Impact of Lumbar Surgery on Pharmacological Treatment for Patients with Lumbar Spinal Canal Stenosis: A Single-Center Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Ethics Approval
2.3. Data Collection
2.4. Statistical Analyses
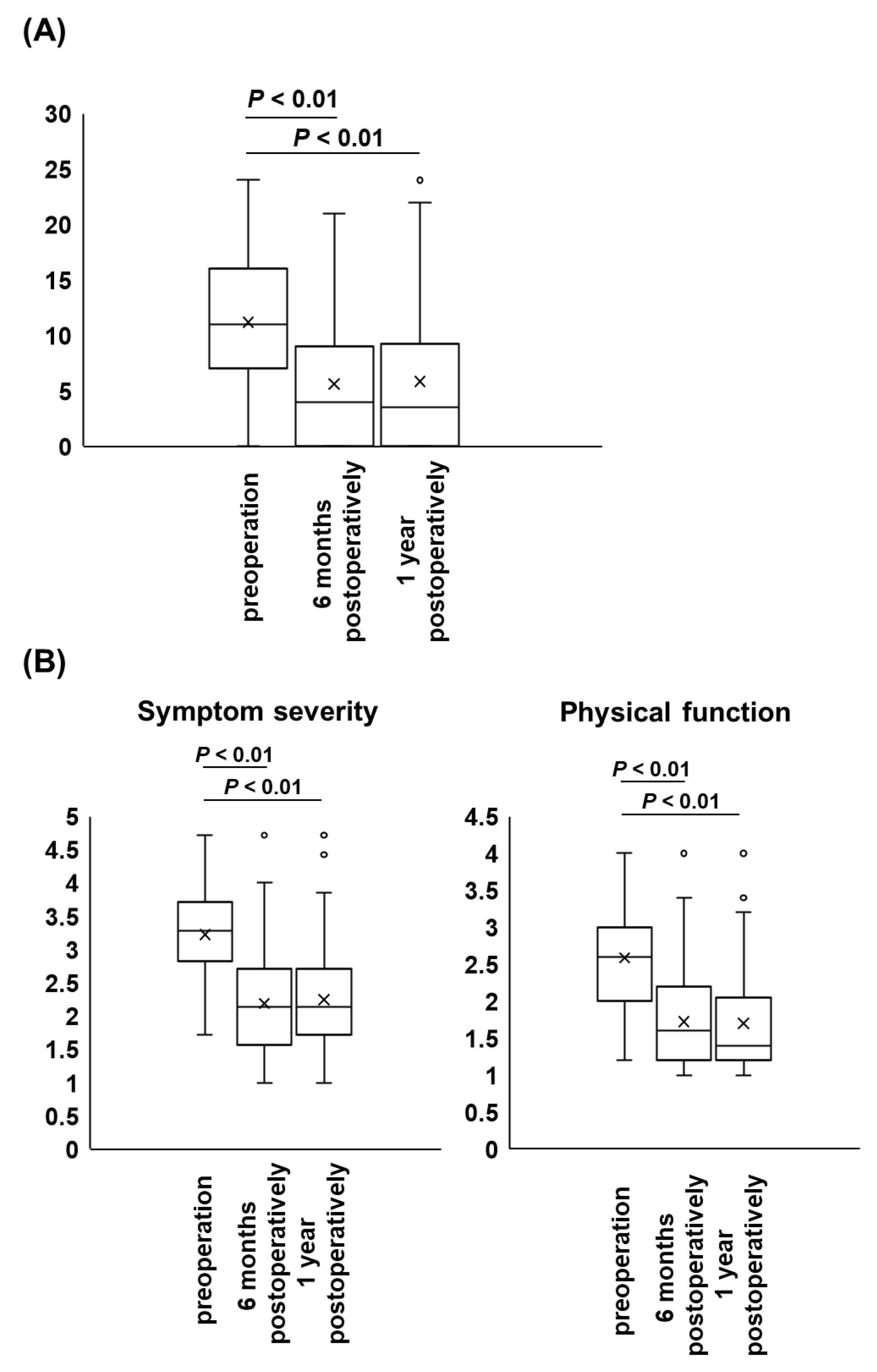
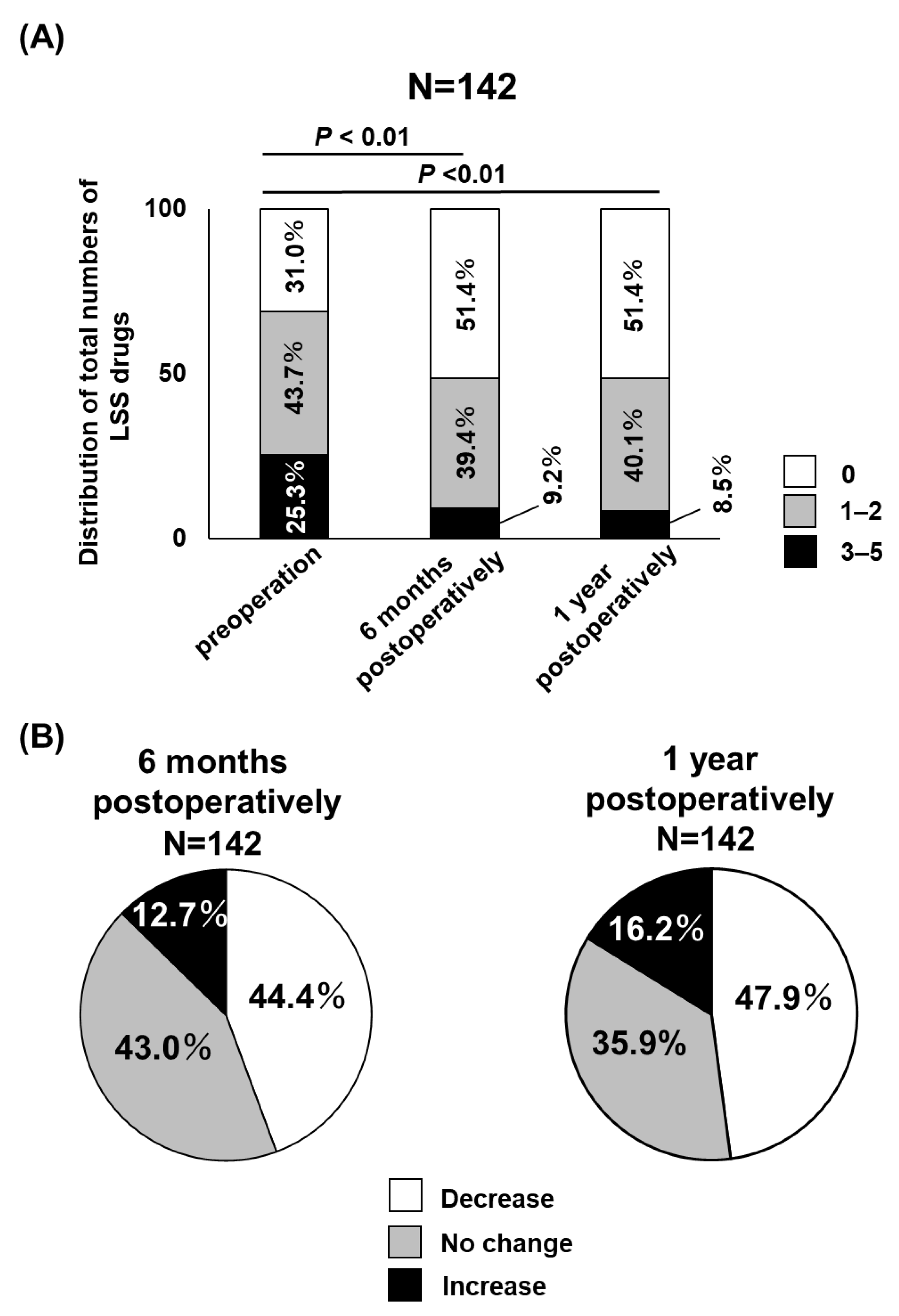
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kawakami, M.; Takeshita, K.; Inoue, G.; Sekiguchi, M.; Fujiwara, Y.; Hoshino, M.; Kaito, T.; Kawaguchi, Y.; Minetama, M.; Orita, S.; et al. Japanese Orthopaedic Association (JOA) Clinical Practice Guidelines on the Management of Lumbar Spinal Stenosis, 2021—Secondary Publication. J. Orthop. Sci. 2023, 28, 46–91. [Google Scholar] [CrossRef]
- Kreiner, D.S.; Shaffer, W.O.; Baisden, J.L.; Gilbert, T.J.; Summers, J.T.; Toton, J.F.; Hwang, S.W.; Mendel, R.C.; Reitman, C.A.; North American Spine Society. An Evidence-based Clinical Guideline for the Diagnosis and Treatment of Degenerative Lumbar Spinal Stenosis (Update). Spine J. 2013, 13, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Kalichman, L.; Cole, R.; Kim, D.H.; Li, L.; Suri, P.; Guermazi, A.; Hunter, D.J. Spinal Stenosis Prevalence and Association with Symptoms: The Framingham Study. Spine J. 2009, 9, 545–550. [Google Scholar] [CrossRef]
- Ishimoto, Y.; Yoshimura, N.; Muraki, S.; Yamada, H.; Nagata, K.; Hashizume, H.; Takiguchi, N.; Minamide, A.; Oka, H.; Kawaguchi, H.; et al. Prevalence of Symptomatic Lumbar Spinal Stenosis and Its Association with Physical Performance in a Population-based Cohort in Japan: The Wakayama Spine Study. Osteoarthr. Cartil. 