Abstract
This study aimed to analyze the differences in severity and clinical characteristics of COVID-19 in infants hospitalized in Poland in 2021, when the dominance of variants of concern (VOCs) alpha and delta was reported, compared to 2020, when original (wild) SARS-CoV-2 was dominant (III–IV vs. I–II waves of the pandemic, respectively). In addition, the influence of the presence of comorbidities on the clinical course of COVID-19 in infants was studied. This multicenter study, based on the pediatric part of the national SARSTer database (SARSTer-PED), included 940 infants with COVID-19 diagnosed between March 1, 2020, and December 31, 2021, from 13 Polish inpatient centers. An electronic questionnaire, which addressed epidemiological and clinical data, was used. The number of hospitalized infants was significantly higher in 2021 than in 2020 (651 vs. 289, respectively). The analysis showed similar lengths of infant hospitalization in 2020 and 2021, but significantly more children were hospitalized for more than 7 days in 2020 (p < 0.009). In both analyzed periods, the most common route of infection for infants was household contact. There was an increase in the percentage of comorbidities, especially prematurity, in children hospitalized in 2021 compared to 2020. Among the clinical manifestations, fever was predominant among children hospitalized in 2021 and 2020. Cough, runny nose, and loss of appetite were significantly more frequently observed in 2021 (p < 0.0001). Severe and critical conditions were significantly more common among children with comorbidities. More infants were hospitalized during the period of VOCs dominance, especially the delta variant, compared to the period of wild strain dominance, even though indications for hospitalization did not include asymptomatic patients during that period. The course of COVID-19 was mostly mild, characterized mainly by fever and respiratory symptoms. Comorbidities, particularly from the cardiovascular system and prematurity, were associated with a more severe course of the disease in infants.
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the etiologic agent of coronavirus disease 2019 (COVID-19), rapidly spread worldwide, causing a global pandemic. According to the data published by the National Institute of Public Health NIH—National Research Institute, there were 2,834,287 cases of COVID-19 reported in Poland in 2020 and 1,289,293 in 2021 [1]. The proportion of infected children remains unknown. Although the effects of COVID-19 have been more significant in adults, children are also infected with SARS-CoV-2, and COVID-19 can lead to severe outcomes in pediatric patients, such as severe multisystem inflammatory syndrome in children (MIS-C) [2]. According to the study by Nikolopoulou et al., serologic surveys indicate that half of the children who tested positive for SARS-CoV-2 reported no symptoms, and children with COVID-19 are at lower risk of hospitalization and life-threatening complications compared to adults [3].
Our previous analysis of the clinical course of COVID-19 in 300 infants, selected from 1283 children diagnosed with COVID-19 between March and December 2020, showed that COVID-19 in infants usually manifests as a mild gastrointestinal or respiratory infection, but pneumonia is also observed with falls in oxygen saturation, requiring oxygen therapy. Gastrointestinal symptoms are common in infants infected with SARS-CoV-2, and loss of appetite in infants may lead to hospitalization [4].
Symptoms associated with SARS-CoV-2 infection are generally milder in children than in adults. In addition, several risk factors for severity have been identified. Schober et al., who performed multivariable ordinal logistic regression analyses adjusted for age, chest imaging findings, laboratory-confirmed bacterial and/or viral coinfection, and MIS-C diagnosis in 403 hospitalized children in a median age of 3.78 years, revealed that the presence of comorbidities, obesity, and chromosomal disorders are independent risk factors for COVID-19 severity. Age was not an independent risk factor, but different age-specific comorbidities were associated with more severe disease in age-stratified adjusted analyses: cardiac and non-asthma pulmonary disorders in children <12 years old and obesity in adolescents ≥12 years old. Among infants < 1 year old, neurological and cardiac disorders were independent predictors of severe disease [5].
Since the start of the SARS-CoV-2 pandemic, children aged ≤12 years have always been defined as underrepresented in terms of SARS-CoV-2 infections’ frequency and severity [6,7]. Increased transmissibility across all age groups has been reported for SARS-CoV-2 variants of concern (VOCs), including the delta and omicron variants [8]. According to our recent observations, it seems that infants may play a particular role in the transmission of SARS-COV-2 infections in households, despite mild or asymptomatic courses [4].
