Clinical Evaluation of Bilateral Multiple Gingival Recession Treatment with Autogenous Connective Tissue Graft Associated with Low-Level Laser Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Aspects
2.2. Study Population and Sample Size
2.3. Periodontal and Clinical Parameters Evaluation
2.4. Allocation and Blinding
2.5. Periodontal Surgical and Post-Operative Treatment
2.6. Laser Protocol
2.7. Statistical Analysis
3. Results
3.1. Sample Sociodemographic and Clinical Characteristics
3.2. Dental Hypersensitivity
3.3. Dental Biofilm Control
3.4. Probing Depth and Clinical Attachment Level
3.5. Gingival Recession
3.6. Keratinized Tissue Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mester, E.; Ludany, G.; Sellyei, M.; Szende, B. On the biologic effect of laser rays. Bull. Soc. Int. Chir. 1968, 27, 68–73. [Google Scholar]
- Mester, E.; Ludány, G.; Sellyei, M.; Szende, B.; Gyenes, G.; Tota, G.J. Studies on the inhibiting and activating effects of laser beams. Langenbecks Arch. Chir. 1968, 322, 1022–1027. [Google Scholar] [CrossRef] [PubMed]
- Mester, E.; Szende, B.; Gärtner, P. The effect of laser beams on the growth of hair in mice. Radiobiol. Radiother. 1968, 9, 621–626. [Google Scholar]
- Mester, E.; Spiry, T.; Szende, B.; Tota, J.G. Effect of laser rays on wound healing. Am. J. Surg. 1971, 122, 532–535. [Google Scholar] [CrossRef]
- Bereiter-Hahn, J. Laser as a micromanipulator in biology and medicine. 2. Methods and use of laser microirradiation of biological matters. Microsc. Acta 1972, 72, 1–33. [Google Scholar]
- Mester, E.; Spiry, T.; Szende, B. Effect of laser rays on wound healing. Bull. Soc. Int. Chir. 1973, 32, 169–173. [Google Scholar] [CrossRef]
- Kara, O.; Dirihan, R.S.; Ozel, G.S.; Mutluay, A.T.; Usumez, A. Inhibition of cathepsin-K and matrix metalloproteinase by photodynamic therapy. Dent. Mater. 2021, 37, e485–e492. [Google Scholar] [CrossRef]
