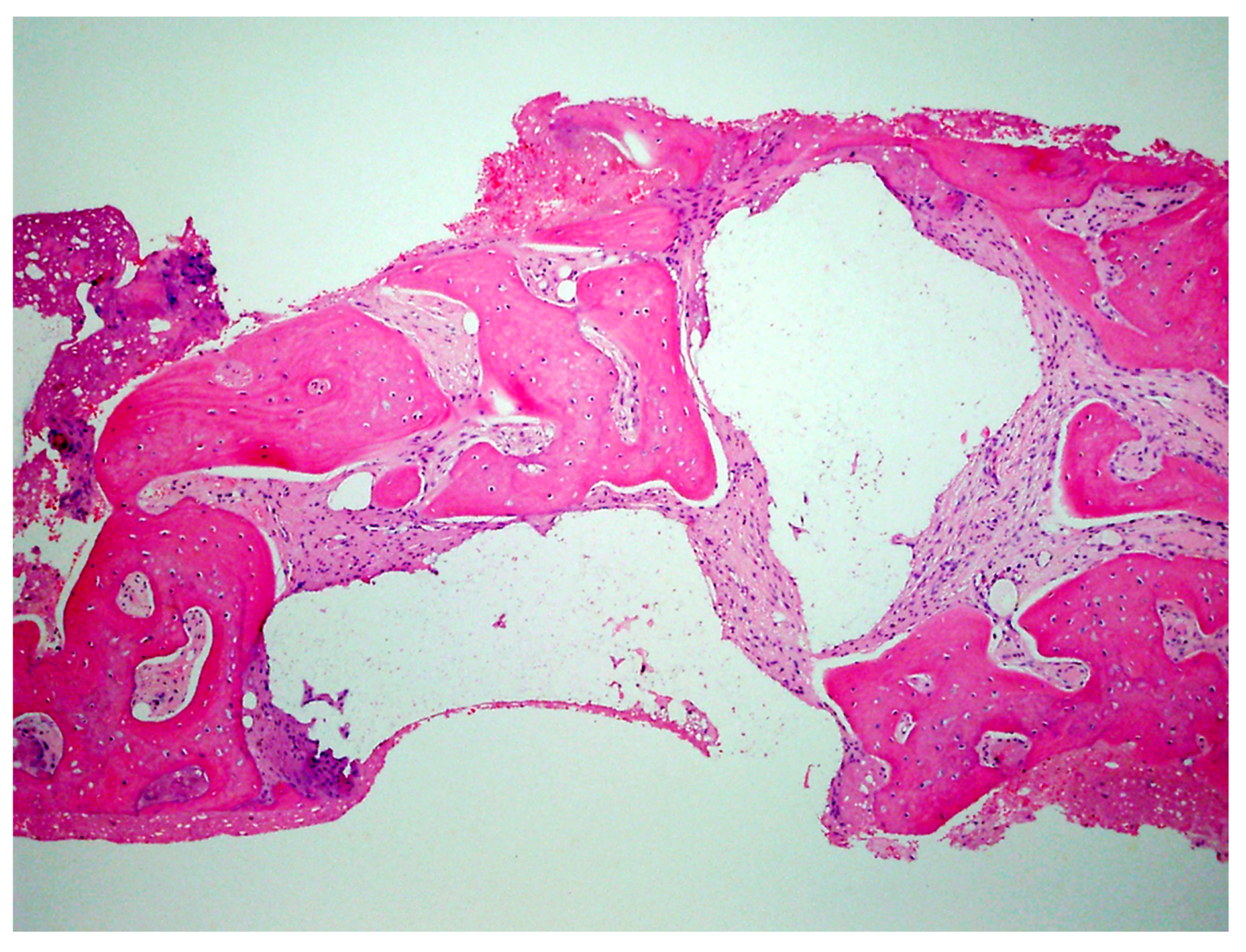
| Case No | Gender | Age (Years) | Smoker (Y/N) | Perio Tooth Loss | Flap Raised (Y/N) | Quantity in Socket Graft | Horizontal Measurement at Placement | Vertical Measurement at Placement | Type of Implant Placed | NCM at Placement | Sinus Augmentation Protocol | Loaded at 12 Weeks | Loaded Vertical Measurement | 2 Year Post op Vertical, Measurement of PA X-ray | 2 Year post op PES Score and Papillae | Complications |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 54 | N | Yes | Y | 1 cc | 8.6 mm | 6.6 mm | Paltop | 70 Ncm | Internal D/V | Y | 9.3 mm | 9.2 mm | 13 | Nil |
| 2 | F | 64 | n | Yes | Y | 1 cc | 7 mm | 5 mm | Anyridge | 65 Ncm | Internal D/V | N14 weeks | 8.8 mm | 9.1 mm | 12 | Nil |
| 3 | M | 80 | N | Yes | Y | 1.5 cc | 7 mm | 9.5 mm | Paltop | 50 Ncm | Internal D | Y | 12mm | 12.3 mm | 12 | NIl |
| 4 | M | 82 | P | Yes | Y | 1 cc | 6.5 mm | 8.6 mm | Dio SM | 50 ncm | Internal D | Y | 11.1mm | 10.9 mm | 11 | NII |
| 5 | M | 79 | N | Yes | Y | 1 cc | 9 mm | 8.5 mm | Anyridge | 40 ncm | Internal D/V | Y | 12.4mm | 11.9 mm | 12 | Perio, distal |
| 6 | F | 43 | N | Yes | Y | 1 cc | 10 mm | 7 mm | Dio SM | 45 ncm | Internal D | Y | 8.2mm | 9 mm | 12 | Nil |
| 7 | F | 75 | P | Yes | Y | 1 cc | 7.5 mm | 7.1 mm | Anyridge | 40 ncm | Internal V | Y | 9.6mm | 10.5 mm | 14 | Nil |
| 8 | F | 81 | N | Yes | Y | 1 cc | 8 mm | 8 mm | Anyridge | 55 ncm | Internal D | Y | 10.7mm | 11.2 mm | 9 | Nil |
