Vesicoureteral Reflux and Innate Immune System: Physiology, Physiopathology, and Clinical Aspects
Abstract
1. Introduction
2. Pathophysiology of VUR
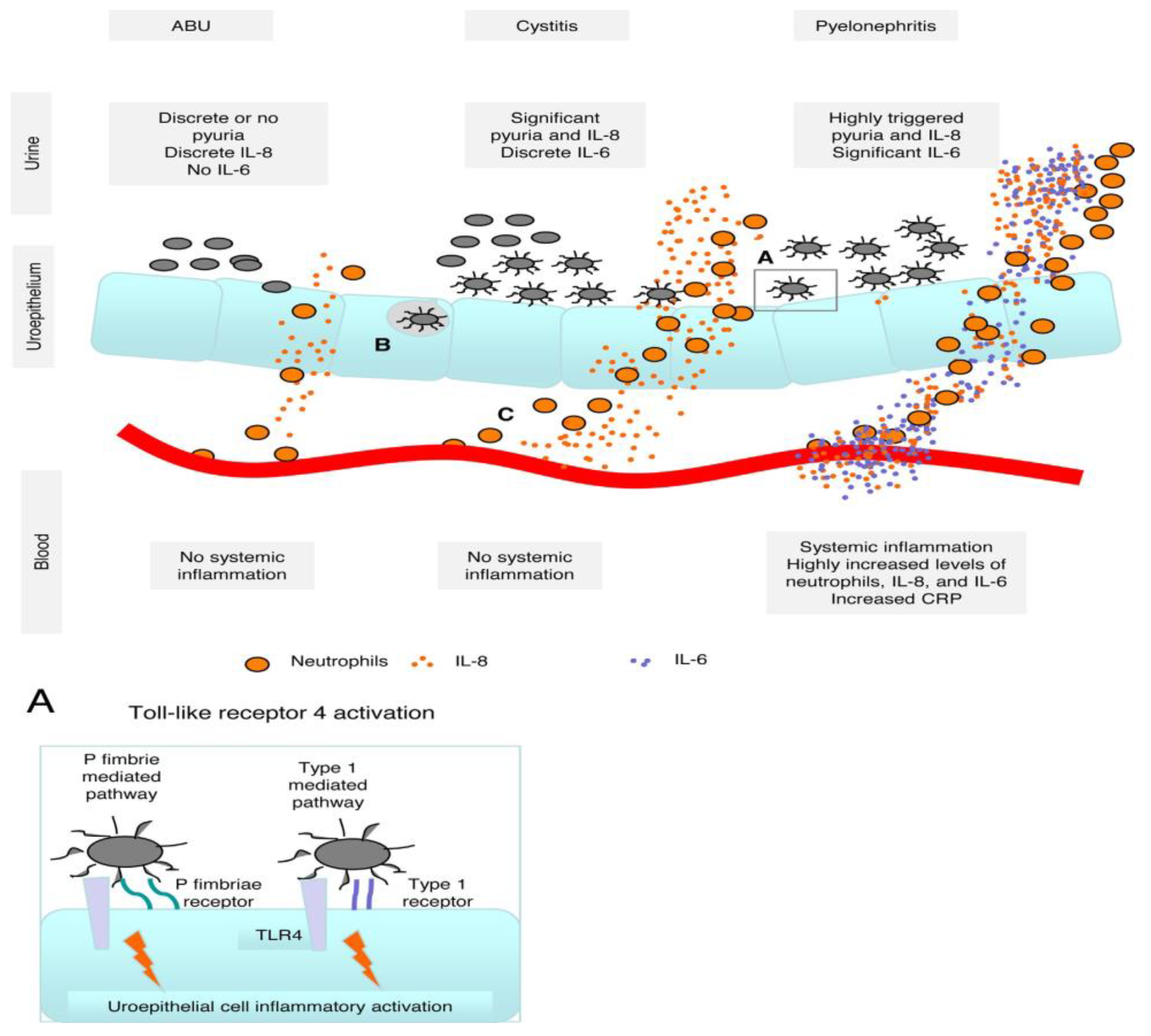
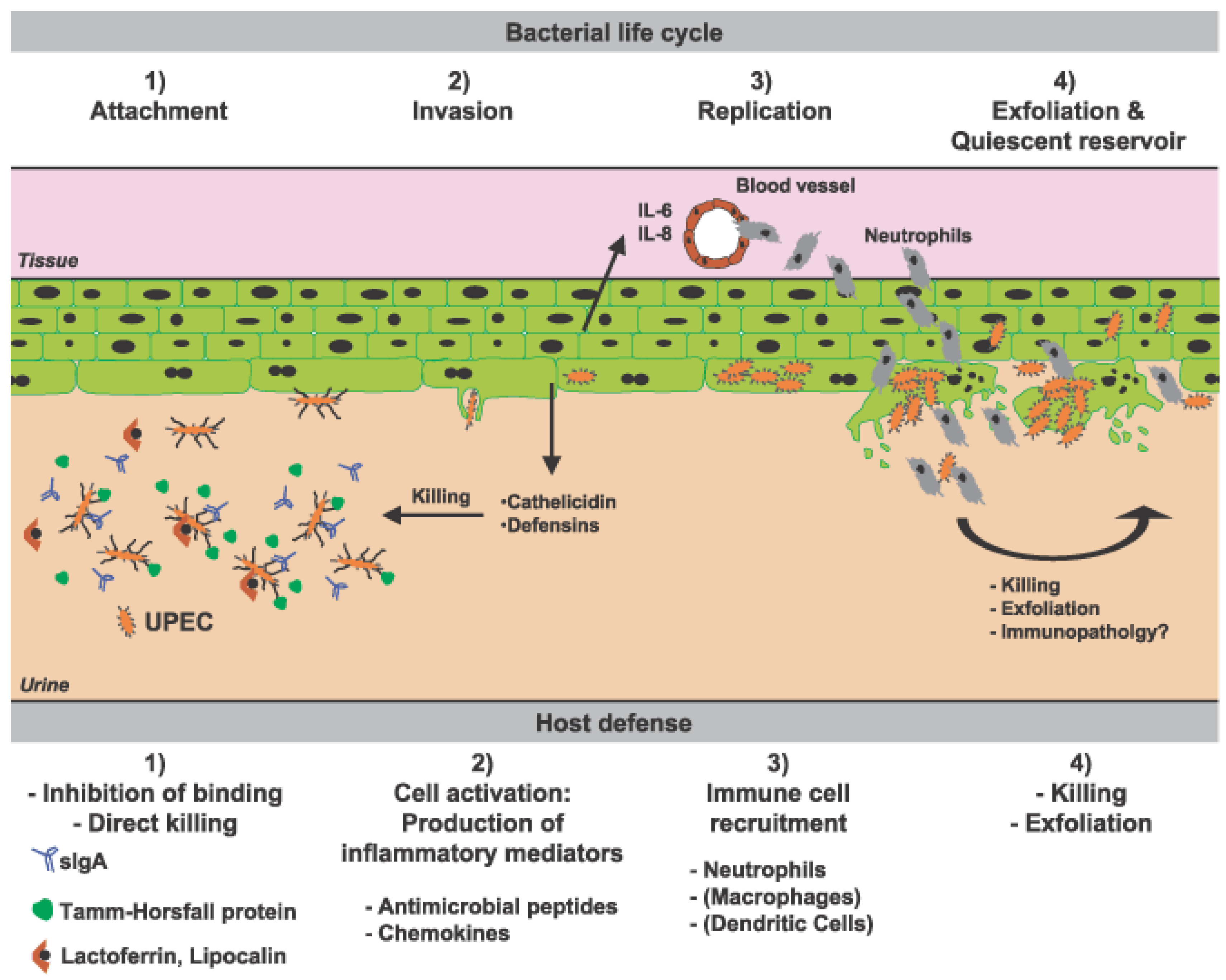
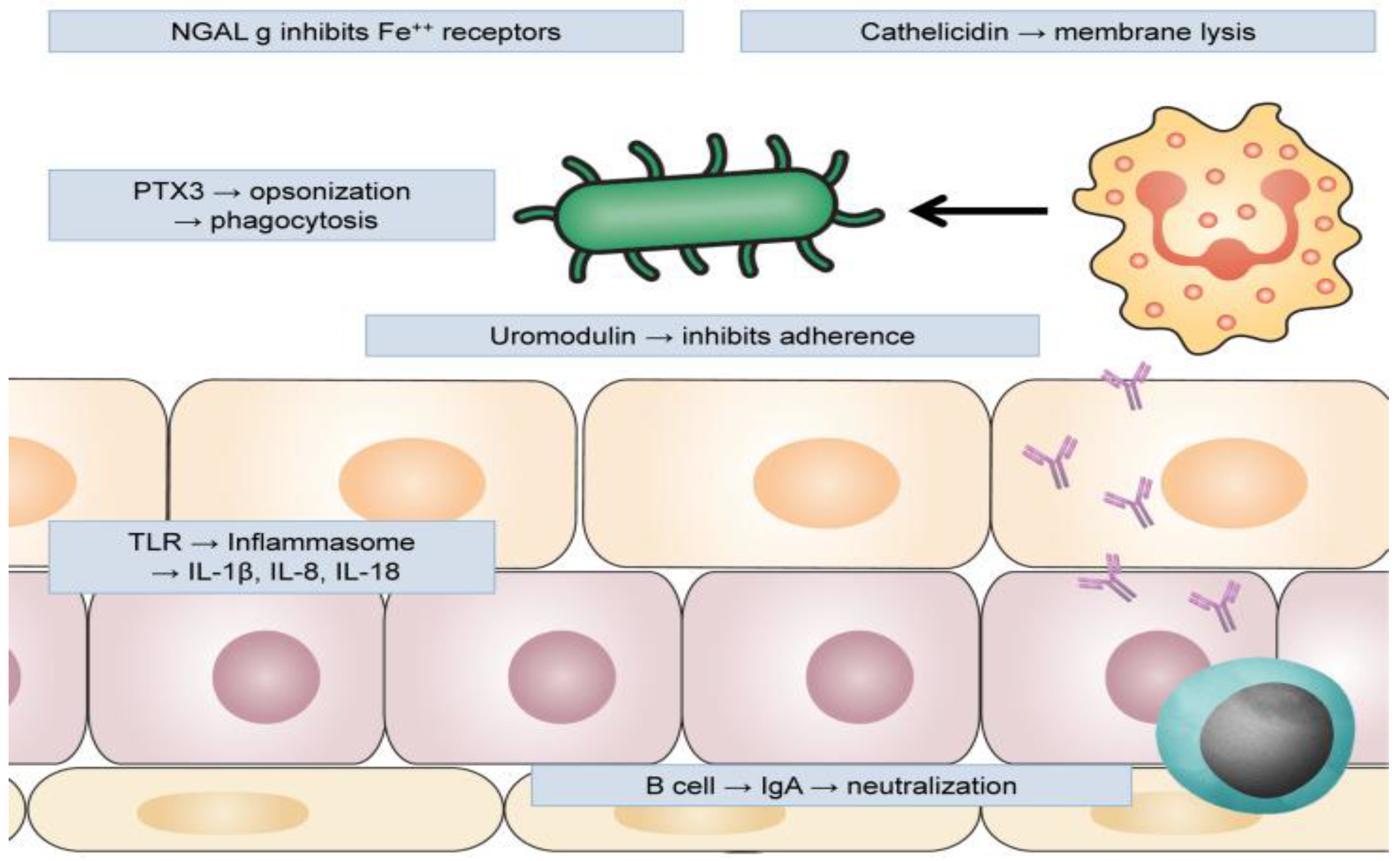
3. Urinary Tract Infections and Innate Immune System
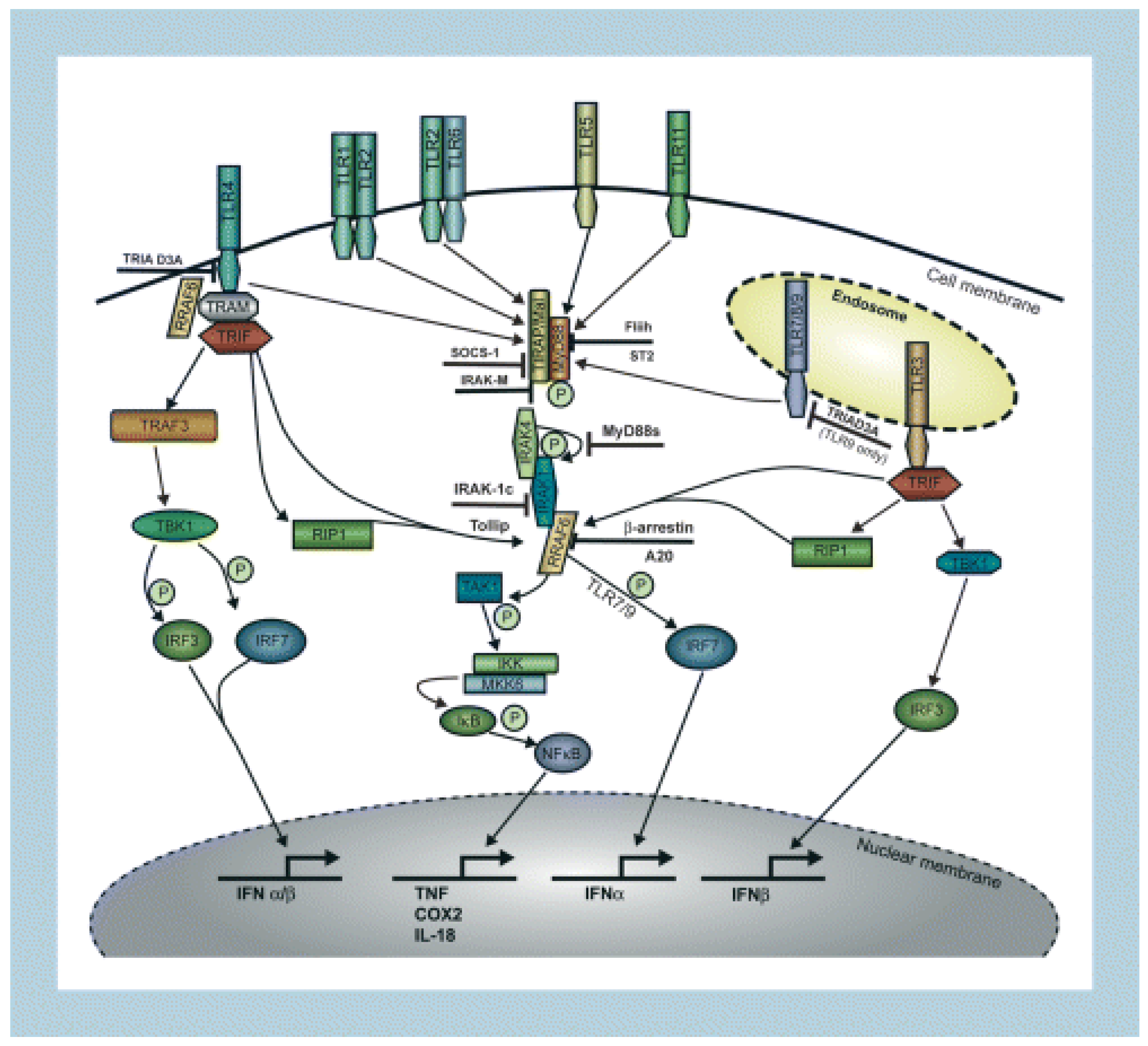
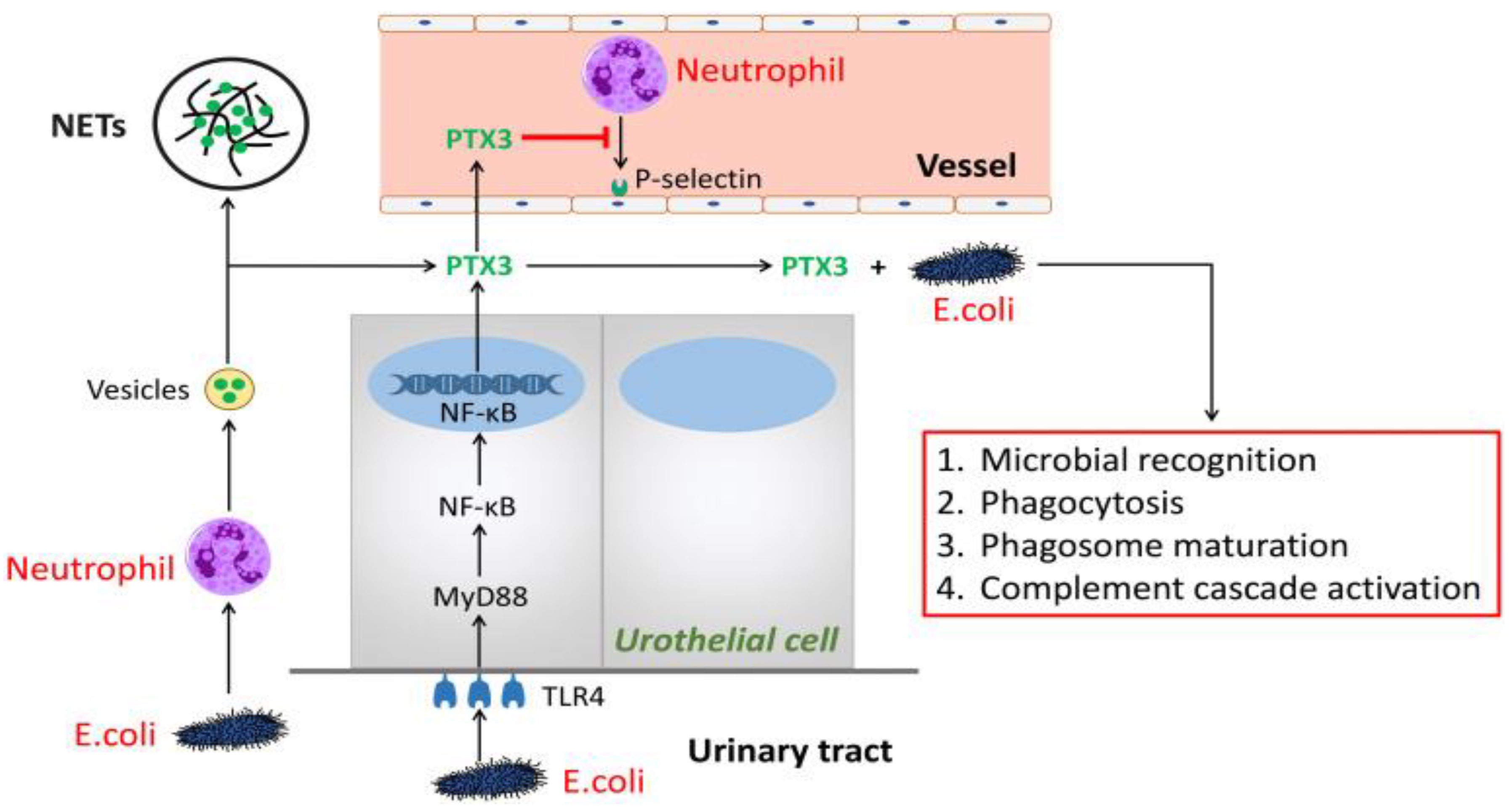
3.1. Toll-Like Receptors
3.2. Transcription Factors
3.3. Cytokines and Chemokines
3.4. Antimicrobial Peptides
3.4.1. Cathelicidin
3.4.2. Defensins
3.4.3. Metal-Binding AMPs
3.4.4. Ribonuclease
3.5. Antimicrobial Proteins
3.5.1. Uromodulin
3.5.2. Pentraxin 3