Alteri et al., correlating SARS-CoV-2 transmission dynamics with clinical and virological features in 612 SARS-CoV-2 positive patients aged ≤12 years, demonstrated a sizeable circulation of different SARS-CoV-2 lineages over the four pandemic waves in the pediatric population, sustained by local transmission chains. They revealed that age <5 years, the highest viral load, and gamma and delta clades positively influence this local transmission. No correlations between COVID-19 manifestations and lineages or transmission chains are seen, except for a negative correlation between B.1.1.7 and hospitalization [9].
This study aimed to analyze the differences in severity and clinical characteristics of COVID-19 in infants hospitalized in Poland in 2021, when the dominance of VOCs alpha and delta was reported, compared to 2020, when original (wild) SARS-CoV-2 was dominant (III–IV vs. I–II waves of the pandemic, respectively). In addition, the influence of the presence of comorbidities on the clinical course of COVID-19 in infants was studied.
2. Material and Methods
The multicenter pediatric part of the national SARSTer database (SARSTer-PED) includes clinical and epidemiological data on children and adolescents (0 to 18 years) hospitalized in Poland with COVID-19 and diagnosed between 1 March 2020 and 31 December 2021. Fourteen Polish inpatient centers dedicated to pediatric patients with COVID-19 reported their consecutive cases using an electronic questionnaire. In this study, we extracted data on infants (0–12 months), which were available from 13 centers. Diagnosis of COVID-19 was based either on a positive real-time polymerase chain reaction (RT-PCR) or antigen testing on a nasopharyngeal swab performed in certified diagnostics laboratories. Epidemiologic data included known exposure to a person with confirmed SARS-CoV-2 infection (in the household or otherwise), the duration of symptoms before presentation, duration of hospitalization, and any chronic comorbidity. As immunization against COVID-19 for children younger than 5 years of age was introduced in 2022, the vaccination was not available for infants in the studied period and, thus, was not analyzed. We recorded all clinical symptoms present at the time of admission or during hospitalization. We divided the analyzed period into two parts: the first from 1 March 2020 to 31 December 2020 (which corresponded to the first and the second waves of pandemic, when original, wild SARS-CoV-2 dominated), and the second from 1 January 2021 to 31 December 2021 (corresponding to the third and fourth waves, with a dominance of B.1.1.7—alpha, and B.1.617.2—delta variants).
The clinical course of COVID-19 was defined as follows: grade 0—asymptomatic, when no complaints or symptoms were present, and no abnormalities were found on physical examination; grade 1—mild, when signs of upper respiratory tract infection were present, with or without fever and other complaints, but without pneumonia; grade 2—moderate, when pneumonia without hypoxemia was present; grade 3—severe, when pneumonia manifesting with dyspnea and oxygen desaturation <94% was present; and grade 4—critical, when acute respiratory distress syndrome, shock, or any organ failure occurred.
The indications for hospitalization were mainly based on the clinical status of the child; however, there was a trend for referring to a hospital for all infants below 6 months of age with confirmed SARS-CoV-2 infection. In addition, during the first months of the pandemic, testing for COVID-19 was available in Poland only in hospital settings; thus, children with suspected SARS-CoV-2 infection were referred to the hospital for confirmation of the infection, irrespective of their clinical presentation.
Statistical analysis was performed using MedCalc Statistical Software version 20.123 (MedCalc, Ostend, Belgium, https://www.medcalc.org, accessed on 10 November 2022). A two-sided p-value <0.05 was considered significant. Continuous variables were presented as numbers (percentages) or the medians with interquartile ranges (IQRs) and were compared using the Mann–Whitney test. Categorical variables were presented as numbers with percentages and were compared using the chi-square test.
Ethical Statement
This study was performed in accordance with the ethical standards presented in the 1964 Declaration of Helsinki and its later amendments. It was approved by the local ethics committee of the Regional Medical Chamber in Warsaw (No KB/1270/20; date of approval: 3 April 2020).
