Topical Application of Peptide Nucleic Acid Antisense Oligonucleotide for MMP-1 and Its Potential Anti-Aging Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of PNA-20 CEF
2.2. Cell Culture
2.3. Enzyme-Linked Immunosorbent Assay (ELISA)
2.4. Quantitative Reverse Transcription-PCR (qRT-PCR)
2.5. Western Blotting
2.6. Immunofluorescence Staining
2.7. Participants
2.8. Evaluation of Clinical Efficacy
2.9. Statistical Analysis
3. Results
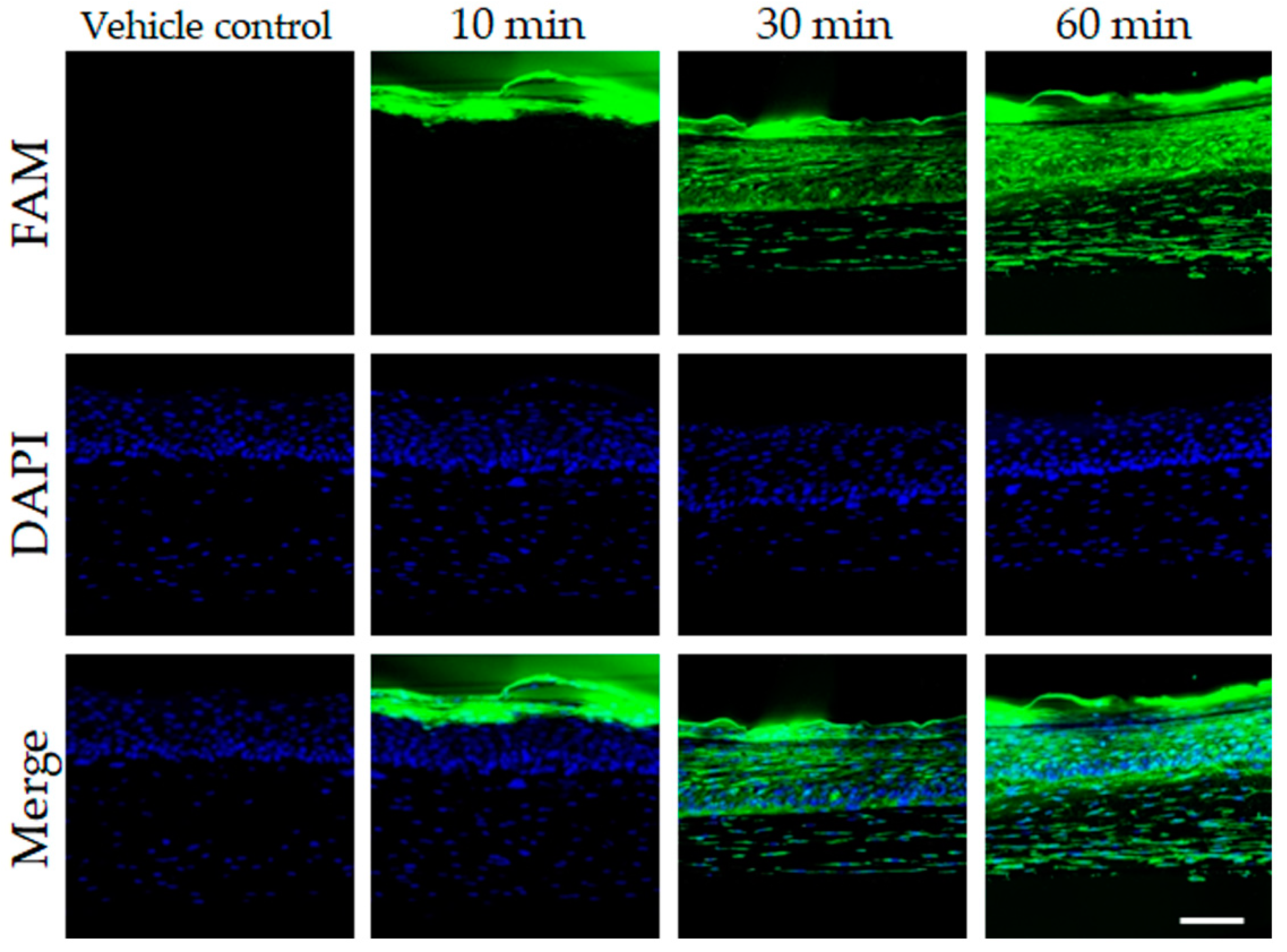
3.1. In Vitro and 3D Skin Assessments of the Cellular Anti-Aging Effect of PNA-20 CEF
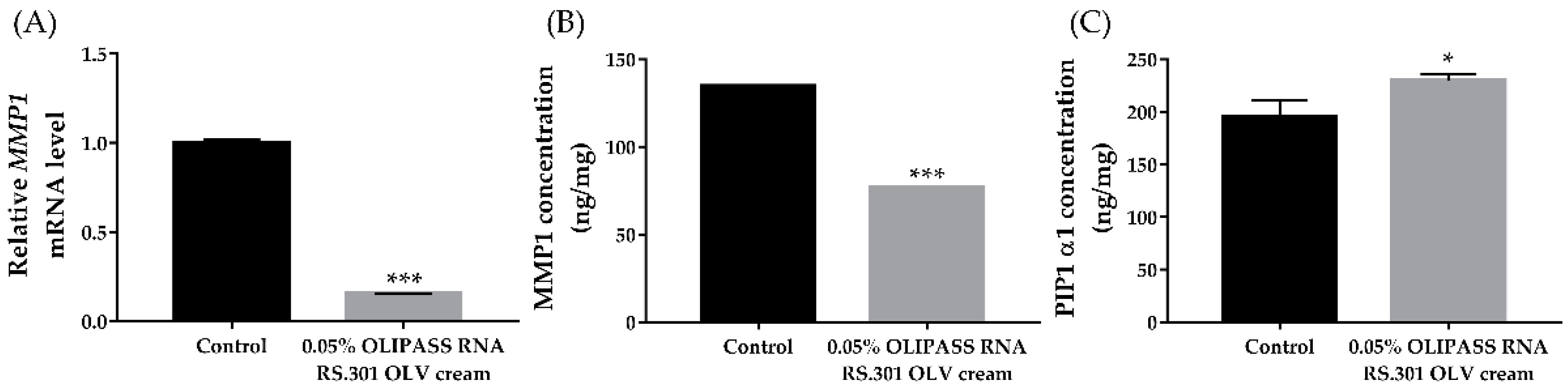
3.2. In Vitro Assessment of the Cellular Anti-Aging Effect of OliPass RNA RS.301 OLV Cream
3.3. Participant Characteristics of the Clinical Trial
3.4. Clinical Efficacy of OliPass RNA RS.301 OLV Cream on Skin Aging
3.5. Assessment of Safety and Adverse Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, Y.I.; Choi, S.; Roh, W.S.; Lee, J.H.; Kim, T.G. Cellular senescence and inflammaging in the skin microenvironment. Int. J. Mol. Sci. 2021, 22, 3849. [Google Scholar] [CrossRef] [PubMed]
- Calles, C.; Schneider, M.; Macaluso, F.; Benesova, T.; Krutmann, J.; Schroeder, P. Infrared a radiation influences the skin fibroblast transcriptome: Mechanisms and consequences. J. Investig. Dermatol. 2010, 130, 1524–1536. [Google Scholar] [CrossRef]
- Schroeder, P.; Calles, C.; Krutmann, J. Prevention of infrared-a radiation mediated detrimental effects in human skin. Ski. Ther. Lett. 2009, 14, 4–5. [Google Scholar]
- Krutmann, J.; Schikowski, T.; Morita, A.; Berneburg, M. Environmentally-induced (extrinsic) skin aging: Exposomal factors and underlying mechanisms. J. Investig. Dermatol. 2021, 141, 1096–1103. [Google Scholar] [CrossRef]
- Milosheska, D.; Roškar, R. Use of Retinoids in Topical Antiaging Treatments: A Focused Review of Clinical Evidence for Conventional and Nanoformulations. Adv. Ther. 2022, 39, 5351–5375. [Google Scholar] [CrossRef] [PubMed]
- Enescu, C.D.; Bedford, L.M.; Potts, G.; Fahs, F. A review of topical vitamin C derivatives and their efficacy. J. Cosmet. Dermatol. 2022, 21, 2349–2359. [Google Scholar] [CrossRef]
- Lee, Y.I.; Lee, S.G.; Jung, I.; Suk, J.; Lee, M.H.; Kim, D.U.; Lee, J.H. Effect of a Topical Collagen Tripeptide on Antiaging and Inhibition of Glycation of the Skin: A Pilot Study. Int. J. Mol. Sci. 2022, 23, 1101. [Google Scholar] [CrossRef]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef]
