Incidence and Predictors of Switching and Dose Change of Direct Oral Anticoagulants among Elderly Patients with Nonvalvular Atrial Fibrillation: A 5-Year Analysis of a Large Administrative Database
Abstract
1. Introduction
2. Methods
2.1. Data Source
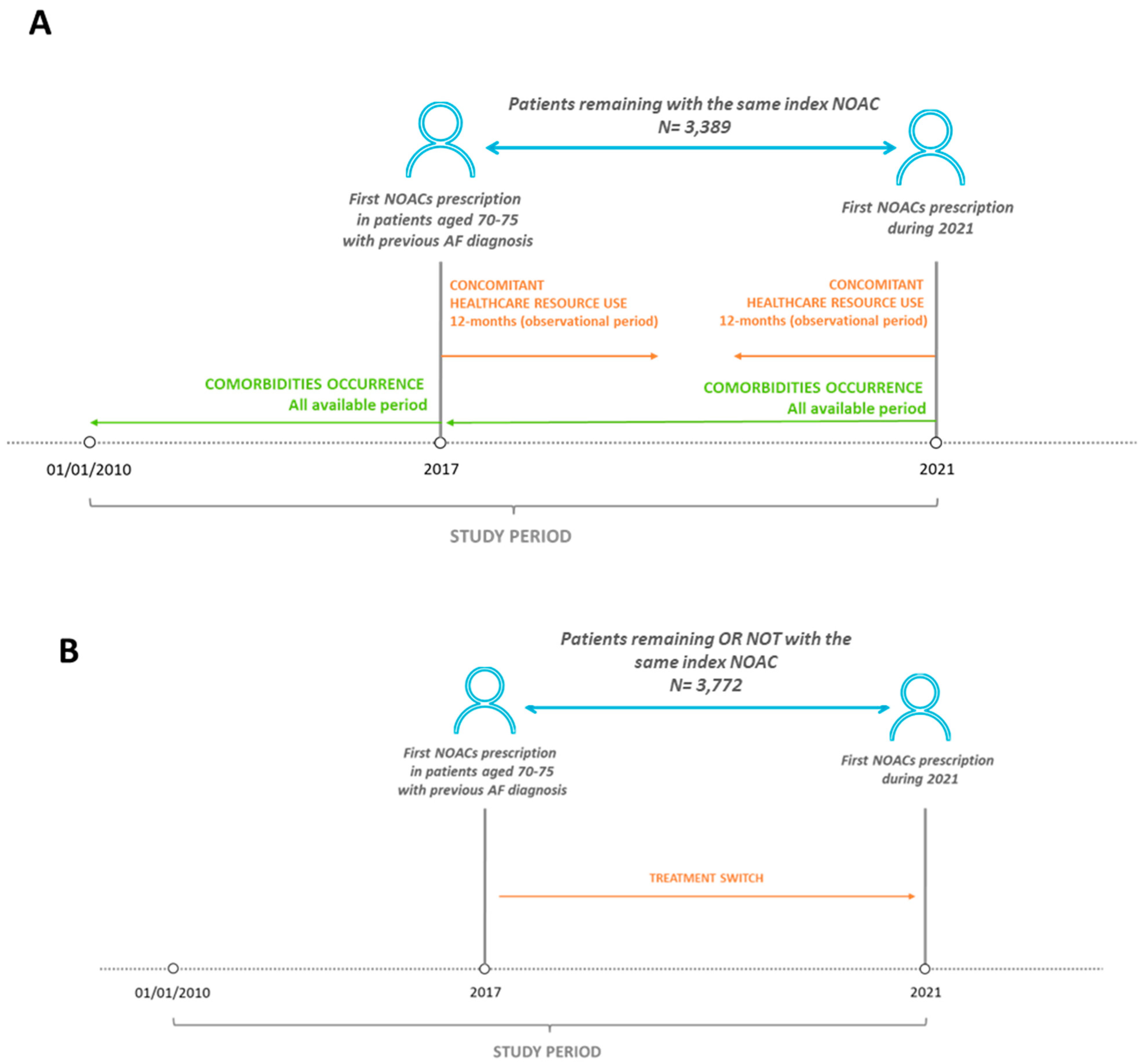
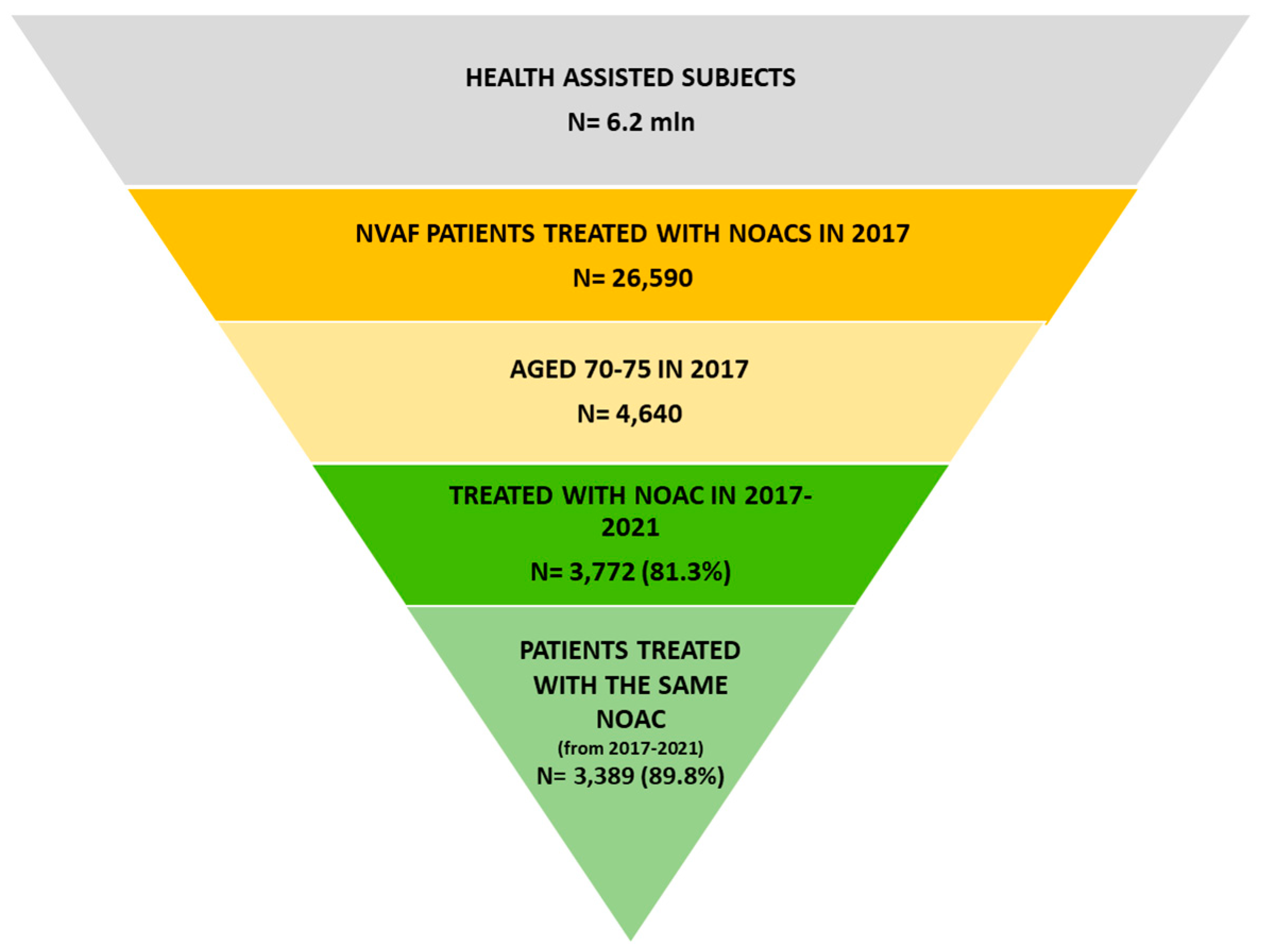
2.2. Study Design and Study Population
2.3. Evaluation of Clinical Burden and Healthcare Resource Use Analysis
2.4. Evaluation of Treatment Switch
2.5. Statistical Analysis
3. Results
4. Discussion
5. Study Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Disclosure
References
- Di Carlo, A.; Bellino, L.; Consoli, D.; Mori, F.; Zaninelli, A.; Baldereschi, M.; Cattarinussi, A.; D’Alfonso, M.G.; Gradia, C.; Sgherzi, B.; et al. Prevalence of atrial fibrillation in the Italian elderly population and projections from 2020 to 2060 for Italy and the European Union: The FAI Project. Europace 2019, 21, 1468–1475. [Google Scholar] [CrossRef]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, R.P.; Ruff, C.T.; Braunwald, E.; Murphy, S.A.; Wiviott, S.D.; Halperin, J.L.; Waldo, A.L.; Ezekowitz, M.D.; Weitz, J.I.; Spinar, J.; et al. Edoxaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2013, 369, 2093–2104. [Google Scholar] [CrossRef] [PubMed]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Avezum, A.; et al. Apixaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2011, 365, 981–992. [Google Scholar] [CrossRef]
- Patel, M.R.; Mahaffey, K.W.; Garg, J.; Pan, G.; Singer, D.E.; Hacke, W.; Breithardt, G.; Halperin, J.L.; Hankey, G.J.; Piccini, J.P.; et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N. Engl. J. Med. 2011, 365, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Ruff, C.T.; Giugliano, R.P.; Braunwald, E.; Hoffman, E.B.; Deenadayalu, N.; Ezekowitz, M.D.; Camm, A.J.; Weitz, J.I.; Lewis, B.S.; Parkhomenko, A.; et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: A meta-analysis of randomised trials. Lancet 2014, 383, 955–962. [Google Scholar] [CrossRef]