3. Results
3.1. Study Group
Among 2771 pediatric and 9458 adult patients with COVID-19 included in the SARSTer-PED database during 2020 and 2021, there were 940 infants (aged 0–12 months): 289 were hospitalized in 2020 and 651 in 2021 (Figure 1). Demographic and epidemiological characteristics of infants included in the study are presented in Table 1. There were no significant differences between the patients hospitalized in 2021 and 2020 concerning the median age, the proportion of hospitalized newborns (0–28 days), sex, source of infection, number of children with comorbidities, and duration of hospitalization (Table 1). However, fewer patients were hospitalized for over 7 days in 2021 compared to 2020 (16% vs. 22%, p = 0.009). The most commonly reported potential source of infection in infants was an infected family member (mainly a parent), which was reported in 54% of cases (52% in 2021 and 58% in 2020). Among the studied infants, 107 (11%) suffered from comorbidities, with cardiovascular diseases and prematurity as the most common (Table 2).
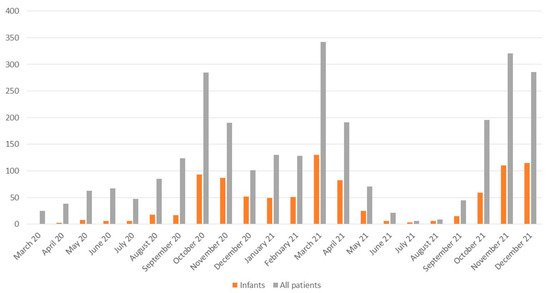
Figure 1.
Number of infants diagnosed with COVID-19 in the subsequent months of 2020 and 2021 included in the analysis among the total number of hospitalized pediatric patients (0–18 years).

Table 1.
Demographic and epidemiological characteristics of 940 infants included in the study.

Table 2.
Comorbidities among infants included in the study group.
3.2. Clinical Presentation of COVID-19
The clinical symptoms of COVID-19 in the studied group are presented in Table 3. The most commonly reported symptom was fever (>38 °C), both in 2021 (64%) and 2020 (66%). Cough, rhinitis, loss of appetite, and dyspnea were more frequently reported among infants hospitalized in 2021 compared to those in 2020 (Table 3), whereas no significant differences were found concerning other reported symptoms. There were fewer asymptomatic children hospitalized in 2021 compared to 2020 (3% vs. 10%, p < 0.0001), which may be due to different indications for hospitalization in both these periods. In a total of 209 (22%) hospitalized children, pneumonia was diagnosed based on clinical evaluation or a chest X-ray examination, without significant differences among infants hospitalized in 2021 (21%) and 2020 (24%). One six-month-old infant hospitalized in December 2021 died due to respiratory failure. The child suffered from underlying diseases, including microsomia, hypotrophia, Hashimoto disease, and suspicion of interstitial lung disease. This one case makes the fatality rate 0.15% in 2021 and 0.11% overall.

Table 3.
Clinical symptoms of COVID-19 in the study group.
3.3. Severity of COVID-19
The clinical course of COVID-19 in this cohort was most frequently described as mild (71%), both in 2021 (75%) and in 2020 (65%). However, there was a significant difference in the clinical course assessment between 2020 and 2021, with more severely ill infants in 2021 and more asymptomatic patients in 2020 (admitted mainly during the first weeks of the pandemic for epidemiological reasons; Table 4). In total, 4% of patients in our cohort were reported as severely or critically ill (grades 3 to 4), including 4% of the participants hospitalized in 2021 and 3% in 2020. We found a significant influence of the underlying comorbidities on the clinical course of the disease (Table 5). Infants with comorbidities were more prone to present with a severe or critical course of COVID-19 (grade 3 to 4) compared to patients without any comorbidity, which was observed for the whole cohort (10% vs. 3%, p = 0.0002) for infants hospitalized in 2021 (11% vs. 3%, p = 0.01) and in 2020 (9% vs. 2%, p = 0.01).

Table 4.
The clinical course of COVID-19.

Table 5.
The clinical course of COVID-19 according to the presence of comorbidities.
3.4. Treatment
Most infants received symptomatic treatment. Only three patients hospitalized in 2021 received the anti-SARS-CoV-2 treatment with remdesivir (Table 6). About one-third of patients (37% in 2021 and 30% in 2020; p = 0.03) received empirical antibiotics due to accompanying bacterial infections. Only a small proportion of infants required oxygen therapy (including high-flow nasal oxygen), mechanical ventilation, as well as treatment in the intensive care unit (Table 6).

Table 6.
Treatment of COVID-19 implemented in the study group in 2020 and 2021.
