One-Year Outcomes after Myval Implantation in Patients with Bicuspid Aortic Valve Stenosis—A Multicentre Real-World Experience
Abstract
1. Introduction
2. Patients and Methods
2.1. Procedural Characteristics
2.2. Endpoints and Definitions
2.3. Statistical Analysis
3. Results
3.1. Study Population and Baseline Characteristics
3.2. Clinical Outcomes
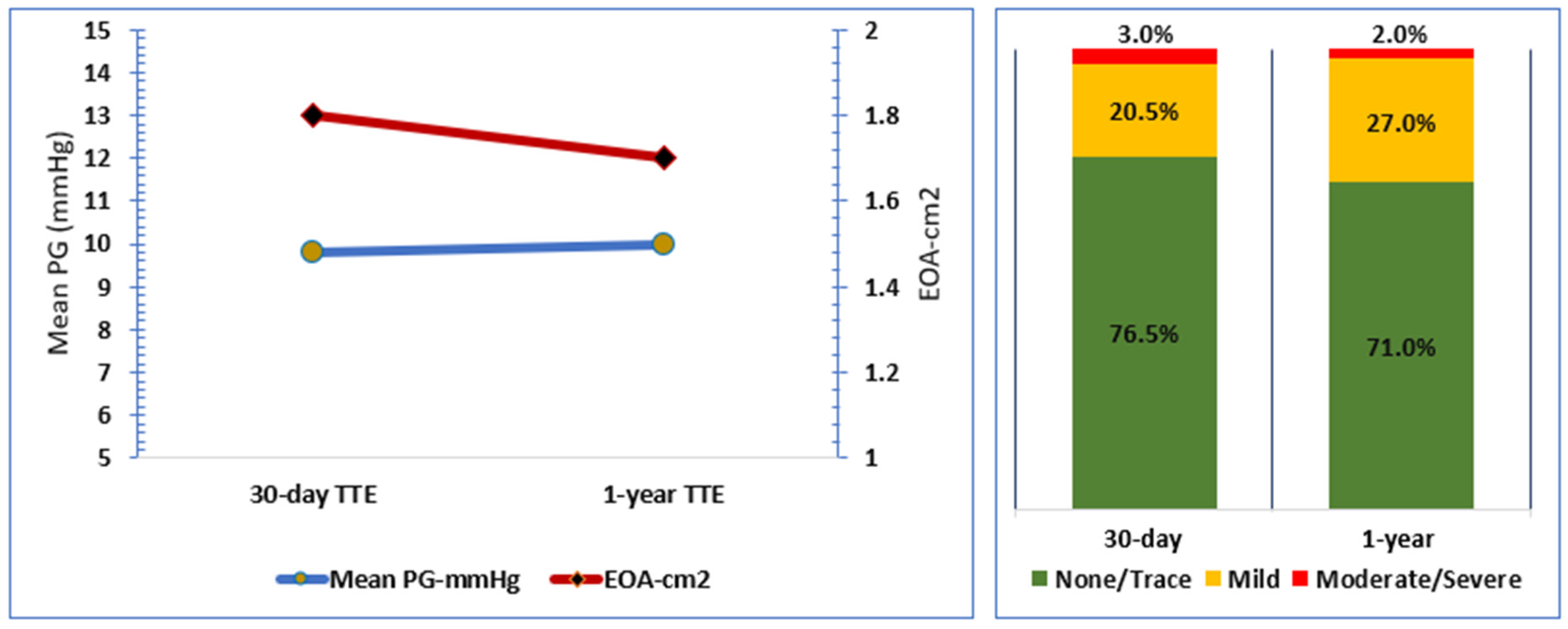
3.3. Echocardiographic Outcome
3.4. Bioprosthetic Valve Deterioration
- Stage I, with morphological valve deterioration in the form of clinical valve thrombosis, was diagnosed by multidetector computed tomography (MDCT) scan in 1 (2.1%) patient.
- Stage II haemodynamic valve deterioration was detected in 3 (6.4%) patients.
- Stage III haemodynamic valve deterioration was detected in 1 (2.1%) patient.