2012, 20, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Yabuki, S.; Fukumori, N.; Takegami, M.; Onishi, Y.; Otani, K.; Sekiguchi, M.; Wakita, T.; Kikuchi, S.; Fukuhara, S.; Konno, S. Prevalence of Lumbar Spinal Stenosis, Using the Diagnostic Support Tool, and Correlated Factors in Japan: A Population-based Study. J. Orthop. Sci. 2013, 18, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Shirado, O.; Arai, Y.; Iguchi, T.; Imagama, S.; Kawakami, M.; Nikaido, T.; Ogata, T.; Orita, S.; Sakai, D.; Sato, K.; et al. Formulation of Japanese Orthopaedic Association (JOA) Clinical Practice Guideline for the Management of Low Back Pain- The Revised 2019 Edition. J. Orthop. Sci. 2022, 27, 3–30. [Google Scholar] [CrossRef] [PubMed]
- Fujita, N.; Michikawa, T.; Miyamoto, A.; Sakurai, A.; Otaka, Y.; Suzuki, S.; Tsuji, O.; Nagoshi, N.; Okada, E.; Yagi, M.; et al. Lumbar Spinal Surgery Improves Locomotive Syndrome in Elderly Patients with Lumbar Spinal Canal Stenosis: A Multicenter Prospective Study. J. Orthop. Sci. 2020, 25, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Clinical Outcomes Committee of the Japanese Orthopaedic Association, Subcommittee on Evaluation of Back Pain and Cervical Myelopathy; Subcommittee on Low Back Pain and Cervical Myelopathy Evaluation of the Clinical Outcome Committe of the Japanese Orthopaedic Association; Fukui, M.; Chiba, K.; Kawakami, M.; Kikuchi, S.; Konno, S.; Miyamoto, M.; Seichi, A.; Shimamura, T.; et al. JOA Back Pain Evaluation Questionnaire: Initial Report. J. Orthop. Sci. 2007, 12, 443–450. [Google Scholar] [CrossRef]
- Nagai, S.; Inagaki, R.; Michikawa, T.; Kawabata, S.; Ito, I.; Hachiya, K.; Takeda, H.; Ikeda, D.; Kaneko, S.; Yamada, S.; et al. Efficacy of surgical treatment on polypharmacy of elderly patients with lumbar spinal canal stenosis: Retrospective exploratory research. BMC Geriatr. 2023; in press. [Google Scholar]