- Rose, D.K.; Bar, B. Direct oral anticoagulant agents: Pharmacologic profile, indications, coagulation monitoring, and reversal agents. J. Stroke Cerebrovasc. Dis. 2018, 27, 2049–2058. [Google Scholar] [CrossRef]
- Zhu, J.; Alexander, G.C.; Nazarian, S.; Segal, J.B.; Wu, A.W. Trends and variation in oral anticoagulant choice in patients with atrial fibrillation, 2010–2017. Pharmacotherapy 2018, 38, 907–920. [Google Scholar] [CrossRef]
- Malik, A.H.; Yandrapalli, S.; Aronow, W.S.; Panza, J.A.; Cooper, H.A. Meta-Analysis of Direct-Acting Oral Anticoagulants Compared With Warfarin in Patients >75 Years of Age. Am. J. Cardiol. 2019, 123, 2051–2057. [Google Scholar] [CrossRef]
- Steffel, J.; Collins, R.; Antz, M.; Cornu, P.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; Rowell, N.; et al. 2021 European Heart Rhythm Association Practical Guide on the Use of Non-Vitamin K Antagonist Oral Anticoagulants in Patients with Atrial Fibrillation. Europace 2021, 23, 1612–1676. [Google Scholar] [CrossRef]
- Degli Esposti, L.; Dovizio, M.; Leogrande, M.; Perrone, V.; De Ponti, R. Evaluation of the Impact of Catheter Ablation Procedure on Outcomes and Economic Burden in Patients with Atrial Fibrillation: Real-World Data from Italian Administrative Databases. Healthcare 2022, 10, 2561. [Google Scholar] [CrossRef]
- Perrone, V.; Veronesi, C.; Dovizio, M.; Ancona, D.D.; Bartolini, F.; Ferrante, F.; Lupi, A.; Palcic, S.; Re, D.; Terlizzi, A.P.; et al. The Influence of Iron-Deficiency Anaemia (IDA) Therapy on Clinical Outcomes and Healthcare Resource Consumptions in Chronic Kidney Disease Patients Affected by IDA: A Real-Word Evidence Study among the Italian Population. J. Clin. Med. 2022, 11, 5820. [Google Scholar] [CrossRef] [PubMed]
- Friberg, L.; Gasparini, A.; Carrero, J.J. A scheme based on ICD-10 diagnoses and drug prescriptions to stage chronic kidney disease severity in healthcare administrative records. Clin. Kidney J. 2018, 11, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Erlbaum: Hillsdale, NJ, USA, 2013. [Google Scholar]