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AR | Aortic regurgitation |
| AS | Aortic stenosis |
| AV | Aortic valve |
| BAV | Bicuspid aortic valve |
| EOA | Effective orifice area |
| THV | Transcatheter heart valve |
References
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2021, 77, e25–e197. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2021, 43, 561–632. [Google Scholar] [CrossRef]
- Xiong, T.Y.; Ali, W.B.; Feng, Y.; Hayashida, K.; Jilaihawi, H.; Latib, A.; Lee, M.K.; Leon, M.B.; Makkar, R.R.; Modine, T.; et al. Transcatheter aortic valve implantation in patients with bicuspid valve morphology: A roadmap towards standardization. Nat. Rev. Cardiol. 2022, 20, 52–67. [Google Scholar] [CrossRef]
- Masri, A.; Svensson, L.G.; Griffin, B.P.; Desai, M.Y. Contemporary natural history of bicuspid aortic valve disease: A systematic review. Heart 2017, 103, 1323–1330. [Google Scholar] [CrossRef]
- Roberts, W.C.; Ko, J.M. Frequency by decades of unicuspid, bicuspid, and tricuspid aortic valves in adults having isolated aortic valve replacement for aortic stenosis, with or without associated aortic regurgitation. Circulation 2005, 111, 920–925. [Google Scholar] [CrossRef]
- Van Belle, E.; Vincent, F. Durability of transcatheter aortic valve implantation in bicuspid aortic valve stenosis: The last missing piece? EuroIntervention 2022, 18, 185–187. [Google Scholar] [CrossRef]
- Michelena, H.I.; Della Corte, A.; Evangelista, A.; Maleszewski, J.J.; Edwards, W.D.; Roman, M.J.; Devereux, R.B.; Fernandez, B.; Asch, F.M.; Barker, A.J.; et al. International Consensus Statement on Nomenclature and Classification of the Congenital Bicuspid Aortic Valve and Its Aortopathy, for Clinical, Surgical, Interventional and Research Purposes. Ann. Thorac. Surg. 2021, 112, e203–e235. [Google Scholar] [CrossRef]
- Zhou, D.; Yidilisi, A.; Fan, J.; Zhang, Y.; Dai, H.; Zhu, G.; Guo, Y.; He, Y.; Zhu, Q.; Lin, X.; et al. Three-year outcomes of transcatheter aortic valve implantation for bicuspid versus tricuspid aortic stenosis. EuroIntervention 2022, 18, 193–202. [Google Scholar] [CrossRef]
- Majmundar, M.; Kumar, A.; Doshi, R.; Shariff, M.; Krishnaswamy, A.; Reed, G.W.; Brockett, J.; Lahorra, J.A.; Svensson, L.G.; Puri, R.; et al. Early outcomes of transcatheter versus surgical aortic valve implantation in patients with bicuspid aortic valve stenosis. EuroIntervention 2022, 18, 23–32. [Google Scholar] [CrossRef]
- Elkoumy, A.; Jose, J.; Terkelsen, C.J.; Nissen, H.; Gunasekaran, S.; Abdelshafy, M.; Seth, A.; Elzomor, H.; Kumar, S.; Bedogni, F.; et al. Safety and Efficacy of Myval Implantation in Patients with Severe Bicuspid Aortic Valve Stenosis—A Multicenter Real-World Experience. J. Clin. Med. 2022, 11, 443. [Google Scholar] [CrossRef]
- Vincent, F.; Ternacle, J.; Denimal, T.; Shen, M.; Redfors, B.; Delhaye, C.; Simonato, M.; Debry, N.; Verdier, B.; Shahim, B.; et al. Transcatheter Aortic Valve Replacement in Bicuspid Aortic Valve Stenosis. Circulation 2021, 143, 1043–1061. [Google Scholar] [CrossRef]
- Sa, M.; Simonato, M.; Van den Eynde, J.; Cavalcanti, L.R.P.; Alsagheir, A.; Tzani, A.; Fovino, L.N.; Kampaktsis, P.N.; Gallo, M.; Laforgia, P.L.; et al. Balloon versus self-expandable transcatheter aortic valve implantation for bicuspid aortic valve stenosis: A meta-analysis of observational studies. Catheter. Cardiovasc. Interv. 2021, 98, E746–E757. [Google Scholar] [CrossRef]