4. Discussion
The COVID-19 pandemic causes a progressive increase in childhood morbidity worldwide. Many reports indicate that the clinical picture may vary depending on the circulating strain of the virus [10,11,12]. It has been proven that successive variants of SARS-CoV-2 differ in their infectivity and induce a specific clinical course of COVID-19 in all age groups. This study attempts to answer the question of whether infection with specific variants of SARS-CoV-2 determines its course in infants. We compared the epidemiology and clinical features of COVID-19 in infants hospitalized in 2020 and 2021 based on observations from 13 centers in Poland. Available epidemiological data show that the SARS-CoV-2 wild-type strain dominated in 2020, while VOCs emerged in 2021, with alpha (B.1.1.7) dominating in the first half of the year and delta (B.1.617.2) in the second half of 2021 [13]. In the retrospective analysis of 945 Ukrainian children hospitalized due to COVID-19 from April 2020 to February 2022, children aged 1–12 months predominated during wave IV caused by the delta variant [14].
Among the clinical manifestations of COVID-19 in infants, fever was predominant among both the children hospitalized in 2021 and those in 2020. Cough, rhinitis, and loss of appetite were significantly more frequently observed in 2021 (p < 0.0001). A similar course of COVID-19 in infants was described by Canadian authors [15]. The course of the disease was mild in most of the children we analyzed, although infants hospitalized in 2020 were more likely to have an asymptomatic course of SARS-CoV-2 infection, which could be due to the early period of the pandemic and slightly different indications for hospitalization.
In 3% of children hospitalized in 2021, the clinical course of the disease was classified as severe, in 1% as critical; in 2020, these numbers were 2% and 1%, respectively. These data are consistent with the observations of Canadian authors. Our study confirmed that severe and critical conditions were significantly more common in children with comorbidities, especially cardiovascular disease. Schober et al. also highlight the impact of comorbidities on the severity of COVID-19 in infants. Piche-Renoaud et al., based on an analysis of SARS-CoV-2 infection in 531 children, also highlight comorbidities and the younger age of children as risk factors for a more severe course of COVID-19 [15].
Chronic comorbidities pose a risk factor for the severe course of COVID-19 in both adult and pediatric patients and consequently increase hospitalization rates. Numerous meta-analyses showed a progressive increase in the number of hospitalizations of children with comorbidities during subsequent waves of the epidemic compared to the initial period of the pandemic [15,16]. Schober et al., in an age-adjusted multivariate logistic regression analysis, showed that comorbidities were one of the independent risk factors for a severe course of COVID-19 in children [5]. Age was not an independent risk factor, but various age-dependent comorbidities were associated with a more severe course of the disease in age-adjusted analyses: heart and lung disease in children <12 years of age and obesity in children >12 years of age. Among infants, neurological disorders and cardiac conditions were independent predictors of severe disease. In our study, the most common comorbidities included cardiovascular diseases and prematurity, then allergies, kidney diseases, lung diseases, genetically determined diseases, and gastrointestinal diseases. Their increase was also identified in the percentage of children hospitalized in 2021 compared to 2020. In 2021, immunosuppressive conditions, diabetes, and cancer were additionally observed among comorbidities. The high percentage of preterm births (21%) among children hospitalized in 2021 may reflect the impact of the pandemic on pregnant women and infections in this group caused by variants with higher infectivity. Preterm labor is one of the more common complications of SARS-CoV-2 infection in pregnant women, and the number of vaccinated pregnant women in 2021 was relatively low [17,18].
In our study, the number of hospitalized infants was significantly higher in 2021 than in 2020 (651 vs. 289, respectively). In contrast to the observations of Schober et al., there was no increase in the number of SARS-CoV-2 infections in newborns, which accounted for about 30% of hospitalized infants in the cited study [5]. It is estimated that about 7% of patients with COVID-19 in Poland require hospitalization [1]. However, data on the proportion of hospitalized children are unavailable. A study from the U.S. conducted between 1 March 2020, and 14 August 2021, identified 49.7 COVID-19-related hospitalizations per 100,000 children and adolescents. The highest rates were reported for children aged 0–4 years (69.2/100,000). During the period of delta variant dominance, the weekly rate of hospitalizations due to COVID-19 among children aged 0–4 years was almost 10 times higher compared to the period before delta dominance [19].