- Panwar, P.; Butler, G.S.; Jamroz, A.; Azizi, P.; Overall, C.M.; Bromme, D. Aging-associated modifications of collagen affect its degradation by matrix metalloproteinases. Matrix Biol. 2018, 65, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Fagot, D.; Asselineau, D.; Bernerd, F. Matrix metalloproteinase-1 production observed after solar-simulated radiation exposure is assumed by dermal fibroblasts but involves a paracrine activation through epidermal keratinocytes. Photochem. Photobiol. 2004, 79, 499–505. [Google Scholar] [CrossRef]
- Lee, J.E.; Oh, J.; Song, D.; Lee, M.; Hahn, D.; Boo, Y.C.; Kang, N.J. Acetylated resveratrol and oxyresveratrol suppress uvb-induced mmp-1 expression in human dermal fibroblasts. Antioxidants 2021, 10, 1252. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.I.; Lee, S.G.; Kim, E.; Jung, I.; Suk, J.; Kim, J.; Lee, J.H. Anti-aging effect of an oral disintegrating collagen film: A prospective, single-arm study. Int. J. Dermatol. 2022, 61, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.H.; Rhie, S.J.; Kim, Y.C. Antioxidant and skin anti-aging effects of marigold methanol extract. Toxicol. Res. 2018, 34, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Oh, J.; Averilla, J.N.; Kim, H.J.; Kim, J.S.; Kim, J.S. Grape peel extract and resveratrol inhibit wrinkle formation in mice model through activation of nrf2/ho-1 signaling pathway. J. Food Sci. 2019, 84, 1600–1608. [Google Scholar] [CrossRef]
- Patel, R.R.; Sundin, G.W.; Yang, C.H.; Wang, J.; Huntley, R.B.; Yuan, X.; Zeng, Q. Exploration of using antisense peptide nucleic acid (pna)-cell penetrating peptide (cpp) as a novel bactericide against fire blight pathogen erwinia amylovora. Front. Microbiol. 2017, 8, 687. [Google Scholar] [CrossRef]
- Coull, J.M.; Egholm, M.; Hodge, R.P.; Ismail, M.; Rajur, S.B. Improved Synthons for the Synthesis and Deprotection of Peptide Nucleic Acids under Mild Conditions. EP0840736B1, 7 October 2009. p. 138. [Google Scholar]
- Marchesi, E.; Bovolenta, M.; Preti, L.; Capobianco, M.L.; Mamchaoui, K.; Bertoldo, M.; Perrone, D. Synthesis and exon-skipping properties of a 3′-ursodeoxycholic acid-conjugated oligonucleotide targeting dmd pre-mrna: Pre-synthetic versus post-synthetic approach. Molecules 2021, 26, 7662. [Google Scholar] [CrossRef]
