Characteristics of Patients with Heart Failure and Advanced Chronic Kidney Disease (Stages 4–5) Not Undergoing Renal Replacement Therapy (ERCA-IC Study)
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
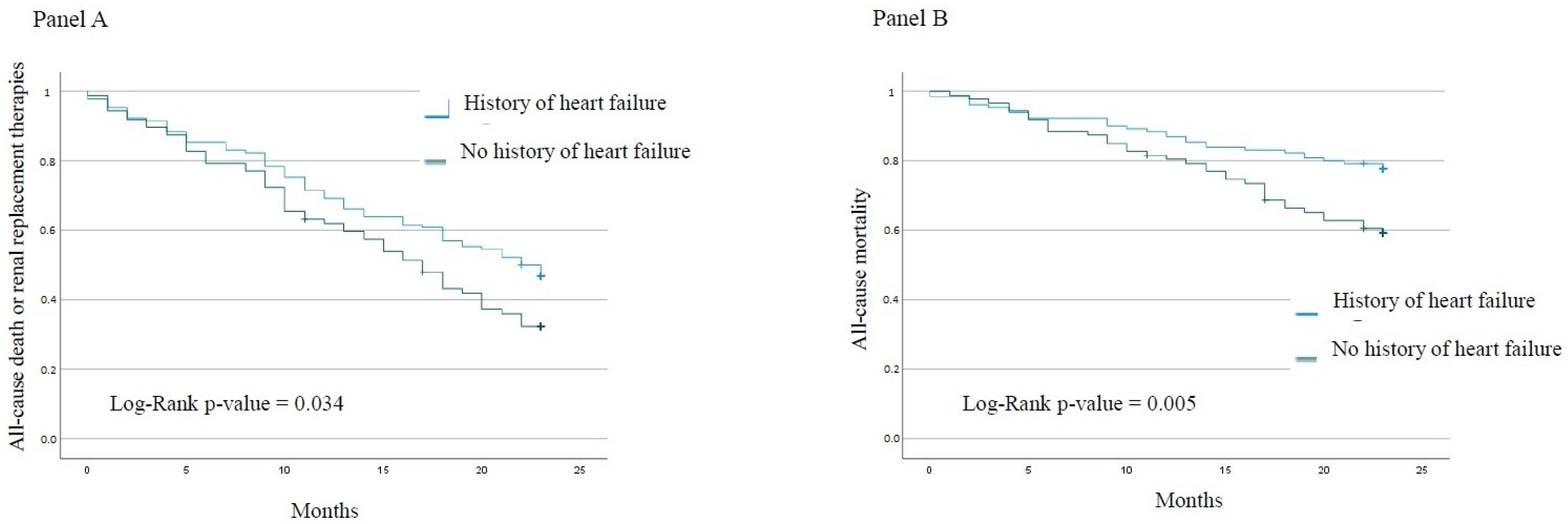
3. Results
3.1. Baseline Characteristics
3.2. Outcomes
Patients without HF History
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- House, A.A.; Wanner, C.; Sarnak, M.J.; Piña, I.L.; McIntyre, C.W.; Komenda, P.; Kasiske, B.L.; Deswal, A.; deFilippi, C.R.; Cleland, J.G.F.; et al. Heart failure in chronic kidney disease: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2019, 95, 1304–1317. [Google Scholar] [CrossRef]
- McCullough, P.A.; Bakris, G.L.; Owen, W.F.; Klassen, P.S.; Califf, R.M. Slowing the progression of diabetic nephropathy and its cardiovascular consequences. Am. Heart J. 2004, 148, 243–251. [Google Scholar]
- Kottgen, A.; Russell, S.D.; Loehr, L.R.; Crainiceanu, C.M.; Rosamond, W.D.; Chang, P.P.; Chambless, L.E.; Coresh, J. Reduced Kidney Function as a Risk Factor for Incident Heart Failure: The Atherosclerosis Risk in Communities (ARIC) Study. J. Am. Soc. Nephrol. 2007, 18, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C. Chronic Kidney Disease and the Risks of Death, Cardiovascular Events, and Hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef]
- Ronco, C.; Haapio, M.; House, A.A.; Anavekar, N.; Bellomo, R. Cardiorenal Syndrome. J. Am. Coll. Cardiol. 2008, 52, 1527–1539. [Google Scholar] [CrossRef] [PubMed]
- Zannad, F.; Rossignol, P. Cardiorenal syndrome revisited. Circulation 2018, 138, 929–944. [Google Scholar] [CrossRef]
- Quiroga, B.; Ortiz, A.; Navarro-González, J.F.; Santamaría, R.; de Sequera, P.; Díez, J. From cardiorenal syndromes to cardionephrology: A reflection by nephrologists on renocardiac syndromes. Clin. Kidney J. 2022, 16, 19–29. [Google Scholar] [CrossRef]
- Krishnamoorthy, A.; Greiner, M.A.; Sharma, P.P.; DeVore, A.D.; Johnson, K.W.; Fonarow, G.C.; Curtis, L.H.; Hernandez, A.F. Heart Failure Transient and persistent worsening renal function during hospitalization for acute heart failure. Am. Heart J. 2014, 168, 891–900. [Google Scholar] [CrossRef]
- Löfman, I.; Szummer, K.; Dahlström, U.; Jernberg, T.; Lund, L.H. Associations with and prognostic impact of chronic kidney disease in heart failure with preserved, mid-range, and reduced ejection fraction. Eur. J. Heart Fail. 2017, 19, 1606–1614. [Google Scholar] [CrossRef]
- Bethesda. USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. Available online: https://adr.usrds.org/2021 (accessed on 7 August 2022).