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mattoo, T.K.; Mohammad, D. Primary Vesicoureteral Reflux and Renal Scarring. Pediatr. Clin. N. Am. 2022, 69, 1115–1129. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.; Fletcher, J.T.; Alexer, S.I.; Craig, J.C. Vesicoureteral Reflux. J. Am. Soc. Nephrol. 2008, 19, 847–862. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.; Rushton, H.G. Pediatric Urology Vesicoureteral Reflux Associated Renal Damage: Congenital Reflux Nephropathy and Acquired Renal Scarring. J. Urol. 2010, 184, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Miyakita, H.; Hayashi, Y.; Mitsui, T.; Okawada, M.; Kinoshita, Y.; Kimata, T.; Koikawa, Y.; Sakai, K.; Satoh, H.; Tokunaga, M.; et al. Guidelines for the medical management of pediatric vesicoureteral reflux. Int. J. Urol. 2020, 27, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Läckgren, G.; Cooper, C.S.; Neveus, T.; Kirsch, A.J. Management of Vesicoureteral Reflux: What Have We Learned Over the Last 20 Years? Front. Pediatr. 2021, 9, 650326. [Google Scholar] [CrossRef]
- Hewitt, I.; Montini, G. Vesicoureteral reflux is it important to find? Pediatr. Nephrol. 2021, 36, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Greenbaum, L.A.; Mesrobian, H.G.O. Vesicoureteral Reflux. Pediatr. Clin. 2006, 53, 413–427. [Google Scholar] [CrossRef]
- Tekgül, S.; Riedmiller, H.; Hoebeke, P.; Kočvara, R.; Nijman, R.J.; Radmayr, C.; Stein, R.; Dogan, H.S. EAU Guidelines on Vesicoureteral Reflux in Children. Eur. Urol. 2012, 62, 534–542. [Google Scholar] [CrossRef]
- Mattoo, T.K. Vesicoureteral Reflux and Reflux Nephropathy. Adv. Chronic Kidney Dis. 2011, 18, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Fillion, M.-L.; Watt, C.L.; Gupta, I.R. Vesicoureteric reflux and reflux nephropathy: From mouse models to childhood disease. Pediatr. Nephrol. 2014, 29, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Brakeman, P. Vesicoureteral Reflux, Reflux Nephropathy, and End-Stage Renal Disease. Adv. Urol. 2008, 2008, 1–7. [Google Scholar] [CrossRef]
- Morozova, O.M.; Lakomova, D.L.; Zakharova, N.Z.; Maltseva, L.M.; Manasova, Z.M.; Morozov, D.M. Reflux nephropathy in children: Pathogenesis and prognosis. Part Urol. 2021, 3, 2021. [Google Scholar] [CrossRef]
- Eddula, N.R.; Baradhi, K.M. Reflux Nephropathy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK526055/ (accessed on 1 February 2023).