- Makkar, R.R.; Yoon, S.H.; Chakravarty, T.; Kapadia, S.R.; Krishnaswamy, A.; Shah, P.B.; Kaneko, T.; Skipper, E.R.; Rinaldi, M.; Babaliaros, V.; et al. Association Between Transcatheter Aortic Valve Replacement for Bicuspid vs Tricuspid Aortic Stenosis and Mortality or Stroke Among Patients at Low Surgical Risk. JAMA 2021, 326, 1034–1044. [Google Scholar] [CrossRef]
- Yoon, S.H.; Kim, W.K.; Dhoble, A.; Milhorini Pio, S.; Babaliaros, V.; Jilaihawi, H.; Pilgrim, T.; De Backer, O.; Bleiziffer, S.; Vincent, F.; et al. Bicuspid Aortic Valve Morphology and Outcomes After Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2020, 76, 1018–1030. [Google Scholar] [CrossRef]
- Colombo, A.; Mangieri, A. Surgery Versus TAVR for Bicuspid Aortic Valve Disease: The Time Has Come for a Randomized Study. JACC Cardiovasc. Interv. 2020, 13, 1028–1029. [Google Scholar] [CrossRef]
- VARC-3 Writing Committee; Genereux, P.; Piazza, N.; Alu, M.C.; Nazif, T.; Hahn, R.T.; Pibarot, P.; Bax, J.J.; Leipsic, J.A.; Blanke, P.; et al. Valve Academic Research Consortium 3: Updated endpoint definitions for aortic valve clinical research. Eur. Heart J. 2021, 42, 1825–1857. [Google Scholar] [CrossRef]
- Kappetein, A.P.; Head, S.J.; Genereux, P.; Piazza, N.; van Mieghem, N.M.; Blackstone, E.H.; Brott, T.G.; Cohen, D.J.; Cutlip, D.E.; van Es, G.A.; et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: The Valve Academic Research Consortium-2 consensus document (VARC-2). Eur. J. Cardiothorac. Surg. 2012, 42, S45–S60. [Google Scholar] [CrossRef]
- Lancellotti, P.; Pibarot, P.; Chambers, J.; Edvardsen, T.; Delgado, V.; Dulgheru, R.; Pepi, M.; Cosyns, B.; Dweck, M.R.; Garbi, M.; et al. Recommendations for the imaging assessment of prosthetic heart valves: A report from the European Association of Cardiovascular Imaging endorsed by the Chinese Society of Echocardiography, the Inter-American Society of Echocardiography, and the Brazilian Department of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 589–590. [Google Scholar] [CrossRef]
- Zamorano, J.L.; Badano, L.P.; Bruce, C.; Chan, K.L.; Goncalves, A.; Hahn, R.T.; Keane, M.G.; La Canna, G.; Monaghan, M.J.; Nihoyannopoulos, P.; et al. EAE/ASE recommendations for the use of echocardiography in new transcatheter interventions for valvular heart disease. Eur. Heart J. 2011, 32, 2189–2214. [Google Scholar] [CrossRef]
- Zoghbi, W.A.; Asch, F.M.; Bruce, C.; Gillam, L.D.; Grayburn, P.A.; Hahn, R.T.; Inglessis, I.; Islam, A.M.; Lerakis, S.; Little, S.H.; et al. Guidelines for the Evaluation of Valvular Regurgitation After Percutaneous Valve Repair or Replacement: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Angiography and Interventions, Japanese Society of Echocardiography, and Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2019, 32, 431–475. [Google Scholar] [CrossRef]
- Mylotte, D.; Lefevre, T.; Sondergaard, L.; Watanabe, Y.; Modine, T.; Dvir, D.; Bosmans, J.; Tchetche, D.; Kornowski, R.; Sinning, J.M.; et al. Transcatheter aortic valve replacement in bicuspid aortic valve disease. J. Am. Coll. Cardiol. 2014, 64, 2330–2339. [Google Scholar] [CrossRef]