- Ozaki, M.; Fujita, N.; Miyamoto, A.; Suzuki, S.; Tsuji, O.; Nagoshi, N.; Okada, E.; Yagi, M.; Tsuji, T.; Nakamura, M.; et al. Impact of knee osteoarthritis on surgical outcomes of lumbar spinal canal stenosis. J. Neurosurg. Spine 2019, 32, 710–715. [Google Scholar] [CrossRef]
- Fujita, N.; Ishihara, S.; Michikawa, T.; Suzuki, S.; Tsuji, O.; Nagoshi, N.; Okada, E.; Yagi, M.; Tsuji, T.; Kono, H.; et al. Negative impact of spinal epidural lipomatosis on the surgical outcome of posterior lumbar spinous-splitting decompression surgery: A multicenter retrospective study. Spine J. 2019, 19, 1977–1985. [Google Scholar] [CrossRef]
- Fritsch, C.G.; Ferreira, M.L.; Maher, C.G.; Herbert, R.D.; Pinto, R.Z.; Koes, B.; Ferreira, P.H. The Clinical Course of Pain and Disability following Surgery for Spinal Stenosis: A Systematic Review and Meta-analysis of Cohort Studies. Eur. Spine J. 2017, 26, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Prasad, B.C.; Ramesh Chandra, V.V.; Devi, B.V.; Chivukula, S.S.; Pundarikakshaiah, K. Clinical, Radiological, and Functional Evaluation of Surgical Treatment in Degenerative Lumbar Canal Stenosis. Neurol. India 2016, 64, 677–683. [Google Scholar] [PubMed]
- Gates, M.; Tang, A.R.; Godil, S.S.; Devin, C.J.; McGirt, M.J.; Zuckerman, S.L. Defining the Relative Utility of Lumbar Spine Surgery: A Systematic Literature Review of Common Surgical Procedures and Their Impact on Health States. J. Clin. Neurosci. 2021, 93, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Carreon, L.Y.; Jespersen, A.B.; Støttrup, C.C.; Hansen, K.H.; Andersen, M.O. Is the Hospital Anxiety and Depression Scale Associated with Outcomes after Lumbar Spine Surgery? Global Spine J. 2020, 10, 266–271. [Google Scholar] [CrossRef]