- Cendron, M. Reflux nephropathy. J. Pediatr. Urol. 2008, 4, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Ragnarsdóttir, B.; Svanborg, C. Susceptibility to acute pyelonephritis or asymptomatic bacteriuria: Host-pathogen interaction in urinary tract infections. Pediatr. Nephrol. 2012, 27, 2017–2029. [Google Scholar] [CrossRef]
- Svanborg, C. Urinary tract infections in children: Microbial virulence versus host susceptibility. Adv. Exp. Med. Biol. 2012, 764, 205–210. [Google Scholar] [CrossRef]
- Nielubowicz, G.R.; Mobley, H. Host–pathogen interactions in urinary tract infection. Nat. Rev. Urol. 2010, 7, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Köves, B.; Wullt, B. The roles of the host and the pathogens in urinary tract infections. Eur. Urol. Suppl. 2016, 15, 88–94. [Google Scholar] [CrossRef]
- Perez-Carrasco, V.; Soriano-Lerma, A.; Soriano, M.; Gutiérrez-Fernández, J.; Garcia-Salcedo, J.A. Urinary Microbiome: Yin and Yang of the Urinary Tract. Front. Cell. Infect. Microbiol. 2021, 11, 617002. [Google Scholar] [CrossRef]
- Kawalec, A.; Zwolińska, D. Emerging Role of Microbiome in the Prevention of Urinary Tract Infections in Children. Int. J. Mol. Sci. 2022, 23, 870. [Google Scholar] [CrossRef]
- Garofalo, L.; Nakama, C.; Hanes, D.; Zwickey, H. Whole-Person, Urobiome-Centric Therapy for Uncomplicated Urinary Tract Infection. Antibiotics 2022, 11, 218. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.; Murphy, C.P.; Sleator, R.D.; Culligan, E.P. The urobiome, urinary tract infections, and the need for alternative therapeutics. Microb. Pathog. 2021, 161, 105295. [Google Scholar] [CrossRef]
- Abraham, S.N.; Miao, Y. The nature of immune responses to urinary tract infections. Nat. Rev. Immunol. 2015, 15, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.D.; Schwaderer, A.L.; Becknell, B.; Watson, J.; Hains, D. The innate immune response during urinary tract infection and pyelonephritis. Pediatr. Nephrol. 2013, 29, 1139–1149. [Google Scholar] [CrossRef]
- Weichhart, T.; Haidinger, M.; Hörl, W.H.; Säemann, M.D. Current concepts of molecular defence mechanisms operative during urinary tract infection. Eur. J. Clin. Investig. 2008, 38, 29–38. [Google Scholar] [CrossRef]
- Martell, J.A.O. Immunology of urinary tract infections. GMS Infect. Dis. 2020, 8, Doc21. [Google Scholar]
- Ching, C.; Schwartz, L.; Spencer, J.D.; Becknell, B. Innate immunity and urinary tract infection. Pediatr. Nephrol. 2019, 35, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Ragnarsdóttir, B.; Lutay, N.; Grönberg-Hernandez, J.; Köves, B.; Svanborg, C. Genetics of innate immunity and UTI susceptibility. Nat. Rev. Urol. 2011, 8, 449–468. [Google Scholar] [CrossRef]
- Kaisho, T.; Akira, S. Toll-like receptor function and signaling. J. Allergy Clin. Immunol. 2006, 117, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, E.; Behzadi, P. The role of toll-like receptors (TLRs) in urinary tract infections (UTIs). Cent. Eur. J. Urol. 2016, 69, 404–410. [Google Scholar] [CrossRef]
- Samuelsson, P.; Hang, L.; Wullt, B.; Irjala, H.; Svanborg, C. Toll-Like Receptor 4 Expression and Cytokine Responses in the Human Urinary Tract Mucosa. Infect. Immun. 2004, 72, 3179–3186. [Google Scholar] [CrossRef]
- Scherberich, J.E.; Hartinger, A. Impact of Toll-like receptor signalling on urinary tract infection. Int. J. Antimicrob. Agents 2008, 31, 9–14. [Google Scholar] [CrossRef]
- Albiger, B.; Dahlberg, S.; Henriques-Normark, B.; Normark, S. Role of the innate immune system in host defense against bacterial infections: Focus on the toll-like receptors. J. Intern. Med. 2007, 261, 511–528. [Google Scholar] [CrossRef] [PubMed]
- Becknell, B.; Schwaderer, A.; Hains, D.; Spencer, J.D. Amplifying renal immunity: The role of antimicrobial peptides in pyelonephritis. Nat. Rev. Nephrol. 2015, 11, 642–655. [Google Scholar] [CrossRef]
- Yin, X.; Hou, T.; Liu, Y.; Chen, J.; Yao, Z.; Ma, C.; Yang, L.; Wei, L. Association of Toll-Like Receptor 4 Gene Polymorphism and Expression with Urinary Tract Infection Types in Adults. PLoS ONE 2010, 5, e14223. [Google Scholar] [CrossRef]
- Ragnarsdóttir, B.; Samuelsson, M.; Gustafsson, M.; Leijonhufvud, I.; Karpman, D.; Svanborg, C. Reduced Toll-Like Receptor 4 Expression in Children with Asymptomatic Bacteriuria. J. Infect. Dis. 2007, 196, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Otto, G.; Burdick, M.; Strieter, R.; Godaly, G. Chemokine response to febrile urinary tract infection. Kidney Int. 2005, 68, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef]
- Borish, L.C.; Steinke, J.W. Cytokines and chemokines. J. Allergy Clin. Immunol. 2003, 111, S460–S475. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta 2014, 1843, 2563–2582. [Google Scholar] [CrossRef]