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Official Journal of the International Society of nephrology KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. 2013. Available online: www.publicationethics.org (accessed on 20 November 2022).
- Romero-González, G.; Ravassa, S.; González, O.; Lorenzo, I.; Rojas, M.A.; García-Trigo, I.; García-Fernández, N.; Lavilla, J.; Martín, P.L.; López, B.; et al. Burden and challenges of heart failure in patients with chronic kidney disease. A call to action. Nefrología 2020, 40, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Trespalacios, F.C.; Taylor, A.J.; Agodoa, L.Y.; Bakris, G.L.; Abbott, K.C. Heart failure as a cause for hospitalization in chronic dialysis patients. Am. J. Kidney Dis. 2003, 41, 1267–1277. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lv, J.; He, K.; Wang, F.; Gao, B.; Zhao, M.H.; Zhang, L. Longitudinal Follow-Up and Outcomes for Chinese Patients with Stage 1–4 Chronic Kidney Disease. Kidney Dis. 2022, 8, 72–81. [Google Scholar] [CrossRef]
- Oh, K.-H.; Park, S.K.; Kim, J.; Ahn, C. The KoreaN Cohort Study for Outcomes in Patients with Chronic Kidney Disease (KNOW-CKD): A Korean Chronic Kidney Disease Cohort. J. Prev. Med. Public Health 2022, 55, 313–320. [Google Scholar] [CrossRef]
- Roy, S.; Schweiker-Kahn, O.; Jafry, B.; Masel-Miller, R.; Raju, R.S.; O’Neill, L.M.O.; Correia, C.R.; Trivedi, A.; Johnson, C.; Pilot, C.; et al. Risk Factors and Comorbidities Associated with Diabetic Kidney Disease. J. Prim. Care Community Health 2021, 12, 215013272110485. [Google Scholar] [CrossRef]
- Tuegel, C.; Bansal, N. Heart failure in patients with kidney disease. Heart 2017, 103, 1848–1853. [Google Scholar] [CrossRef]
- Beldhuis, I.E.; Lam, C.S.; Testani, J.M.; Voors, A.A.; Van Spall, H.G.; Ter Maaten, J.M.; Damman, K. Evidence-Based Medical Therapy in Patients with Heart Failure with Reduced Ejection Fraction and Chronic Kidney Disease. Circulation 2022, 145, 693–712. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, S.; Mehta, S.; Khwaja, A.; Cleland, J.G.; Ives, N.; Brettell, E.; Chadburn, M.; Cockwell, P. Renin–Angiotensin System Inhibition in Advanced Chronic Kidney Disease. N. Engl. J. Med. 2022, 387, 2021–2032. [Google Scholar] [CrossRef]
- Bansal, N.; Katz, R.; Robinson-Cohen, C.; Odden, M.C.; Dalrymple, L.; Shlipak, M.G.; Sarnak, M.J.; Siscovick, D.S.; Zelnick, L.; Psaty, B.M.; et al. Absolute Rates of Heart Failure, Coronary Heart Disease, and Stroke in Chronic Kidney Disease. JAMA Cardiol. 2017, 2, 314. [Google Scholar] [CrossRef]