- Chambrone, L.; Wang, H.-L.; Romanos, G.E. Antimicrobial photodynamic therapy for the treatment of periodontitis and peri-implantitis: An American Academy of Periodontology best evidence review. J. Periodontol. 2018, 89, 783–803. [Google Scholar]
- Butera, A.; Maiorani, C.; Morandini, A.; Simonini, M.; Colnaghi, A.; Morittu, S.; Barbieri, S.; Ricci, M.; Guerrisi, G.; Piloni, D.; et al. Assessment of Oral Microbiome Changes in Healthy and COVID-19-Affected Pregnant Women: A Narrative Review. Microorganisms 2021, 9, 2385. [Google Scholar] [CrossRef]
- Colombo, M.; Gallo, S.; Garofoli, A.; Poggio, C.; Arciola, C.; Scribante, A. Ozone Gel in Chronic Periodontal Disease: A Randomized Clinical Trial on the Anti-Inflammatory Effects of Ozone Application. Biology 2021, 10, 625. [Google Scholar] [CrossRef]
- Ren, C.; McGrath, C.; Jin, L.; Zhang, C.; Yang, Y. The effectiveness of low-level laser therapy as an adjunct to non-surgical periodontal treatment: A meta-analysis. J. Periodontal Res. 2017, 52, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Theodoro, L.H.; Marcantonio, R.A.C.; Wainwright, M.; Garcia, V.G. LASER in periodontal treatment: Is it an effective treatment or science fiction? Braz. Oral Res. 2021, 24 (Suppl. S2), e099. [Google Scholar] [CrossRef] [PubMed]
- Giorgetti, A.P.O.; De Matos, R.; Casarin, R.C.V.; Pimentel, S.P.; Cirano, F.R.; Ribeiro, F.V. Preemptive and Postoperative Medication Protocols for Root Coverage Combined with Connective Tissue Graft. Braz. Dent. J. 2018, 29, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Toledano-Osorio, M.; Muñoz-Soto, E.; Toledano, M.; Vallecillo-Rivas, M.; Vallecillo, C.; Ramos-García, P.; Osorio, R. Treating Gingival Recessions Using Coronally Advanced Flap or Tunnel Techniques with Autografts or Polymeric Substitutes: A Systematic Review and Meta-Analysis. Polymers 2022, 14, 1453. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.-L.; Fu, E.; Tu, Y.-K.; Shen, E.-C.; Chiu, H.-C.; Huang, R.-Y.; Yuh, D.-Y.; Chiang, C.-Y. Root coverage by coronally advanced flap with connective tissue graft and/or enamel matrix derivative: A meta-analysis. J. Periodontal Res. 2015, 50, 220–230. [Google Scholar] [CrossRef]