- Mak, R.H.; Kuo, H.J. Pathogenesis of urinary tract infection: An update. Curr. Opin. Pediatr. 2006, 18, 148–152. [Google Scholar] [CrossRef]
- Simões e Silva, A.C. Valério, F.C.; Vasconcelos, M.A.; Miranda, D.M.; Oliveira, E.A. Interactions between Cytokines, Congenital Anomalies of Kidney and Urinary Tract and Chronic Kidney Disease. J. Immunol. Res. 2013, 2013, 1–14. [Google Scholar] [CrossRef]
- Su, H.; Lei, C.-T.; Zhang, C. Interleukin-6 Signaling Pathway and Its Role in Kidney Disease: An Update. Front. Immunol. 2017, 8, 405. [Google Scholar] [CrossRef]
- Abdelaal, A.; AlHamshary, A.A.; El Shaer, O.; Khalil, M.; Younes, A. Urinary Interleukin-6 as biomarker for diagnosis of acute pyelonephritis in children. Geget 2019, 14, 41–46. [Google Scholar] [CrossRef]
- Heinrich, P.C.; Behrmann, I.; Haan, S.; Hermanns, H.M.; Müller-Newen, G.; Schaper, F. Principles of interleukin [IL]-6-type cytokine signalling and its regulation. Biochem. J. 2003, 374, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Sheu, J.-N.; Chen, M.-C.; Lue, K.-H.; Cheng, S.-L.; Lee, I.-C.; Chen, S.-M.; Tsay, G.J. Serum and urine levels of interleukin-6 and interleukin-8 in children with acute pyelonephritis. Cytokine 2006, 36, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Gokce, I.; Alpay, H.; Biyikli, N.; Unluguzel, G.; DeDe, F.; Topuzoglu, A. Urinary levels of interleukin-6 and interleukin-8 in patients with vesicoureteral reflux and renal parenchymal scar. Pediatr. Nephrol. 2010, 25, 905–912. [Google Scholar] [CrossRef]
- Renata, Y.; Jassar, H.; Katz, R.; Hochberg, A.; Nir, R.-R.; Klein-Kremer, A. Urinary concentration of cytokines in children with acute pyelonephritis. Eur. J. Pediatr. 2013, 172, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Valério, F.C.; Lemos, R.D.; Reis, A.L.D.C.; Pimenta, L.P.; Vieira, É.L.M.; Silva, A.C.S.E. Biomarkers in vesicoureteral reflux: An overview. Biomark. Med. 2020, 14, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Bickel, M. The role of interleukin-8 in inflammation and mechanisms of regulation. J. Periodontol. 1993, 64, 456–460. [Google Scholar] [PubMed]
- Galanakis, E.; Bitsori, M.; Dimitriou, H.; Giannakopoulou, C.; Karkavitsas, N.S.; Kalmanti, M. Urine Interleukin-8 as a Marker of Vesicoureteral Reflux in Infants. Pediatrics 2006, 117, e863–e867. [Google Scholar] [CrossRef] [PubMed]
- Zasloff, M. Antimicrobial Peptides, Innate Immunity, and the Normally Sterile Urinary Tract. J. Am. Soc. Nephrol. 2007, 18, 2810–2816. [Google Scholar] [CrossRef] [PubMed]
- Alford, M.A.; Baquir, B.; Santana, F.L.; Haney, E.F.; Hancock, R.E.W. Cathelicidin Host Defense Peptides and Inflammatory Signaling: Striking a Balance. Front. Microbiol. 2020, 11, 1902. [Google Scholar] [CrossRef]
- Chung, C.; Silwal, P.; Kim, I.; Modlin, R.L.; Jo, E.-K. Vitamin D-Cathelicidin Axis: At the Crossroads between Protective Immunity and Pathological Inflammation during Infection. Immune Netw. 2020, 20, e12. [Google Scholar] [CrossRef]
- Wang, G.; Narayana, J.L.; Mishra, B.; Zhang, Y.; Wang, F.; Wang, C.; Zarena, D.; Lushnikova, T.; Wang, X. Design of Antimicrobial Peptides: Progress Made with Human Cathelicidin LL-37. Adv. Exp. Med. Biol. 2019, 1117, 215–240. [Google Scholar] [CrossRef] [PubMed]
- Fruitwala, S.; El-Naccache, D.W.; Chang, T.L. Multifaceted immune functions of human defensins and underlying mechanisms. Semin. Cell Dev. Biol. 2019, 88, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Scudiero, O.; Brancaccio, M.; Mennitti, C.; Laneri, S.; Lombardo, B.; De Biasi, M.G.; De Gregorio, E.; Pagliuca, C.; Colicchio, R.; Salvatore, P.; et al. Human Defensins: A Novel Approach in the Fight against Skin Colonizing Staphylococcus aureus. Antibiotics 2020, 9, 198. [Google Scholar] [CrossRef]
- Tikhonov, I.; Rebenok, A.; Chyzh, A. Nephrology Dialysis Transplantation A study of interleukin-8 and defensins in urine and plasma of patients with pyelonephritis and glomerulonephritis. Nephrol Dial Transplant. 1997, 12, 2557–2561. [Google Scholar] [CrossRef]
- Xu, D.; Lu, W. Defensins: A Double-Edged Sword in Host Immunity. Front. Immunol. 2020, 11, 764. [Google Scholar] [CrossRef]
- Choi, N.; Rigatto, C.; Zappitelli, M.; Gao, A.; Christie, S.; Hiebert, B.; Arora, R.C.; Ho, J. Urinary Hepcidin-25 Is Elevated in Patients That Avoid Acute Kidney Injury Following Cardiac Surgery. Can. J. Kidney Health Dis. 2018, 5. [Google Scholar] [CrossRef]
- Baker, E.; Baker, H.M. Lactoferrin. Cell. Mol. Life Sci. 2005, 62, 2531–2539. [Google Scholar] [CrossRef]
- Devarajan, P. Neutrophil gelatinase-associated lipocalin (NGAL): A new marker of kidney disease. Scand. J. Clin. Lab. Investig. 2008, 68, 89–94. [Google Scholar] [CrossRef]
- Decavele, A.-S.C.; Dhondt, L.; De Buyzere, M.L.; Delanghe, J.R. Increased urinary neutrophil gelatinase associated lipocalin in urinary tract infections and leukocyturia. Clin. Chem. Lab. Med. 2011, 49, 999–1003. [Google Scholar] [CrossRef]