Our analysis showed similar lengths of infant hospitalization in 2020 and 2021, but significantly more children were hospitalized for longer than 7 days in 2020 (p < 0.009). Similar observations on the shortening of hospitalization were described by Ukrainian authors [14]. It seems that with the increase in the duration of the pandemic, some knowledge and experience in the management of patients infected with SARS-CoV-2 was acquired, which was reflected in the development of recommendations and, consequently, updating of routine clinical practice also with regard to indications and duration of hospitalization. In addition, at the onset of the 2020 pandemic, infants with COVID-19 with an asymptomatic course were also hospitalized for epidemiological reasons.
The dependence of infection prevalence in groups of the youngest children on specific SARS-CoV-2 variants was also confirmed by other authors regarding the Omicron variant wave [20,21]. Between December 2021 and February 2022, the overall seroprevalence in the U.S. increased from 33.5% to 57.7%, whereas in the group of children aged 0–11 years, it increased from 44.2% to 75.2%. As of February 2022, about 75% of children and adolescents had serologic evidence of prior SARS-CoV-2 infection, and about one-third had become seropositive as of December 2021. These findings illustrate the high rate of Omicron variant infection, especially among children [20]. Franczak et al., evaluating the seroprevalence of SARS-CoV-2 IgG antibodies in 686 children aged 2 weeks to 18 years hospitalized for reasons other than COVID-19, showed the presence of these antibodies in 392 (57%) children. As of December 2021, an increase in the percentage of children with positive anti-SARS-CoV-2 antibody titters was observed, to 87.5% of patients hospitalized in April 2022. 69% of the children with detected antibodies were under 5 years old. The study showed an increase in the percentage of those children during the fourth and fifth waves of COVID-19 in Poland caused by the delta and omicron variants, respectively. The vast majority of parents of the studied children had no knowledge of their offspring being infected with COVID-19, which may indicate an asymptomatic or mild course of the disease [21].
Our study group showed no significant differences in the gender distribution of hospitalized children, with a slight consistent predominance of boys (56% in both 2020 and 2021), which corresponds to Sierakova’s observations of Ukrainian children [14]. In both periods analyzed, the most common route of infection for infants was family contact, confirmed in a slightly higher percentage of infants hospitalized in 2020 (58% and 52%, respectively). These observations are also confirmed by Canadian authors, who, based on an analysis of 531 infants infected with SARS-CoV-2, confirmed family contact in 69% of infants hospitalized for COVID-19 [15].
In the treatment of the analyzed infants, mainly antipyretics were used, and patient hydration was carried out. Only three patients hospitalized in 2021 required remdesivir, 30 (5%) required oxygen therapy, 44 (7%) received systemic corticosteroids, and 141 (21%) received corticosteroid inhalation. One child required mechanical ventilation and transfer to the Intensive Care Unit. Azithromycin was used significantly less often than in 2020, which was due to the updated recommendations for the management of a child infected with SARS-CoV-2 [22].
This paper is one of the few studies of SARS-CoV-2 infections in infants available and, to our knowledge, the first one covering such an extensive material of 940 children. This research, however, has some limitations, such as the lack of determination of SARS-CoV-2 variants in samples from individual patients and the use of the data on the identification of individual strains from epidemiological surveillance. Another limitation is that the analyses were performed only in a group of hospitalized children.
Concluding, more infants were hospitalized during the period of dominance of VOCs, especially the delta variant, compared to the period of dominance of the wild strain, despite the fact that indications for hospitalization did not include hospitalization of asymptomatic patients in that period. The course of COVID-19 was mild in most cases, characterized mainly by fever and respiratory symptoms. The presence of comorbidities, particularly on the cardiovascular side and prematurity, was associated with a more severe course of the disease in infants.