- Huisman, M.V.; Rothman, K.J.; Paquette, M.; Teutsch, C.; Diener, H.C.; Dubner, S.J.; Halperin, J.L.; Ma, C.S.; Zint, K.; Elsaesser, A.; et al. The changing landscape for stroke prevention in AF: Findings from the GLORIA-AF Registry Phase 2. J. Am. Coll. Cardiol. 2017, 69, 777–785. [Google Scholar] [CrossRef]
- Alalwan, A.A.; Voils, S.A.; Hartzema, A.G. Trends in utilization of warfarin and direct oral anticoagulants in older adult patients with atrial fibrillation. Am. J. Health Syst. Pharm. 2017, 74, 1237–1244. [Google Scholar] [CrossRef]
- Bezabhe, W.M.; Bereznicki, L.R.; Radford, J.; Wimmer, B.C.; Curtain, C.; Salahudeen, M.S.; Peterson, G.M. Ten-year trends in the use of oral anticoagulants in Australian general practice patients with atrial fibrillation. Front. Pharmacol. 2021, 12, 586370. [Google Scholar] [CrossRef] [PubMed]
- Kefale, A.T.; Peterson, G.M.; Bezabhe, W.M.; Bereznicki, L.R. Switching of oral anticoagulants in patients with nonvalvular atrial fibrillation: A narrative review. Br. J. Clin. Pharmacol. 2022, 88, 514–534. [Google Scholar] [CrossRef]
- Nielsen, P.B.; Skjøth, F.; Søgaard, M.; Kjældgaard, J.N.; Lip, G.Y.; Larsen, T.B. Effectiveness and safety of reduced dose non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: Propensity weighted nationwide cohort study. BMJ 2017, 356, j510. [Google Scholar] [CrossRef]
- Steinberg, B.A.; Shrader, P.; Pieper, K.; Thomas, L.; Allen, L.A.; Ansell, J.; Chan, P.S.; Ezekowitz, M.D.; Fonarow, G.C.; Freeman, J.V.; et al. Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) II Investigators. Frequency and Outcomes of Reduced Dose Non-Vitamin K Antagonist Anticoagulants: Results From ORBIT-AF II (The Outcomes Registry for Better Informed Treatment of Atrial Fibrillation II). J. Am. Heart. Assoc. 2018, 7, e007633. [Google Scholar]
- Fosbøl, E.L.; Vinding, N.E.; Lamberts, M.; Staerk, L.; Gundlund, A.; Gadsbøll, K.; Køber, L.; Gislason, G.H.; Olesen, J.B. Shifting to a non-vitamin K antagonist oral anticoagulation agent from vitamin K antagonist in atrial fibrillation. Europace 2018, 20, e78–e86. [Google Scholar] [CrossRef]
- Martinez, C.; Katholing, A.; Wallenhorst, C.; Freedman, S.B. Therapy persistence in newly diagnosed non-valvular atrial fibrillation treated with warfarin or NOAC. A cohort study. Thromb. Haemost. 2016, 115, 31–39. [Google Scholar] [PubMed]