- Forster, C.S.; Johnson, K.; Patel, V.; Wax, R.; Rodig, N.; Barasch, J.; Bachur, R.; Lee, R.S. Urinary NGAL deficiency in recurrent urinary tract infections. Pediatr. Nephrol. 2017, 32, 1077–1080. [Google Scholar] [CrossRef] [PubMed]
- Bolignano, D.; Lacquaniti, A.; Coppolino, G.; Donato, V.; Campo, S.; Fazio, M.R.; Nicocia, G.; Buemi, M. Neutrophil Gelatinase-Associated Lipocalin (NGAL) and Progression of Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2009, 4, 337–344. [Google Scholar] [CrossRef]
- Haase, M.; Bellomo, R.; Devarajan, P.; Schlattmann, P.; Haase-Fielitz, A.; NGAL Meta-Analysis Investigator Group. Accuracy of Neutrophil Gelatinase-Associated Lipocalin (NGAL) in Diagnosis and Prognosis in Acute Kidney Injury: A Systematic Review and Meta-analysis. Am. J. Kidney Dis. 2009, 54, 1012–1024. [Google Scholar] [CrossRef] [PubMed]
- Bolignano, D.; Donato, V.; Coppolino, G.; Campo, S.; Buemi, A.; Lacquaniti, A.; Buemi, M. Neutrophil Gelatinase–Associated Lipocalin (NGAL) as a Marker of Kidney Damage. Am. J. Kidney Dis. 2008, 52, 595–605. [Google Scholar] [CrossRef]
- Yilmaz, A.; Sevketoglu, E.; Gedikbasi, A.; Karyağar, S.; Kiyak, A.; Mulazimoglu, M.; Aydoğan, G.; Ozpacaci, T.; Hatipoglu, S.; Kıyak, A. Early prediction of urinary tract infection with urinary neutrophil gelatinase associated lipocalin. Pediatr. Nephrol. 2009, 24, 2387–2392. [Google Scholar] [CrossRef] [PubMed]
- Seibert, F.S.; Sitz, M.; Passfall, J.; Haesner, M.; Laschinski, P.; Buhl, M.; Bauer, F.; Babel, N.; Pagonas, N.; Westhoff, T.H. Prognostic Value of Urinary Calprotectin, NGAL and KIM-1 in Chronic Kidney Disease. Kidney Blood Press. Res. 2018, 43, 1255–1262. [Google Scholar] [CrossRef]
- Heller, F.; Frischmann, S.; Grünbaum, M.; Zidek, W.; Westhoff, T.H. Urinary Calprotectin and the Distinction between Prerenal and Intrinsic Acute Kidney Injury. Clin. J. Am. Soc. Nephrol. 2011, 6, 2347–2355. [Google Scholar] [CrossRef] [PubMed]
- Boix, E.; Nogués, M.V. Mammalian antimicrobial proteins and peptides: Overview on the RNase A superfamily members involved in innate host defence. Mol. Biosyst. 2007, 3, 317–335. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, H.F. RNase A ribonucleases and host defense: An evolving story. J. Leukoc. Biol. 2008, 83, 1079–1087. [Google Scholar] [CrossRef]
- Spencer, J.D.; Schwaderer, A.L.; Wang, H.; Bartz, J.; Kline, J.; Eichler, T.; DeSouza, K.R.; Sims-Lucas, S.; Baker, P.; Hains, D.S. Ribonuclease 7, an antimicrobial peptide upregulated during infection, contributes to microbial defense of the human urinary tract. Kidney Int. 2013, 83, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.D.; Schwaderer, A.L.; DiRosario, J.D.; McHugh, K.M.; McGillivary, G.; Justice, S.S.; Carpenter, A.R.; Baker, P.B.; Harder, J.; Hains, D. Ribonuclease 7 is a potent antimicrobial peptide within the human urinary tract. Kidney Int. 2011, 80, 174–180. [Google Scholar] [CrossRef]
- Becknell, B.; Eichler, T.E.; Beceiro, S.; Li, B.; Easterling, R.S.; Carpenter, A.R.; James, C.L.; McHugh, K.M.; Hains, D.; Partida-Sanchez, S.; et al. Ribonucleases 6 and 7 have antimicrobial function in the human and murine urinary tract. Kidney Int. 2015, 87, 151–161. [Google Scholar] [CrossRef]
- Säemann, M.D.; Weichhart, T.; Zeyda, M.; Staffler, G.; Schunn, M.; Stuhlmeier, K.M.; Sobanov, Y.; Stulnig, T.M.; Akira, S.; von Gabain, A.; et al. Tamm-Horsfall glycoprotein links innate immune cell activation with adaptive immunity via a Toll-like receptor-4–dependent mechanism. J. Clin. Investig. 2005, 115, 468–475. [Google Scholar] [CrossRef]
- Iorember, F.M.; Vehaskari, V.M. Uromodulin: Old friend with new roles in health and disease. Pediatr. Nephrol. 2013, 29, 1151–1158. [Google Scholar] [CrossRef]
- Wu, T.-H.; Li, K.-J.; Yu, C.-L.; Tsai, C.-Y. Tamm–Horsfall Protein is a Potent Immunomodulatory Molecule and a Disease Biomarker in the Urinary System. Molecules 2018, 23, 200. [Google Scholar] [CrossRef] [PubMed]
- Devuyst, O.; Olinger, E.; Rampoldi, L. Uromodulin: From physiology to rare and complex kidney disorders. Nat. Rev. Nephrol. 2017, 13, 525–544. [Google Scholar] [CrossRef]
- Rampoldi, L.; Scolari, F.; Amoroso, A.; Ghiggeri, G.; Devuyst, O. The rediscovery of uromodulin [Tamm–Horsfall protein]: From tubulointerstitial nephropathy to chronic kidney disease. Kidney Int. 2011, 80, 338–347. [Google Scholar] [CrossRef]
- Devuyst, O.; Dahan, K.; Pirson, Y. Tamm–Horsfall protein or uromodulin: New ideas about an old molecule. Nephrol. Dial. Transpl. 2005, 20, 1290–1294. [Google Scholar] [CrossRef] [PubMed]
- Vyletal, P.; Bleyer, A.J.; Kmoch, S. Uromodulin Biology and Pathophysiology—An Update. Kidney Blood Press. Res. 2010, 33, 456–475. [Google Scholar] [CrossRef]