- Ross, S.B.; Wu, P.E.; Atique, A.; Papillon-Ferland, L.; Tamblyn, R.; Lee, T.C.; McDonald, E.G. Adverse Drug Events in Older Adults: Review of Adjudication Methods in Deprescribing Studies. J. Am. Geriatr. Soc. 2020, 68, 1594–1602. [Google Scholar] [CrossRef]
- Postacchini, R.; Ferrari, E.; Cinotti, G.; Menchetti, P.P.; Postacchini, F. Aperius Interspinous Implant Versus Open Surgical Decompression in Lumbar Spinal Stenosis. Spine J. 2011, 11, 933–939. [Google Scholar] [CrossRef]
- Gu, Y.; Chen, L.; Yang, H.L.; Chen, X.Q.; Dong, R.B.; Han, G.S.; Tang, T.S.; Zhang, Z.M. Efficacy of Surgery and Type of Fusion in Patients with Degenerative Lumbar Spinal Stenosis. J. Clin. Neurosci. 2009, 16, 1291–1295. [Google Scholar] [CrossRef]
- Kojima, T.; Mizukami, K.; Tomita, N.; Arai, H.; Ohrui, T.; Eto, M.; Takeya, Y.; Isaka, Y.; Rakugi, H.; Sudo, N.; et al. Screening Tool for Older Persons’ Appropriate Prescriptions for Japanese: Report of the Japan Geriatrics Society Working Group on “Guidelines for Medical Treatment and Its Safety in the Elderly”. Geriatr. Gerontol. Int. 2016, 16, 983–1001. [Google Scholar] [CrossRef]
- Fick, D.M.; Cooper, J.W.; Wade, W.E.; Waller, J.L.; Maclean, J.R.; Beers, M.H. Updating the Beers Criteria for Potentially Inappropriate Medication Use in Older Adults: Results of a US Consensus Panel of Experts. Arch. Intern. Med. 2003, 163, 2716–2724. [Google Scholar] [CrossRef]
- O’Mahony, D.; O’Sullivan, D.; Byrne, S.; O’Connor, M.N.; Ryan, C.; Gallagher, P. STOPP/START Criteria for Potentially Inappropriate Prescribing in Older People: Version 2. Age Ageing 2014, 44, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Yamaguchi, S.; Suzuki, T.; Kato, J.; Chiba, S.; Hirakawa, N.; Yamaguchi, K.; Tanabe, Y.; Takatsuna, H.; Kenyoshi, Y.; et