- Zucchelli, G.; Mele, M.; Stefanini, M.; Mazzotti, C.; Marzadori, M.; Montebugnoli, L.; de Sanctis, M. Patient morbidity and root coverage outcome after subepithelial connective tissue and de-epithelialized grafts: A comparative randomized-controlled clinical trial. J. Clin. Periodontol. 2010, 37, 728–738. [Google Scholar] [CrossRef]
- Gobbato, L.; Nart, J.; Bressan, E.; Mazzocco, F.; Paniz, G.; Lops, D. Patient morbidity and root coverage outcomes after the application of a subepithelial connective tissue graft in combination with a coronally advanced flap or via a tunneling technique: A randomized controlled clinical trial. Clin. Oral Investig. 2016, 20, 2191–2202. [Google Scholar] [CrossRef]
- Fernandes-Dias, S.B.; de Marco, A.C.; Santamaria, M., Jr.; Kerbauy, W.D.; Jardini, M.A.; Santamaria, M.P. Connective tissue graft associated or not with low laser therapy to treat gingival recession: Randomized clinical trial. J. Clin. Periodontol. 2015, 42, 54–61. [Google Scholar] [CrossRef]
- Santamaria, M.; Fernandes-Dias, S.B.; Araujo, C.; Neves, F.L.D.S.; Mathias, I.F.; Andere, N.M.R.B.; Jardini, M. 2-Year Assessment of Tissue Biostimulation with Low-Level Laser on the Outcomes of Connective Tissue Graft in the Treatment of Single Gingival Recession: A Randomized Clinical Trial. J. Periodontol. 2017, 88, 320–328. [Google Scholar] [CrossRef]
- Lafzi, A.; Kadkhodazadeh, M.; Mojahedi, S.M.; Amid, R.; Shidfar, S.; Baghani, M.T. The Clinical Evaluation of the Effects of Low-Level Laser Therapy on the Donor and Recipient Sites of the Free Gingival Graft: A Case Series. J. Lasers Med. Sci. 2019, 10, 355–360. [Google Scholar] [CrossRef]
- Lavu, V.; Gutknecht, N.; Vasudevan, A.; SK, B.; Hilgers, R.-D.; Franzen, R. Laterally closed tunnel technique with and without adjunctive photobiomodulation therapy for the management of isolated gingival recession—A randomized controlled assessor-blinded clinical trial. Lasers Med. Sci. 2022, 37, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.C.-Y.; Nevins, M.; Kim, D.M. Laser De-epithelialization of Autogenous Gingival Graft for Root Coverage and Soft Tissue Augmentation Procedures. Int. J. Periodontics Restor. Dent. 2018, 38, 405–411. [Google Scholar] [CrossRef]