- Weiss, G.L.; Stanisich, J.J.; Sauer, M.M.; Lin, C.-W.; Eras, J.; Zyla, D.S.; Trück, J.; Devuyst, O.; Aebi, M.; Pilhofer, M.; et al. Architecture and function of human uromodulin filaments in urinary tract infections. Science 2020, 369, 1005–1010. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, C.; Devuyst, O.; Rampoldi, L. Uromodulin: Roles in Health and Disease. Annu. Rev. Physiol. 2021, 83, 477–501. [Google Scholar] [CrossRef] [PubMed]
- Bleyer, A.J.; Ẑivná, M.; Kmoch, S. Uromodulin-Associated Kidney Disease. Nephron Clin. Practice. 2011, 118, c31–c36. [Google Scholar] [CrossRef] [PubMed]
- Lhotta, K. Uromodulin and Chronic Kidney Disease. Kidney Blood Press. Res. 2010, 33, 393–398. [Google Scholar] [CrossRef]
- Kunes, P.; Holubcova, Z.; Kolackova, M.; Krejsek, J. Pentraxin 3(PTX 3): An Endogenous Modulator of the Inflammatory Response. Mediat. Inflamm. 2012, 2012, 1–10. [Google Scholar] [CrossRef]
- Qiu, C.; Liu, T.; Luo, D.; Xie, Z. Pentraxin 3: A powerful orchestrator in urinary tract infection. Nanotheranostics 2021, 5, 445–447. [Google Scholar] [CrossRef]
- Pang, Y.; Tan, Y.; Li, Y.; Zhang, J.; Guo, Y.; Guo, Z.; Zhang, C.; Yu, F.; Zhao, M.H. Pentraxin 3 Is Closely Associated with Tubulointerstitial Injury in Lupus Nephritis: A Large Multicenter Cross-Sectional Study. Medicine 2016, 95, e2520. [Google Scholar] [CrossRef] [PubMed]
- Gursu, M.; Celik, K.; Ozturk, S.; Turkmen, A.; Gorcin, S.; Kocak, B.; Sari, S.; Koldas, M.; Feyizoglu, H.; Kazancioglu, R. Pentraxin 3 and C-Reactive Protein As Inflammatory Markers After a Kidney Transplant. Exp. Clin. Transpl. Off. J. Middle East Soc. Organ Transplant. 2014, 12, 295–299. [Google Scholar]
- Ge, W.; Wang, H.-L.; Sun, R.-P. Pentraxin 3 as a novel early biomarker for the prediction of Henoch-Schönlein purpura nephritis in children. Eur. J. Pediatr. 2013, 173, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Becerir, T.; Yüksel, S.; Evrengül, H.; Ergin, A.; Enli, Y. Urinary excretion of pentraxin-3 correlates with the presence of renal scar following acute pyelonephritis in children. Int. Urol. Nephrol. 2019, 51, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Suliman, M.E.; Yilmaz, M.I.; Carrero, J.J.; Qureshi, A.R.; Saglam, M.; Ipcioglu, O.M.; Yenicesu, M.; Tong, M.; Heimbürger, O.; Barany, P.; et al. Novel Links between the Long Pentraxin 3, Endothelial Dysfunction, and Albuminuria in Early and Advanced Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2008, 3, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Tong, M.; Carrero, J.J.; Qureshi, A.R.; Anderstam, B.; Heimbürger, O.; Bárány, P.; Axelsson, J.; Alvestrand, A.; Stenvinkel, P.; Lindholm, B.; et al. Plasma Pentraxin 3 in Patients with Chronic Kidney Disease: Associations with Renal Function, Protein-Energy Wasting, Cardiovascular Disease, and Mortality. Clin. J. Am. Soc. Nephrol. 2007, 2, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Imai, N.; Nishi, S.; Yoshita, K.; Ito, Y.; Osawa, Y.; Takahashi, K.; Nakagawa, Y.; Saito, K.; Takahashi, K.; Narita, I. Pentraxin-3 expression in acute renal allograft rejection. Clin. Transpl. 2012, 26, 25–31. [Google Scholar] [CrossRef]
- Morozova, O.; Morozov, D.; Pervouchine, D.; Einav, Y.; Lakomova, D.; Zakharova, N.; Severgina, L.; Maltseva, L.; Budnik, I. Urinary biomarkers of latent inflammation and fibrosis in children with vesicoureteral reflux. Int. Urol. Nephrol. 2019, 52, 603–610. [Google Scholar] [CrossRef]
- Parmaksız, G.; Noyan, A.; Dursun, H.; Ince, E.; Anarat, R.; Cengiz, N. Role of new biomarkers for predicting renal scarring in vesicoureteral reflux: NGAL, KIM-1, and L-FABP. Pediatr. Nephrol. 2015, 31, 97–103. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colceriu, M.-C.; Aldea, P.L.; Răchișan, A.-L.; Clichici, S.; Sevastre-Berghian, A.; Mocan, T. Vesicoureteral Reflux and Innate Immune System: Physiology, Physiopathology, and Clinical Aspects. J. Clin. Med. 2023, 12, 2380. https://doi.org/10.3390/jcm12062380
Colceriu M-C, Aldea PL, Răchișan A-L, Clichici S, Sevastre-Berghian A, Mocan T. Vesicoureteral Reflux and Innate Immune System: Physiology, Physiopathology, and Clinical Aspects. Journal of Clinical Medicine. 2023; 12(6):2380. https://doi.org/10.3390/jcm12062380
Chicago/Turabian StyleColceriu, Marius-Cosmin, Paul Luchian Aldea, Andreea-Liana Răchișan, Simona Clichici, Alexandra Sevastre-Berghian, and Teodora Mocan. 2023. "Vesicoureteral Reflux and Innate Immune System: Physiology, Physiopathology, and Clinical Aspects" Journal of Clinical Medicine 12, no. 6: 2380. https://doi.org/10.3390/jcm12062380
APA StyleColceriu, M.-C., Aldea, P. L., Răchișan, A.-L., Clichici, S., Sevastre-Berghian, A., & Mocan, T. (2023). Vesicoureteral Reflux and Innate Immune System: Physiology, Physiopathology, and Clinical Aspects. Journal of Clinical Medicine, 12(6), 2380. https://doi.org/10.3390/jcm12062380