- Tang, Z.; Zhao, J.; Pearson, Z.J.; Boskovic, Z.V.; Wang, J. Rna-targeting splicing modifiers: Drug development and screening assays. Molecules 2021, 26, 2263. [Google Scholar] [CrossRef] [PubMed]
- Staroseletz, Y.; Gaponova, S.; Patutina, O.; Bichenkova, E.; Amirloo, B.; Heyman, T.; Chiglintseva, D.; Zenkova, M. Site-selective artificial ribonucleases: Renaissance of oligonucleotide conjugates for irreversible cleavage of rna sequences. Molecules 2021, 26, 1732. [Google Scholar] [CrossRef]
- Ono, Y.; Torii, K.; Fritsche, E.; Shintani, Y.; Nishida, E.; Nakamura, M.; Shirakata, Y.; Haarmann-Stemmann, T.; Abel, J.; Krutmann, J.; et al. Role of the aryl hydrocarbon receptor in tobacco smoke extract-induced matrix metalloproteinase-1 expression. Exp. Dermatol. 2013, 22, 349–353. [Google Scholar] [CrossRef]
- Liebel, F.; Kaur, S.; Ruvolo, E.; Kollias, N.; Southall, M.D. Irradiation of skin with visible light induces reactive oxygen species and matrix-degrading enzymes. J. Investig. Dermatol. 2012, 132, 1901–1907. [Google Scholar] [CrossRef]
- Kim, H.; Jang, J.; Song, M.J.; Kim, G.; Park, C.H.; Lee, D.H.; Lee, S.H.; Chung, J.H. Attenuation of intrinsic ageing of the skin via elimination of senescent dermal fibroblasts with senolytic drugs. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Lee, S.N.; Kim, K.; Joo, D.H.; Shin, S.; Lee, J.; Lee, H.K.; Kim, J.; Kwon, S.B.; Kim, M.J.; et al. Biological effects of rutin on skin aging. Int. J. Mol. Med. 2016, 38, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Njus, D.; Kelley, P.M.; Tu, Y.J.; Schlegel, H.B. Ascorbic acid: The chemistry underlying its antioxidant properties. Free. Radic. Biol. Med. 2020, 159, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Engin, K.N. Alpha-tocopherol: Looking beyond an antioxidant. Mol. Vis. 2009, 15, 855–860. [Google Scholar]
- Rodriguez-Arce, E.; Saldias, M. Antioxidant properties of flavonoid metal complexes and their potential inclusion in the development of novel strategies for the treatment against neurodegenerative diseases. Biomed. Pharmacother. 2021, 143, 112236. [Google Scholar] [CrossRef]