- Behdin, S.; Monje, A.; Lin, G.-H.; Edwards, B.; Othman, A.; Wang, H.-L. Effectiveness of Laser Application for Periodontal Surgical Therapy: Systematic Review and Meta-Analysis. J. Periodontol. 2015, 86, 1352–1363. [Google Scholar] [CrossRef] [PubMed]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.C.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions—Introduction and key changes from the 1999 classification. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S1–S8. [Google Scholar] [CrossRef]
- Miller, P.D., Jr. A classification of marginal tissue recession. Int. J. Periodontics Restor. Dent. 1985, 5, 8–13. [Google Scholar]
- Hefti, A.F.; Preshaw, P.M. Examiner alignment and assessment in clinical periodontal research. Periodontology 2000 2012, 59, 41–60. [Google Scholar] [CrossRef]
- Rocha, M.O.C.; Cruz, A.A.C.F.; Santos, D.O.; Douglas-De-Oliveira, D.W.; Flecha, O.D.; Gonçalves, P.F. Sensitivity and specificity of assessment scales of dentin hypersensitivity—An accuracy study. Braz. Oral Res. 2020, 34, e043. [Google Scholar] [CrossRef]
- Zucchelli, G.; De Sanctis, M. Treatment of Multiple Recession-Type Defects in Patients with Esthetic Demands. J. Periodontol. 2000, 71, 1506–1514. [Google Scholar] [CrossRef]
- Zhao, H.; Hu, J.; Zhao, L. The effect of low-level laser therapy as an adjunct to periodontal surgery in the management of postoperative pain and wound healing: A systematic review and meta-analysis. Lasers Med. Sci. 2020, 36, 175–187. [Google Scholar] [CrossRef]
- Ustaoglu, G.; Ercan, E.; Tunali, M. Low-Level Laser Therapy in Enhancing Wound Healing and Preserving Tissue Thickness at Free Gingival Graft Donor Sites: A Randomized, Controlled Clinical Study. Photomed. Laser Surg. 2017, 35, 223–230. [Google Scholar] [CrossRef]