- Olimpieri, P.P.; Di Lenarda, A.; Mammarella, F.; Gozzo, L.; Cirilli, A.; Cuomo, M.; Gulizia, M.M.; Colivicchi, F.; Murri, G.; Gabrielli, D.; et al. Non-vitamin K antagonist oral anticoagulation agents in patients with atrial fibrillation: Insights from Italian monitoring registries. Int. J. Cardiol. Heart. Vasc. 2020, 26, 100465. [Google Scholar] [CrossRef] [PubMed]
- Santoro, C.; Capone, V.; Canonico, M.E.; Gargiulo, G.; Esposito, R.; Sanna, G.D.; Parodi, G.; Esposito, G. Single, Dual, and Triple Antithrombotic Therapy in Cancer Patients with Coronary Artery Disease: Searching for Evidence and Personalized Approaches. Semin. Thromb. Hemost. 2021, 47, 950–961. [Google Scholar] [CrossRef]
- Hohnloser, S.H.; Basic, E.; Nabauer, M. Changes in oral anticoagulation therapy over one year in 51,000 atrial fibrillation patients at risk for stroke: A practice-derived study. Thromb. Haemost. 2019, 119, 882–893. [Google Scholar] [CrossRef]
- Hellfritzsch, M.; Husted, S.E.; Grove, E.L.; Rasmussen, L.; Poulsen, B.K.; Johnsen, S.P.; Hallas, J.; PottegAard, A. Treatment changes among users of non-vitamin K antagonist oral anticoagulants in atrial fibrillation. Basic. Clin. Pharmacol. Toxicol. 2017, 120, 187–194. [Google Scholar] [CrossRef]
- Baker, C.L.; Dhamane, A.D.; Rajpura, J.; Mardekian, J.; Dina, O.; Russ, C.; Rosenblatt, L.; Lingohr-Smith, M.; Lin, J. Switching to another oral anticoagulant and drug discontinuation among elderly patients with nonvalvular atrial fibrillation treated with different direct oral anticoagulants. Clin. Appl. Thromb. Hemost. 2019, 25, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Boriani, G.; Proietti, M.; Laroche, C.; Diemberger, I.; Popescu, M.I.; Riahi, S.; Shantsila, A.; Dan, G.A.; Tavazzi, L.; Maggioni, A.P.; et al. Changes to oral anticoagulant therapy and risk of death over a 3-year follow-up of a contemporary cohort of European patients with atrial fibrillation final report of the EURObservational Research Programme on Atrial Fibrillation (EORP-AF) pilot general registry. Int. J. Cardiol. 2018, 271, 68–74. [Google Scholar] [PubMed]