- Lee, Y.T.; Yin, W.H.; Tsao, T.P.; Lee, K.C.; Hsiung, M.C.; Tzeng, Y.H.; Wei, J. The Presence of Calcified Raphe Is an Independent Predictor of Adverse Long-Term Clinical Outcomes in Patients with Bicuspid Aortic Stenosis Undergoing Transcatheter Aortic Valve Replacement. Front. Cardiovasc. Med. 2022, 9, 767906. [Google Scholar] [CrossRef]
- Qiu, D.; Barakat, M.; Hopkins, B.; Ravaghi, S.; Azadani, A.N. Transcatheter aortic valve replacement in bicuspid valves: The synergistic effects of eccentric and incomplete stent deployment. J. Mech. Behav. Biomed. Mater. 2021, 121, 104621. [Google Scholar] [CrossRef]
- Finotello, A.; Romarowski, R.M.; Gorla, R.; Bianchi, G.; Bedogni, F.; Auricchio, F.; Morganti, S. Performance of high conformability vs. high radial force devices in the virtual treatment of TAVI patients with bicuspid aortic valve. Med. Eng. Phys. 2021, 89, 42–50. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, T.Y.; Li, Y.M.; Yao, Y.J.; He, J.J.; Yang, H.R.; Zhu, Z.K.; Chen, F.; Ou, Y.; Wang, X.; et al. Patients with Bicuspid Aortic Stenosis Undergoing Transcatheter Aortic Valve Replacement: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 794850. [Google Scholar] [CrossRef]
- Kawashima, H.; Wang, R.; Mylotte, D.; Jagielak, D.; De Marco, F.; Ielasi, A.; Onuma, Y.; den Heijer, P.; Terkelsen, C.J.; Wijns, W.; et al. Quantitative Angiographic Assessment of Aortic Regurgitation after Transcatheter Aortic Valve Implantation among Three Balloon-Expandable Valves. Glob. Heart 2021, 16, 20. [Google Scholar] [CrossRef]
- Delgado-Arana, J.R.; Gordillo-Monge, M.X.; Halim, J.; De Marco, F.; Trani, C.; Martin, P.; Infusino, F.; Ancona, M.; den Heijer, P.; Bedogni, F.; et al. Early clinical and haemodynamic matched comparison of balloon-expandable valves. Heart 2021, 108, 725–732. [Google Scholar] [CrossRef]
- Garcia-Gomez, M.; Delgado-Arana, J.R.; Halim, J.; De Marco, F.; Trani, C.; Martin, P.; Won-Keun, K.; Montorfano, M.; den Heijer, P.; Bedogni, F.; et al. Next-generation balloon-expandable Myval transcatheter heart valve in low-risk aortic stenosis patients. Catheter. Cardiovasc. Interv. 2021, 99, 889–895. [Google Scholar] [CrossRef]
- Veulemans, V.; Nuyens, P.; Goh, S.; Maier, O.; Binnebossel, S.; Heermann, J.; Jung, C.; Westenfeld, R.; Kelm, M.; de Backer, O.; et al. Bioprosthetic valve dysfunction and failure after TAVI in bicuspid aortic valve stenosis during one-year follow-up according to VARC-3. Clin. Res. Cardiol. 2022, 111, 1358–1366. [Google Scholar] [CrossRef]
- Sondergaard, L.; De Backer, O.; Kofoed, K.F.; Jilaihawi, H.; Fuchs, A.; Chakravarty, T.; Kashif, M.; Kazuno, Y.; Kawamori, H.; Maeno, Y.; et al. Natural history of subclinical leaflet thrombosis affecting motion in bioprosthetic aortic valves. Eur. Heart J. 2017, 38, 2201–2207. [Google Scholar] [CrossRef]
- Nakashima, M.; Jilaihawi, H. Transcatheter Aortic Valve Leaflet Thrombosis: Prevalence, Management, and Future Directions. Curr. Cardiol. Rep. 2021, 23, 186. [Google Scholar] [CrossRef]
| Demographic Characteristics | |
|---|---|
| Age | 732 [66.3, 77.0] |
| Men | 45 (72.6%) |
| Women | 17 (27.4%) |
| Body surface area (BSA) m2 | 1.8 ± 0.27 |
| Body mass index (BMI) kg/m2 | 25.3 ± 5.6 |
| Clinical characteristics | |
| STS risk score% | 3.2 ± 2.2 |
| New York Heart Association (NYHA) class III/IV | 40 (64.5) |