- Morshedzadeh, G.; Aslroosta, H.; Vafaei, M. Effect of GaAlAs 940 nm Photobiomodulation on palatal wound healing after free gingival graft surgery: A split mouth randomized controlled clinical trial. BMC Oral Health 2022, 22, 202. [Google Scholar] [CrossRef] [PubMed]
- Ozturan, S.; Durukan, S.A.; Ozcelik, O.; Seydaoglu, G.; Haytac, M.C. Coronally advanced flap adjunct with low intensity laser therapy: A randomized controlled clinical pilot study. J. Clin. Periodontol. 2011, 38, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Pini-Prato, G.P.; Cairo, F.; Nieri, M.; Franceschi, D.; Rotundo, R.; Cortellini, P. Coronally advanced flap versus connective tissue graft in the treatment of multiple gingival recessions: A split-mouth study with a 5-year follow-up. J. Clin. Periodontol. 2010, 37, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Pereira Neto, A.R.L.; de Albuquerque, F.R.; Alves, A.C.B.A.; Fonseca, R.R.S.; de Menezes, S.A.F.; Benfatti, C.A.M. Creeping attachment after autogenous free gingival grafting: Case report. Braz. J. Periodontol. 2019, 29, 24–29. [Google Scholar]
- Karu, T. Photobiology of Low-power Laser Effects. Health Phys. 1989, 56, 691–704. [Google Scholar] [CrossRef]
- Scribante, A.; Butera, A.; Alovisi, M. Customized Minimally Invasive Protocols for the Clinical and Microbiological Management of the Oral Microbiota. Microorganisms 2022, 10, 675. [Google Scholar] [CrossRef]

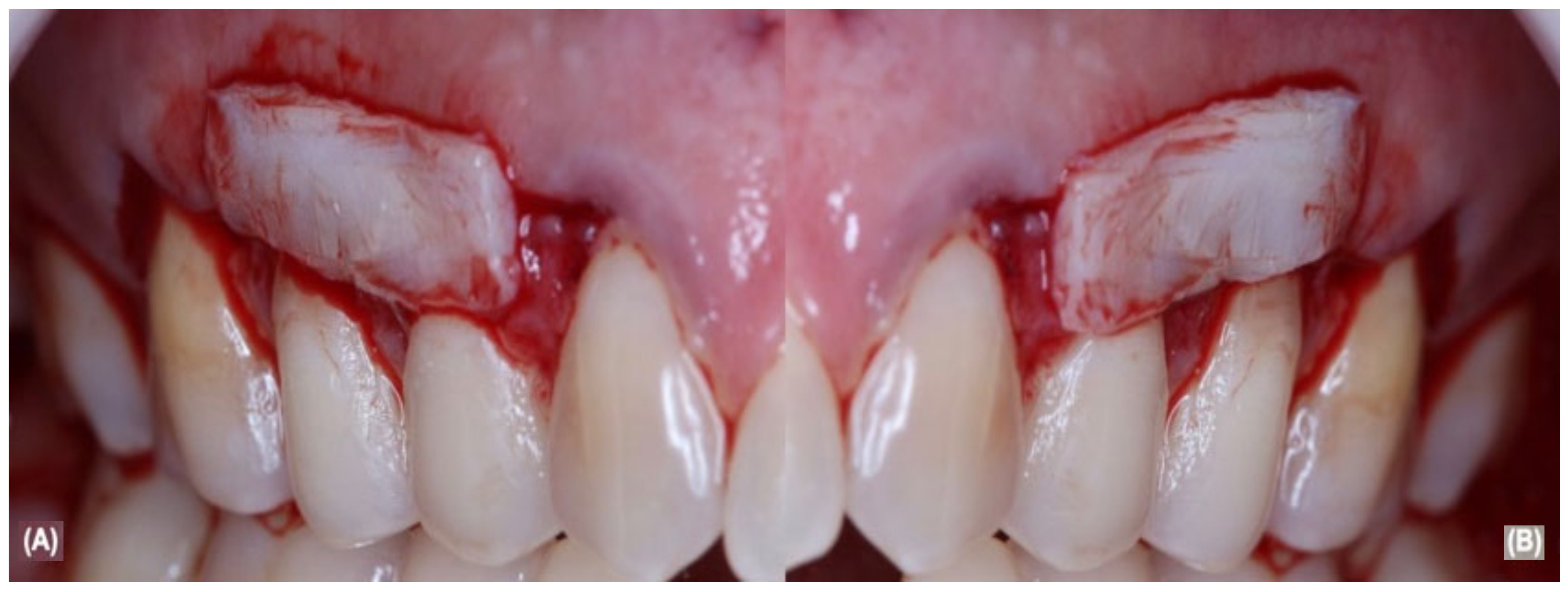
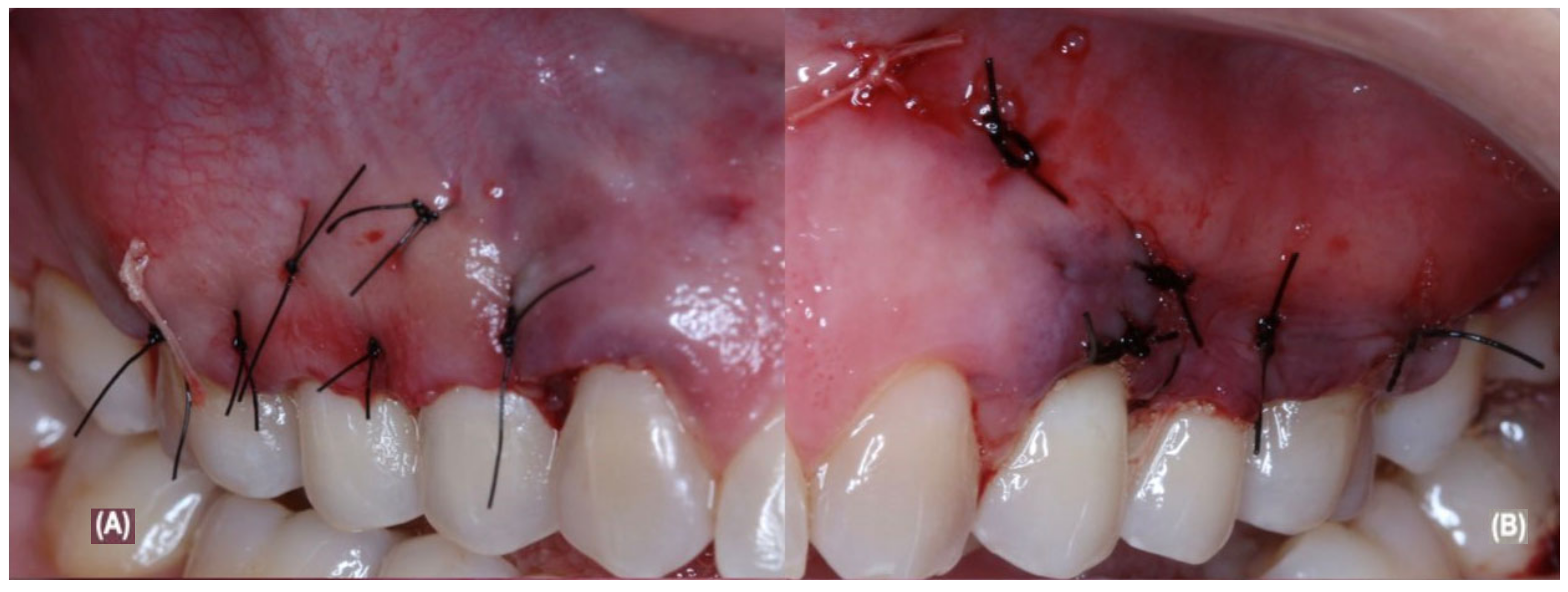
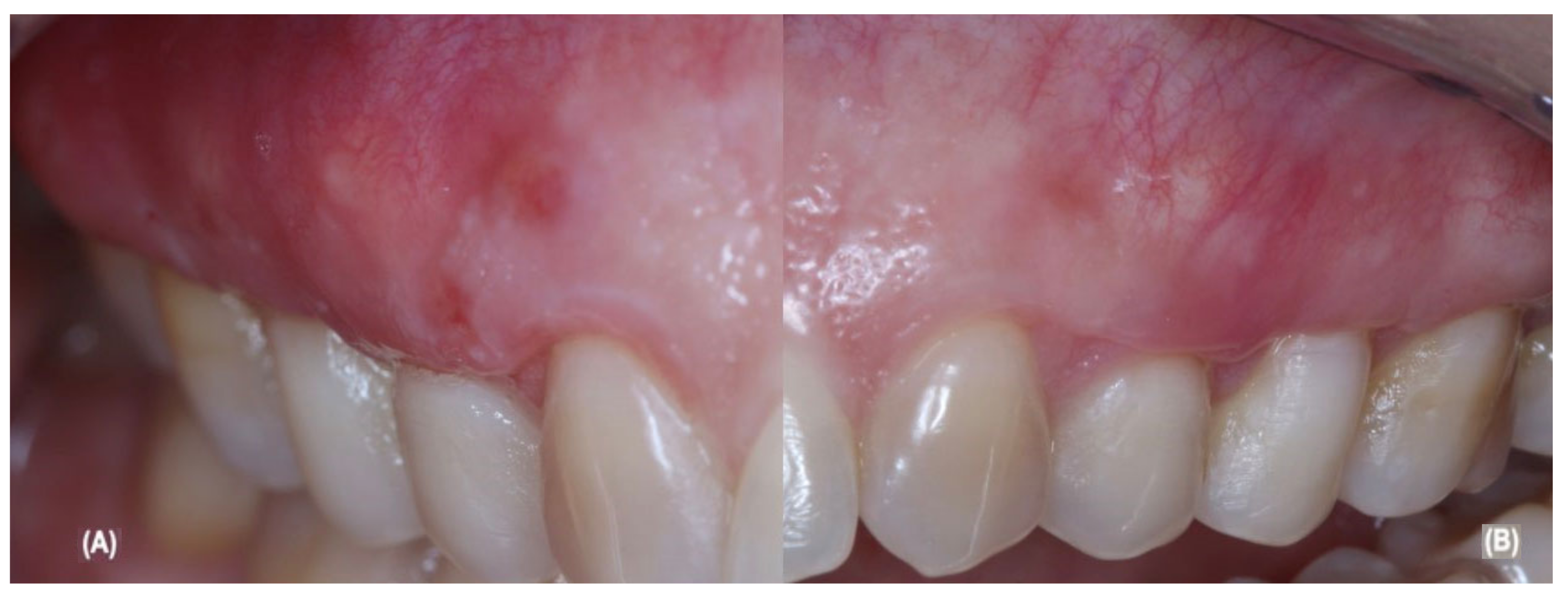
| Parameters | Pre-Treatment | G1: aCTG Treatment | G2: aCTG + LLLT Treatment |
|---|---|---|---|
| Age (years) | 35 years | - | - |
| Sex | Female | - | - |
| Ethnicity † | White | - | - |
| Source | Belém | - | - |
| Educational level | Completed post-graduation | - | - |
| Marital status | Married | - | - |
| Traumatic brushing | Yes | No | No |
| Dental hypersensitivity | Yes | No | No |
| Pain during feeding | Yes | No | No |