- Bikdeli, B.; Tajrishi, F.Z.; Sadeghipour, P.; Talasaz, A.H.; Fanikos, J.; Lippi, G.; Siegal, D.M.; Eikelboom, J.W.; Monreal, M.; Jimenez, D.; et al. Efficacy and Safety Considerations With Dose-Reduced Direct Oral Anticoagulants: A Review. JAMA Cardiol. 2022, 7, 747–759. [Google Scholar] [CrossRef]
- Silverio, A.; Di Maio, M.; Prota, C.; De Angelis, E.; Radano, I.; Citro, R.; Carrizzo, A.; Ciccarelli, M.; Vecchione, C.; Capodanno, D.; et al. Safety and efficacy of non-vitamin K antagonist oral anticoagulants in elderly patients with atrial fibrillation: Systematic review and meta-analysis of 22 studies and 440,281 patients. Eur. Heart J. Cardiovasc. Pharmacother. 2021, 7, f20–f29. [Google Scholar] [CrossRef]
| Dabigatran N = 885 | Rivaroxaban N = 1259 | Apixaban N = 1030 | Edoxaban 215 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2017 | 2021 | Δ% | 2017 | 2021 | Δ% | 2017 | 2021 | Δ% | 2017 | 2021 | Δ% | |
| Antihypertensives use (n, %) | 839 (94.8) | 865 (97.7) | 3.1 | 1127 (89.5) | 1228 (97.5) | 9.0 | 942 (91.5) | 1003 (97.4) | 6.5 | 189 (87.9) | 208 (96.7) | 10.1 |
| Lipid-lowering agents use (n, %) | 454 (51.3) | 522 (59.0) | 15.0 | 610 (48.5) | 737 (58.5) | 20.8 | 491 (47.7) | 596 (57.9) | 21.4 | 104 (48.4) | 128 (59.5) | 23.1 |
| Rheumatoid arthritis (n, %) | 6 (0.7) | 10 (1.1) | 66.7 | 15 (1.2) | 18 (1.4) | 20.0 | 4 (0.4) | 5 (0.5) | 25.0 | <4 | <4 | / |
| Chronic kidney disease (n, %) | 159 (18.0) | 229 (25.9) | 44.0 | 249 (19.8) | 358 (28.4) | 43.8 | 238 (23.1) | 345 (33.5) | 45.0 | 46 (21.4) | 62 (28.8) | 34.8 |
| GFR < 60 (n, %) | 30 (3.4) | 36 (4.1) | 20.0 | 50 (4.0) | 66 (5.2) | 32.0 | 33 (3.2) | 57 (5.5) | 72.7 | 7 (3.3) | 12 (5.6) | 71.4 |
| Osteoporosis medication use (n, %) | 45 (5.1) | 61 (6.9) | 35.6 | 54 (4.3) | 82 (6.5) | 51.9 | 62 (6.0) | 98 (9.5) | 58.1 | 12 (5.6) | 15 (7.0) | 25.0 |
| Trauma (n, %) | 69 (7.8) | 102 (11.5) | 47.8 | 72 (5.7) | 129 (10.2) | 79.2 | 75 (7.3) | 110 (10.7) | 46.7 | 15 (7.0) | 22 (10.2) | 46.7 |