- Shiraishi, T.; Nielsen, P.E. Improved cellular uptake of antisense peptide nucleic acids by conjugation to a cell-penetrating peptide and a lipid domain. Methods Mol. Biol. 2011, 751, 209–221. [Google Scholar]
- Shin, J.W.; Kwon, S.H.; Choi, J.Y.; Na, J.I.; Huh, C.H.; Choi, H.R.; Park, K.C. Molecular mechanisms of dermal aging and antiaging approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef]
- Wlaschek, M.; Maity, P.; Makrantonaki, E.; Scharffetter-Kochanek, K. Connective tissue and fibroblast senescence in skin aging. J. Investig. Dermatol. 2021, 141, 985–992. [Google Scholar] [CrossRef]
- Abd, E.; Yousef, S.A.; Pastore, M.N.; Telaprolu, K.; Mohammed, Y.H.; Namjoshi, S.; Grice, J.E.; Roberts, M.S. Skin models for the testing of transdermal drugs. Clin. Pharmacol. 2016, 8, 163–176. [Google Scholar] [CrossRef]
- Jiang, X.; Dutton, C.M.; Qi, W.N.; Block, J.A.; Garamszegi, N.; Scully, S.P. Sirna mediated inhibition of mmp-1 reduces invasive potential of a human chondrosarcoma cell line. J. Cell. Physiol. 2005, 202, 723–730. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.I.; Lee, S.G.; Jung, I.; Suk, J.; Baeg, C.; Han, S.-Y.; Seo, J.Y.; Jung, D.; Jeon, Y.; Lee, J.H. Topical Application of Peptide Nucleic Acid Antisense Oligonucleotide for MMP-1 and Its Potential Anti-Aging Properties. J. Clin. Med. 2023, 12, 2472. https://doi.org/10.3390/jcm12072472
Lee YI, Lee SG, Jung I, Suk J, Baeg C, Han S-Y, Seo JY, Jung D, Jeon Y, Lee JH. Topical Application of Peptide Nucleic Acid Antisense Oligonucleotide for MMP-1 and Its Potential Anti-Aging Properties. Journal of Clinical Medicine. 2023; 12(7):2472. https://doi.org/10.3390/jcm12072472
Chicago/Turabian StyleLee, Young In, Sang Gyu Lee, Inhee Jung, Jangmi Suk, Chaemin Baeg, Seon-Young Han, Jeong Yeon Seo, Daram Jung, Yeasel Jeon, and Ju Hee Lee. 2023. "Topical Application of Peptide Nucleic Acid Antisense Oligonucleotide for MMP-1 and Its Potential Anti-Aging Properties" Journal of Clinical Medicine 12, no. 7: 2472. https://doi.org/10.3390/jcm12072472
APA StyleLee, Y. I., Lee, S. G., Jung, I., Suk, J., Baeg, C., Han, S.-Y., Seo, J. Y., Jung, D., Jeon, Y., & Lee, J. H. (2023). Topical Application of Peptide Nucleic Acid Antisense Oligonucleotide for MMP-1 and Its Potential Anti-Aging Properties. Journal of Clinical Medicine, 12(7), 2472. https://doi.org/10.3390/jcm12072472