| Exposed dentin root surface | Yes | No | No |
| Parafunctional habit | No | No | No |
| Numerical scale of hypersensitivity | Level 7 | Level 1 | Level 0 |
| Facial pain scale | Moderate pain iconography | Light pain iconography | No pain iconography |
| Toothbrush type | Hard bristles | Soft bristles | Soft bristles |
| Oral Hygiene | Yes | Yes | Yes |
| Dental floss use | Yes | Yes | Yes |
| Simplified oral hygiene index | Level 1 | Level 0 | Level 0 |
| Bleeding on probing * | Absent | Absent | Absent |
| Probing depth * | |||
| Tooth 13 | 1 mm | - | 2 mm |
| Tooth 14 | 2 mm | - | 3 mm |
| Tooth 15 | 2 mm | - | 3 mm |
| Tooth 23 | 1 mm | 3 mm | - |
| Tooth 24 | 2 mm | 3 mm | - |
| Tooth 25 | 2 mm | 3 mm | - |
| Clinical attachment level * | |||
| Tooth 13 | 4 mm | - | 2 mm |
| Tooth 14 | 4 mm | - | 3 mm |
| Tooth 15 | 5 mm | - | 3 mm |
| Tooth 23 | 4 mm | 3 mm | - |
| Tooth 24 | 4 mm | 3 mm | - |
| Tooth 25 | 4 mm | 3 mm | - |
| Gingival recession * | |||
| Tooth 13 | 3 mm | - | Absent |
| Tooth 14 | 2 mm | - | Absent |
| Tooth 15 | 3 mm | - | Absent |
| Tooth 23 | 3 mm | 1 mm | - |
| Tooth 24 | 2 mm | ≤1 mm | - |
| Tooth 25 | 2 mm | ≤1 mm | - |
| Keratinized tissue width * | |||
| Tooth 13 | 1 mm | - | 3 mm |
| Tooth 14 | 1 mm | - | 3 mm |
| Tooth 15 | 1 mm | - | 3 mm |
| Tooth 23 | 1 mm | 3 mm | - |
| Tooth 24 | 1 mm | 3 mm | - |
| Tooth 25 | 1 mm | 3 mm | - |
| Keratinized tissue thickness * | |||
| Tooth 13 | 1 mm | - | 2 mm |
| Tooth 14 | 1 mm | - | 2 mm |
| Tooth 15 | 2 mm | - | 2 mm |
| Tooth 23 | 1 mm | 2 mm | - |
| Tooth 24 | 1 mm | 2 mm | - |
| Tooth 25 | 1 mm | 2 mm | - |
| Parameters (Measure Unit) | Pre-Treatment | G1: aCTG * Treatment | G2: aCTG + LLLT † Treatment |
|---|---|---|---|
| Probing depth (mm) | 1.66 ± 0.51 | 3.00 ± 0.00 | 2.66 ± 0.57 |
| Clinical attachment level (mm) | 4.16 ± 0.40 | 3.00 ± 0.00 | 2.66 ± 0.57 |
| Gingival recession (mm) | 2.50 ± 0.54 | 1.00 ± 0.00 | - |
| Keratinized tissue width (mm) | 1.00 ± 0.00 | 3.00 ± 0.00 | 3.00 ± 0.00 |