| COPD (n, %) | 248 (28.0) | 333 (37.6) | 34.3 | 353 (28.0) | 447 (35.5) | 26.6 | 303 (29.4) | 399 (38.7) | 31.7 | 57 (26.5) | 71 (33.0) | 24.6 |
| Anti-diabetics use (n, %) | 203 (22.9) | 246 (27.8) | 21.2 | 280 (22.2) | 335 (26.6) | 19.6 | 225 (21.8) | 272 (26.4) | 20.9 | 39 (18.1) | 44 (20.5) | 12.8 |
| AMI (n, %) | 211 (23.8) | 252 (28.5) | 19.4 | 255 (20.3) | 301 (23.9) | 18.0 | 212 (20.6) | 252 (24.5) | 18.9 | 53 (24.7) | 60 (27.9) | 13.2 |
| Heart failure (n, %) | 148 (16.7) | 184 (20.8) | 24.3 | 254 (20.2) | 327 (26.0) | 28.7 | 245 (23.8) | 309 (30.0) | 26.1 | 53 (24.7) | 62 (28.8) | 17.0 |
| Cancer (n, %) | 59 (6.7) | 94 (10.6) | 59.3 | 75 (6.0) | 110 (8.7) | 46.7 | 80 (7.8) | 119 (11.6) | 48.8 | 15 (7.0) | 24 (11.2) | 60.0 |
| Liver disease (n, %) | 26 (2.9) | 35 (4.0) | 34.6 | 48 (3.8) | 60 (4.8) | 25.0 | 39 (3.8) | 47 (4.6) | 20.5 | 13 (6.0) | 15 (7.0) | 15.4 |
| Treatment Switch across 2017–2021 | N | Dabigatran, 2021 | Rivaroxaban, 2021 | Apixaban, 2021 | Edoxaban, 2021 |
|---|---|---|---|---|---|
| Dabigatran, 2017 | 1062 | - | 47 (4.4) | 76 (7.2) | 54 (5.1) |
| Rivaroxaban, 2017 | 1373 | 21 (1.5) | - | 53 (3.9) | 40 (2.9) |
| Apixaban, 2017 | 1097 | 9 (0.8) | 20 (1.8) | - | 38 (3.5) |
| Edoxaban, 2017 | 240 | 4 (1.7) | 8 (3.3) | 13 (5.4) | - |
| TOTAL (2017–2021% variation) | 3772 | 919 (−13.5%) | 1334 (−2.8%) | 1172 (+6.8%) | 347 (+44.6%) |
| Treatment Switch across 2017–2021 | N | Dabigatran Reduced Dose, 2021 | Dabigatran Standard Dose, 2021 | Rivaroxaban Low Dose, 2021 | Rivaroxaban Standard Dose, 2021 | Apixaban Low Dose, 2021 | Apixaban Standard Dose, 2021 | Edoxaban Low Dose, 2021 | Edoxaban Standard Dose, 2021 |
|---|---|---|---|---|---|---|---|---|---|
| Dabigatran reduced dose, 2017 | 327 | - | 45 (13.8) | <4 | 7 (2.1) | 10 (3.1) | 15 (4.6) | 8 (2.4) | 7 (2.1) |
| Dabigatran standard dose, 2017 | 735 | 72 (9.8) | - | 5 (0.7) | 32 (4.4) | 4 (0.5) | 47 (6.4) | 8 (1.1) | 31 (4.2) |
| Rivaroxaban low dose, 2017 | 227 | <4 | 0 (0.0) | - | 42 (18.5) | 6 (2.6) | 10 (4.4) | 7 (3.1) | 0 (0.0) |
| Rivaroxaban standard dose, 2017 | 1146 | 7 (0.6) | 13 (1.1) | 111 (9.7) | - | 9 (0.8) | 28 (2.4) | 12 (1.0) | 21 (1.8) |