Author Contributions
Conceptualization and methodology, M.P. (Małgorzata Pawłowska), M.P.-Ś., E.T. and M.M.; formal analysis, M.P. (Małgorzata Pawłowska) and M.P.-Ś.; interpretation of the data, M.P. (Małgorzata Pawłowska), M.P.-Ś. and A.M.; Investigation, M.P. (Małgorzata Pawłowska), M.P.-Ś., E.T., A.M., B.H., E.Ż.-P., M.S. (Magdalena Stankiewicz), M.S. (Martyna Stani), P.F.-C., L.S., I.Z., J.C., E.M.-S., U.D., K.G., M.F., K.M.-M., K.F., P.C., M.P. (Michał Peregrym), J.Ł.-Z., J.R., B.S., I.P.-B., E.R., D.H.-N., K.D.-G., A.S. (Adam Sybilski), I.K., J.F., M.S.-P., E.K., M.W., M.P. (Maria Paryż), B.K., K.T., A.S. (Artur Sulik) and S.N.; writing—original draft preparation, review and editing, M.P. (Małgorzata Pawłowska), M.P.-Ś., E.T., A.M., R.F. and M.M.; visualization, M.P.-Ś.; supervision, R.F. and M.M.; funding acquisition, M.P. (Małgorzata Pawłowska) and R.F. All authors have collected the data. All authors have read and agreed to the published version of the manuscript.
Funding
Medical Research Agency, grant number 2020/ABM/COVID-19/PTEILCHZ, and the Polish Association of Epidemiologists and Infectiologists.
Institutional Review Board Statement
The study was conducted according to the guidelines standards of the Declaration of Helsinki and approved by the Ethics Committee of the Regional Medical Chamber in Warsaw (No KB/1270/20; date of approval: 3 April 2020).
Informed Consent Statement
Due to the retrospective nature of the study, a waiver of consent was in effect.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- National Institute of Public Health NIH—National Research Institute. Epidemiological Reports. Available online: http://wwwold.pzh.gov.pl/oldpage/epimeld/index_p.html (accessed on 9 March 2023).
- Godfred-Cato, S.; Bryant, B.; Leung, J.; Oster, M.E.; Conklin, L.; Abrams, J.; Roguski, K.; Wallace, B.; Prezzato, E.; Koumans, E.H.; et al. California MIS-C Response Team. COVID-19-associated multisystem inflammatory syndrome in children—United States, March–July 2020. MMWR Morb. Mortal Wkly. Rep. 2020, 69, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Nikolopoulou, G.B.; Maltezou, H.C. COVID-19 in Children: Where do we Stand? Arch. Med. Res. 2022, 53, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sobolewska-Pilarczyk, M.; Pokorska-Śpiewak, M.; Stachowiak, A.; Marczyńska, M.; Talarek, E.; Ołdakowska, A.; Kuckarek, I.; Sybilski, A.; Mania, A.; Figlerowicz, M.; et al. COVID-19 infections in infants. Sci. Rep. 2022, 12, 7765. [Google Scholar] [CrossRef] [PubMed]
- Schober, T.; Caya, C.; Barton, M.; Bayliss, A.; Bitnun, A.; Bowes, J.; Brenes-Chacon, H.; Bullard, J.; Cooke, S.; Dewan, T.; et al. Risk factors for severe PCR-positive SARS-CoV-2 infection in hospitalized children. BMJ Paediatr. Open 2022, 6, e001440. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhang, L.; Du, H.; Zhang, J.; Li, Y.Y.; Qu, J.; Zhang, W.; Wang, Y.; Bao, S.; Li, Y.; et al. SARS-CoV-2 Infection in Children. N. Engl. J. Med. 2020, 382, 1663–1665. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020, 109, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Howard-Jones, A.R.; Bowen, A.C.; Danchin, M.; Koirala, A.; Sharma, K.; Yeoh, D.K.; Burgner, D.P.; Crawford, N.W.; Goeman, E.; Gray, P.E.; et al. COVID-19 in children: I. Epidemiology, prevention and indirect impacts. J. Paediatr. Child Health 2022, 58, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Alteri, C.; Scutari, R.; Costabile, V.; Colagrossi, L.; La Rosa, K.Y.; Agolini, E.; Lanari, V.; Chiurchiù, S.; Romani, L.; Merkowich, A.H.; et al. Epidemiological characterization of SARS-CoV-2 variants in children over the four COVID-19 waves and correlation with clinical presentation. Sci. Rep. 2022, 12, 10194. [Google Scholar] [CrossRef] [PubMed]
- Quintero, A.M.; Eisner, M.; Sayegh, R.; Wright, T.; Ramilo, O.; Leber, A.L.; Wang, H.; Mejias, A. Differences in SARS-CoV-2 Clinical Manifestations and Disease Severity in Children and Adolescents by Infecting Variant. Emerg. Infect. Dis. 2022, 28, 2270–2280. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, E.A.; Simões Silva, A.C.; Oliveira, M.C.; Colosimo, E.A.; Mak, R.H.; Vasconcelos, M.A.; Miranda, D.M.; Martelli, D.B.; Silva, L.R.; Pinhati, C.C.; et al. Comparison of the First and second waves of the coronavirus disease 2019 (COVID-19) pandemic in children and adolescents in a middle-income country: Clinical impact associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) gamma lineage. J. Pediatr. 2022, 244, P178–P185. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.; Holfter, A.; Ruebsteck, E.; Gruell, H.; Dewald, F.; Koerner, R.W.; Klein, F.; Lehmann, C.; Huenseler, C.; Weber, L.T. The Alpha Variant (B.1.1.7) of SARS-CoV-2 in children: First experience from 3544 nucleic acid amplification tests in a cohort of children in Germany. Viruses 2021, 13, 1600. [Google Scholar] [CrossRef] [PubMed]
- GISAID Database. Available online: https://nextstrain.org/groups/neherlab/ncov/poland (accessed on 10 December 2022).