al. Switching from Pregabalin to Mirogabalin in Patients with Peripheral Neuropathic Pain: A Multi-Center, Prospective, Single-Arm, Open-Label Study (MIROP Study). Pain Ther. 2021, 10, 711–727. [Google Scholar] [CrossRef]
- Nikaido, T.; Takatsuna, H.; Tabata, S.; Shiosakai, K.; Nakatani, T.; Konno, S. Efficacy and Safety of Add-on Mirogabalin to NSAIDs in Lumbar Spinal Stenosis with Peripheral Neuropathic Pain: A Randomized, Open-Label Study. Pain Ther. 2022, 11, 1195–1214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.F.; Ning, G. Mecobalamin. Expert Opin. Investig. Drugs 2008, 17, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, J.N.; Tosteson, T.D.; Lurie, J.D.; Tosteson, A.N.; Blood, E.; Hanscom, B.; Herkowitz, H.; Cammisa, F.; Albert, T.; Boden, S.D.; et al. Surgical versus nonsurgical therapy for lumbar spinal stenosis. N. Engl. J. Med. 2008, 358, 794–810. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.L.; Zhao, X.W.; Ma, J.X.; Li, F.; Wang, Y.; Lu, B. Effectiveness of surgery versus conservative treatment for lumbar spinal stenosis: A system review and meta-analysis of randomized controlled trials. Int. J. Surg. 2017, 44, 329–338. [Google Scholar] [CrossRef]
| Patients | n = 142 | |
| Gender | Male: 84 Female: 58 | |
| Age (years) | 70.1 ± 10.3 | |
| BMI (kg/m2) | 24.1 ± 3.4 | |
| Medical history | Diabetes mellitus | 38 (26.8%) |
| Hypertension | 76 (53.5%) | |
| Dyslipidemia | 61 (43.0%) | |
| Cardiovascular disease | 42 (29.6%) | |
| Cerebrovascular disease | 12 (8.5%) | |
| Cancer | 21 (14.8%) | |
| Spondylolisthesis | 50 (35.2%) | |
| Degenerative lumbar scoliosis | 16 (11.2%) | |
| FBSS | 11 (7.7%) | |