| Prior atrial fibrillation | 10 (16.1%) |
| Peripheral vascular disease | 8 (12.9%) |
| Bicuspid aortic valve (BAV) phenotype | |
| Type 0 | 10 (3.2%) |
| Type 1-a | 50 (80.6%) |
| Type 2 | 2 (16.1%) |
| Outcome | |
|---|---|
| Follow-up duration, months | 13.5 [12.2, 18.3] |
| All-cause mortality | 7 (11.3%) |
| Cardiovascular mortality | 3 (4.8%) |
| Non-cardiovascular mortality | 4 (6.5%) |
| TAVI-related rehospitalisation | 1 (1.8%) |
| Other cardiovascular rehospitalisation | 5 (8.8%) |
| Non-cardiovascular rehospitalisation | 6 (10.5%) |
| All-stroke | 2 (3.2%) |
| Myocardial infarction | 1 (1.6%) |
| Permanent pacemaker implantation | 5 (8.3%) |
| Major bleeding | 1 (1.6%) |
| Acute kidney injury (AKI) | 0 |
| Endocarditis | 0 |
| Valve thrombosis | 1 (1.6%) |
| Re-intervention to the valve | 0 |
| New York Heart Association (NYHA) Class | |
| NYHA I | 27 (49.1%) |
| NYHA II | 25 (45.5%) |
| NYHA III | 3 (5.5%) |
| SARS COVID-2 infection | 5 (8.2%) |
| Echocardiographic Assessment | |
|---|---|
| Mean pressure gradient (mPG), mmHg | 10 [8, 16.5] |
| Effective orifice area (EOA), cm2 | 1.7 [1.4, 1.9] |
| Transvalvular maximum velocity (Vmax), m/sec | 2.1 [1.6, 2.6] |
| Left ventricle ejection fraction (EF), % | 60 [55, 60] |
| Total aortic regurgitation (AR) | |
| None/Trace | 37 (71%) |
| Mild | 14 (27%) |
| Moderate/Severe | 1 (2%) |
| Moderate/Severe mitral regurgitation | 1 (2.3%) |
| Moderate/Severe tricuspid regurgitation | 4 (9.5%) |
| Systolic pulmonary artery pressure (SPAP), mmHg | 26 [23, 32] |
| Haemodynamic valve deterioration | |
| Stage II haemodynamic deterioration | 3 (6.4%) |
| Stage III haemodynamic deterioration | 1 (2.1%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elkoumy, A.; Jose, J.; Terkelsen, C.J.; Nissen, H.; Gunasekaran, S.; Abdelshafy, M.; Seth, A.; Elzomor, H.; Kumar, S.; Bedogni, F.; et al. One-Year Outcomes after Myval Implantation in Patients with Bicuspid Aortic Valve Stenosis—A Multicentre Real-World Experience. J. Clin. Med. 2023, 12, 2398. https://doi.org/10.3390/jcm12062398
Elkoumy A, Jose J, Terkelsen CJ, Nissen H, Gunasekaran S, Abdelshafy M, Seth A, Elzomor H, Kumar S, Bedogni F, et al. One-Year Outcomes after Myval Implantation in Patients with Bicuspid Aortic Valve Stenosis—A Multicentre Real-World Experience. Journal of Clinical Medicine. 2023; 12(6):2398. https://doi.org/10.3390/jcm12062398
Chicago/Turabian StyleElkoumy, Ahmed, John Jose, Christian Juhl Terkelsen, Henrik Nissen, Sengottuvelu Gunasekaran, Mahmoud Abdelshafy, Ashok Seth, Hesham Elzomor, Sreenivas Kumar, Francesco Bedogni, and et al. 2023. "One-Year Outcomes after Myval Implantation in Patients with Bicuspid Aortic Valve Stenosis—A Multicentre Real-World Experience" Journal of Clinical Medicine 12, no. 6: 2398. https://doi.org/10.3390/jcm12062398
APA StyleElkoumy, A., Jose, J., Terkelsen, C. J., Nissen, H., Gunasekaran, S., Abdelshafy, M., Seth, A., Elzomor, H., Kumar, S., Bedogni, F., Ielasi, A., Arsang-Jang, S., Dora, S. K., Chandra, S., Parikh, K., Unic, D., Baumbach, A., Serruys, P., & Soliman, O. (2023). One-Year Outcomes after Myval Implantation in Patients with Bicuspid Aortic Valve Stenosis—A Multicentre Real-World Experience. Journal of Clinical Medicine, 12(6), 2398. https://doi.org/10.3390/jcm12062398