- Seriakova, I.; Yevtushenko, V.; Kramarov, S.; Palatna, L.; Shpak, I.; Kaminska, T. Clinical course of COVID-19 in hospitalized children of Ukraine in different pandemic periods. Eur. Clin. Respir. J. 2022, 9, 2139890. [Google Scholar] [CrossRef] [PubMed]
- Piché-Renaud, P.P.; Panetta, L.; Farrar, D.L. Clinical manifestations and disease severity of SARS-CoV-2 infection among infants in Canada. PLoS ONE 2022, 17, e0272648. [Google Scholar] [CrossRef] [PubMed]
- Irfan, O.; Muttalib, F.; Tang, K.; Jiang, L.; Lasii, Z.S.; Bhutta, Z. Clinical characteristics, treatment and outcomes of paediatric COVID-19: A systematic review and meta-analysis. Arch. Dis. Child. 2021, 106, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Falsaperla, R.; Leone, G.; Familiari, M.; Ruggieri, M. COVID-19 vaccination in pregnant and lactating women: A systematic review. Expert Rev. Vaccines 2021, 20, 1619–1628. [Google Scholar] [CrossRef] [PubMed]
- Sarantaki, A.; Kalogeropoulou, V.A.; Taskou, C.; Nanou, C.; Lykeridou, A. COVID-19 Vaccination and Related Determinants of Hesitancy among Pregnant Women: A Systematic Review and Meta-Analysis. Vaccines 2022, 10, 2055. [Google Scholar] [CrossRef] [PubMed]
- Delahoy, M.J.; Ujamaa, D.; Whitaker, M.; O’Halloran, A.; Anglin, O.; Burns, E.; Cummings, C.; Holstein, R.; Kambhampati, A.K.; Milucky, J.; et al. Hospitalizations Associated with COVID-19 Among Children and Adolescents—COVID-NET, 14 States, March 1, 2020–August 14, 2021. Morb. Mortal. Wkly. Rep. 2021, 70, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- Clarke, K.; Jones, J.M.; Deng, Y.; Nycz, E.; Lee, A.; Iachan, R.; Gundlapalli, A.V.; Hall, A.J.; MacNeil, A. Seroprevalence of Infection-Induced SARS-CoV-2 Antibodies—United States, September 2021-February 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 606–608. [Google Scholar] [CrossRef] [PubMed]
- Franczak, J.; Moppert, J.; Sobolewska-Pilarczyk MPawłowska, M. The Seroprevalence of SARS-CoV-2 IgG Antibodies in Children Hospitalized for Reasons Other Than COVID-19. J. Clin. Med. 2022, 11, 3819. [Google Scholar] [CrossRef] [PubMed]
- Marczyńska, M.; Pokorska-Śpiewak, M.; Talarek, E.; Figlerowicz, M.; Kalicki, B.; Kuchar, E.; Majda-Stanisławska, E.; Pawłowska, M.; Sulik, A.; Sybilski, A.; et al. Management of a Child with COVID-19 recommendations for pediatricians and family medicine physicians in primary healthcare and hospital settings. Przegl. Ped. 2020, 49, 10–16. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).