| Surgical procedure | decompression | 84 (59.2%) |
| decompression + fusion | 58 (40.8%) | |
| Surgical time (min) | 139.8 ± 90.3 | |
| Surgical blood loss (mL) | 149.5 ± 169.8 | |
| Incidental dural tear | 13 (9.2%) | |
| 6POM | 1POY | |
|---|---|---|
| Pain disorder | 92 (64.1%) | 84 (59.1%) |
| Lumbar function | 75 (52.8%) | 74 (52.1%) |
| Walking ability | 99 (69.7%) | 97 (68.3%) |
| Social life | 68 (47.9%) | 79 (55.6%) |
| Psychological disorder | 43 (30.3%) | 50 (35.2%) |
| Preoperation | 6POM | 1POY | p Value * | ||
|---|---|---|---|---|---|
| Preoperation vs. 6POM | Preoperation vs. 1POY | ||||
| NSAIDs | 53 (37.3%) | 39 (27.5%) | 33 (23.2%) | 0.03 | <0.01 |
| pregabalin/mirogabalin | 51 (35.9%) | 29 (20.4%) | 30 (21.1%) | <0.01 | <0.01 |
| mecobalamin | 30 (21.1%) | 23 (16.2%) | 26 (18.3%) | 0.09 | 0.35 |
| opioids | 24 (16.9%) | 8 (5.6%) | 12 (8.5%) | <0.01 | <0.01 |
| PGE1 | 20 (14.1%) | 6 (4.2%) | 6 (4.2%) | <0.01 | <0.01 |
| acetaminophen | 14 (9.9%) | 12 (8.5%) | 13 (9.2%) | 0.53 | 0.76 |
| SNRIs | 10 (7.0%) | 8 (5.6%) | 6 (4.2%) | 0.48 | 0.16 |
| neurotropin | 8 (5.6%) | 1 (0.7%) | 1 (0.7%) | 0.02 | 0.02 |
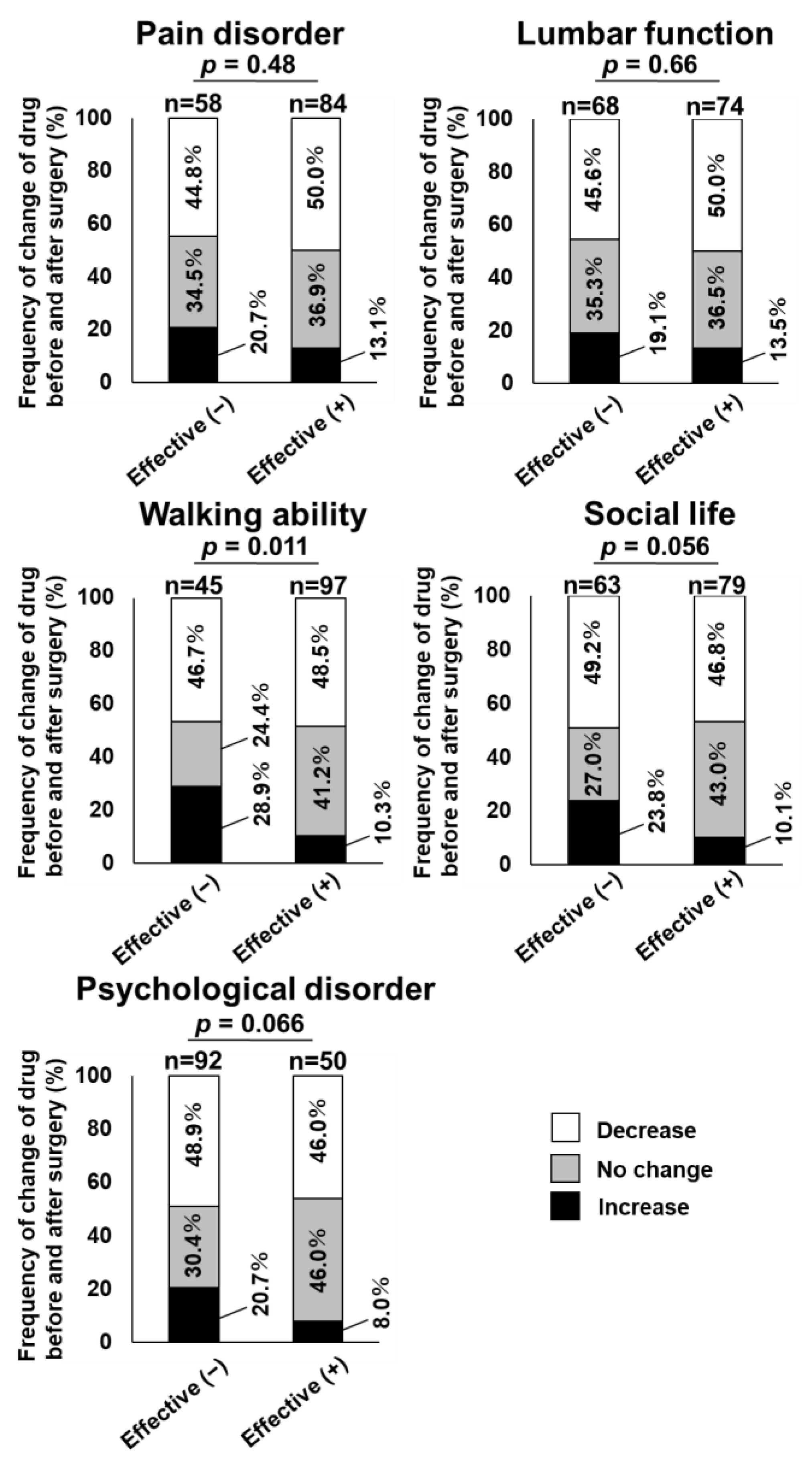
| Total Number | Number of Case | Prevalence of Case | Relative Risk * | 95% Confidence Interval | p-Value | |
|---|---|---|---|---|---|---|
| Pain disorder | ||||||
| Effective (+) | 84 | 11 | 13.1 | Reference | ||
| Effective (−) | 58 | 12 | 20.7 | 1.3 | (0.6–2.7) | 0.49 |
| Lumbar function | ||||||
| Effective (+) | 74 | 10 | 13.5 | Reference | ||
| Effective (−) | 68 | 13 | 19.1 | 1.4 | (0.7–2.9) | 0.34 |
| Walking ability | ||||||
| Effective (+) | 97 | 10 | 10.3 | Reference | ||
| Effective (−) | 45 | 13 | 28.9 | 2.7 | (1.3–5.9) | 0.01 |
| Social life | ||||||