| Keratinized tissue thickness (mm) | 1.16 ± 0.40 | 2.00 ± 0.00 | 2.00 ± 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza Fonseca, R.R.; Silva, C.P.; de Senna Sastre, B.L.; Tanaka, E.B.; Carvalho, T.R.B.; de Oliveira, P.G.F.P.; de Menezes, S.A.F.; Laurentino, R.V.; de Oliveira, R.P.; de Oliveira, R.P.; et al. Clinical Evaluation of Bilateral Multiple Gingival Recession Treatment with Autogenous Connective Tissue Graft Associated with Low-Level Laser Therapy. J. Clin. Med. 2023, 12, 2349. https://doi.org/10.3390/jcm12062349
de Souza Fonseca RR, Silva CP, de Senna Sastre BL, Tanaka EB, Carvalho TRB, de Oliveira PGFP, de Menezes SAF, Laurentino RV, de Oliveira RP, de Oliveira RP, et al. Clinical Evaluation of Bilateral Multiple Gingival Recession Treatment with Autogenous Connective Tissue Graft Associated with Low-Level Laser Therapy. Journal of Clinical Medicine. 2023; 12(6):2349. https://doi.org/10.3390/jcm12062349
Chicago/Turabian Stylede Souza Fonseca, Ricardo Roberto, Camila Pantoja Silva, Beatriz Leal de Senna Sastre, Erich Brito Tanaka, Tábata Resque Beckmann Carvalho, Paula Gabriela Faciola Pessôa de Oliveira, Silvio Augusto Fernandes de Menezes, Rogério Valois Laurentino, Renata Pimentel de Oliveira, Roberta Pimentel de Oliveira, and et al. 2023. "Clinical Evaluation of Bilateral Multiple Gingival Recession Treatment with Autogenous Connective Tissue Graft Associated with Low-Level Laser Therapy" Journal of Clinical Medicine 12, no. 6: 2349. https://doi.org/10.3390/jcm12062349
APA Stylede Souza Fonseca, R. R., Silva, C. P., de Senna Sastre, B. L., Tanaka, E. B., Carvalho, T. R. B., de Oliveira, P. G. F. P., de Menezes, S. A. F., Laurentino, R. V., de Oliveira, R. P., de Oliveira, R. P., Lago, A. D. N., & Almeida Machado, L. F. (2023). Clinical Evaluation of Bilateral Multiple Gingival Recession Treatment with Autogenous Connective Tissue Graft Associated with Low-Level Laser Therapy. Journal of Clinical Medicine, 12(6), 2349. https://doi.org/10.3390/jcm12062349