| Apixaban low dose, 2017 | 112 | 0 (0.0) | 0 (0.0) | <4 | <4 | - | 39 (34.8) | 5 (4.5) | <4 |
| Apixaban standard dose, 2017 | 985 | <4 | 6 (0.6) | 8 (0.8) | 10 (1.0) | 47 (4.8) | - | 9 (0.9) | 23 (2.3) |
| Edoxaban low dose, 2017 | 56 | <4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | <4 | <4 | - | 8 (14.3) |
| Edoxaban standard dose, 2017 | 184 | 0 (0.0) | <4 | 4 (2.2) | 4 (2.2) | <4 | 8 (4.3) | 24 (13.0) | - |
| total (2017–2021 % switch variation) | 3772 | 316 (−3.4) | 603 (−18.0) | 293 (29.1) | 1041 (−9.2) | 143 (27.7) | 1029 (4.5) | 116 (107.1) | 231 (25.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Luca, L.; Dovizio, M.; Sangiorgi, D.; Perrone, V.; Degli Esposti, L. Incidence and Predictors of Switching and Dose Change of Direct Oral Anticoagulants among Elderly Patients with Nonvalvular Atrial Fibrillation: A 5-Year Analysis of a Large Administrative Database. J. Clin. Med. 2023, 12, 2379. https://doi.org/10.3390/jcm12062379
De Luca L, Dovizio M, Sangiorgi D, Perrone V, Degli Esposti L. Incidence and Predictors of Switching and Dose Change of Direct Oral Anticoagulants among Elderly Patients with Nonvalvular Atrial Fibrillation: A 5-Year Analysis of a Large Administrative Database. Journal of Clinical Medicine. 2023; 12(6):2379. https://doi.org/10.3390/jcm12062379
Chicago/Turabian StyleDe Luca, Leonardo, Melania Dovizio, Diego Sangiorgi, Valentina Perrone, and Luca Degli Esposti. 2023. "Incidence and Predictors of Switching and Dose Change of Direct Oral Anticoagulants among Elderly Patients with Nonvalvular Atrial Fibrillation: A 5-Year Analysis of a Large Administrative Database" Journal of Clinical Medicine 12, no. 6: 2379. https://doi.org/10.3390/jcm12062379
APA StyleDe Luca, L., Dovizio, M., Sangiorgi, D., Perrone, V., & Degli Esposti, L. (2023). Incidence and Predictors of Switching and Dose Change of Direct Oral Anticoagulants among Elderly Patients with Nonvalvular Atrial Fibrillation: A 5-Year Analysis of a Large Administrative Database. Journal of Clinical Medicine, 12(6), 2379. https://doi.org/10.3390/jcm12062379