| 9 | M | 57 | N | Yes | Y | 0.5 cc | 7 mm | 6.4 mm | Anyridge | 45 `ncm | Internal D/V | Y | 11.65mm | 11.4 mm | 10 | Perio, distal |
| 10 | F | 92 | Y | Yes | Y | 1 cc | 6 mm | 7 mm | Anyridge | 45 `ncm | Internal D | Y | 9mm | 9.2 mm | 9 | Nil |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fairbairn, P.; Kilner, S.; O’Hooley, D.; Fish, A.; Kurtzman, G.M. Sinus Augmentation for Implant Placement Utilizing a Novel Synthetic Graft Material with Delayed Immediate Socket Grafting: A 2-Year Case Study. J. Clin. Med. 2023, 12, 2485. https://doi.org/10.3390/jcm12072485
Fairbairn P, Kilner S, O’Hooley D, Fish A, Kurtzman GM. Sinus Augmentation for Implant Placement Utilizing a Novel Synthetic Graft Material with Delayed Immediate Socket Grafting: A 2-Year Case Study. Journal of Clinical Medicine. 2023; 12(7):2485. https://doi.org/10.3390/jcm12072485
Chicago/Turabian StyleFairbairn, Peter, Stuart Kilner, Dominic O’Hooley, Andrew Fish, and Gregori M. Kurtzman. 2023. "Sinus Augmentation for Implant Placement Utilizing a Novel Synthetic Graft Material with Delayed Immediate Socket Grafting: A 2-Year Case Study" Journal of Clinical Medicine 12, no. 7: 2485. https://doi.org/10.3390/jcm12072485
APA StyleFairbairn, P., Kilner, S., O’Hooley, D., Fish, A., & Kurtzman, G. M. (2023). Sinus Augmentation for Implant Placement Utilizing a Novel Synthetic Graft Material with Delayed Immediate Socket Grafting: A 2-Year Case Study. Journal of Clinical Medicine, 12(7), 2485. https://doi.org/10.3390/jcm12072485