| Effective (+) | 79 | 8 | 10.1 | Reference | ||
| Effective (−) | 63 | 15 | 23.8 | 2.3 | (1.1–5.0) | 0.03 |
| Psychological disorder | ||||||
| Effective (+) | 50 | 4 | 8.0 | Reference | ||
| Effective (−) | 92 | 19 | 20.7 | 2.3 | (0.7–6.9) | 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imai, T.; Nagai, S.; Michikawa, T.; Inagaki, R.; Kawabata, S.; Ito, K.; Hachiya, K.; Takeda, H.; Ikeda, D.; Yamada, S.; et al. Impact of Lumbar Surgery on Pharmacological Treatment for Patients with Lumbar Spinal Canal Stenosis: A Single-Center Retrospective Study. J. Clin. Med. 2023, 12, 2385. https://doi.org/10.3390/jcm12062385
Imai T, Nagai S, Michikawa T, Inagaki R, Kawabata S, Ito K, Hachiya K, Takeda H, Ikeda D, Yamada S, et al. Impact of Lumbar Surgery on Pharmacological Treatment for Patients with Lumbar Spinal Canal Stenosis: A Single-Center Retrospective Study. Journal of Clinical Medicine. 2023; 12(6):2385. https://doi.org/10.3390/jcm12062385
Chicago/Turabian StyleImai, Takaya, Sota Nagai, Takehiro Michikawa, Risa Inagaki, Soya Kawabata, Kaori Ito, Kurenai Hachiya, Hiroki Takeda, Daiki Ikeda, Shigeki Yamada, and et al. 2023. "Impact of Lumbar Surgery on Pharmacological Treatment for Patients with Lumbar Spinal Canal Stenosis: A Single-Center Retrospective Study" Journal of Clinical Medicine 12, no. 6: 2385. https://doi.org/10.3390/jcm12062385
APA StyleImai, T., Nagai, S., Michikawa, T., Inagaki, R., Kawabata, S., Ito, K., Hachiya, K., Takeda, H., Ikeda, D., Yamada, S., Fujita, N., & Kaneko, S. (2023). Impact of Lumbar Surgery on Pharmacological Treatment for Patients with Lumbar Spinal Canal Stenosis: A Single-Center Retrospective Study. Journal of Clinical Medicine, 12(6), 2385. https://doi.org/10.3390/jcm12062385