- Morales, R.O.; Barbosa, F.; Farre, N. Peritoneal dialysis in heart failure: Focus on kidney and ventricular dysfunction. Rev. Cardiovasc. Med. 2021, 22, 649–657. [Google Scholar] [CrossRef]
- de la Espriella, R.; González, M.; Górriz, J.L. Setting up a cardiorenal clinic. Consensus document of the cardiorenal working groups of the Spanish Society of Cardiology and the Spanish Society of Nephrology. REC CardioClinics 2021, 56, 284–295. [Google Scholar] [CrossRef]
- Ortiz, A.; Navarro-González, J.F.; Núñez, J.; Espriella, R.d.; Cobo, M.; Santamaría, R.; Sequera, P.d.; Díez, J. The unmet need of evidence-based therapy for patients with advanced chronic kidney disease and heart failure. Clin. Kidney J. 2022, 15, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Scheven, L.; de Jong, P.E.; Hillege, H.L.; Heerspink, H.J.L.; van Pelt, L.J.; Kootstra, J.E.; Bakker, S.J.L.; Gansevoort, R.T.; for the PREVEND study group. High-sensitive troponin T and N-terminal pro-B type natriuretic peptide are associated with cardiovascular events despite the cross-sectional association with albuminuria and glomerular filtration rate. Eur. Heart J. 2012, 33, 2272–2281. [Google Scholar] [CrossRef]
- Bansal, N.; Zelnick, L.; Shlipak, M.G.; Anderson, A.; Christenson, R.; Deo, R.; deFilippi, C.; Feldman, H.; Lash, J.; He, J.; et al. Cardiac and Stress Biomarkers and Chronic Kidney Disease Progression: The CRIC Study. Clin. Chem. 2019, 65, 1448–1457. [Google Scholar] [CrossRef] [PubMed]
| No Heart Failure n = 130 (60%) | Heart Failure n = 87 (40%) | p-Value | |
|---|---|---|---|
| Baseline Characteristics | |||
| Age (years) | 72.1 ± 13.1 | 78.2 ± 8.8 | <0.001 |
| Women | 47 (36.2) | 46 (52.9) | 0.015 |
| Hypertension | 128 (98.5) | 85 (97.7) | 0.683 |
| Diabetes mellitus | 58 (44.6) | 61 (70.1) | <0.001 |
| Dyslipidemia | 109 (83.8) | 79 (90.8) | 0.14 |
| BMI | 28.0 ± 5.1 | 29.6 ± 5.7 | 0.016 |
| Never smoker | 84 (64.6) | 57 (65.5) | 0.25 |
| Active smoker | 25 (19.2) | 22 (25.3) | |
| Previous smoker | 21 (16.2) | 8 (9.2) | |
| Stroke/TIA | 16 (12.3) | 13 (14.9) | 0.58 |
| Sleep apnea | 15 (11.5) | 17 (19.5) | 0.10 |
| COPD/Asthma | 19 (14.6) | 19 (21.8) | 0.17 |
| Peripheral vascular disease | 31 (23.8) | 24 (27.6) | 0.54 |
| Cancer | 37 (28.5) | 19 (21.8) | 0.28 |
| Myocardial infarction | 13 (10.0) | 26 (29.9) | <0.001 |
| Percutaneous coronary intervention | 7 (5.5) | 23 (26.4) | <0.001 |
| Moderate-severe valve disease | 3 (2.3) | 15 (17.2) | <0.001 |
| Atrial fibrillation/flutter | 14 (10.8) | 48 (55.2) | <0.001 |
| Baseline Treatment | |||
| ACEi/ARB2/ARNI | 40 (30.8) | 18 (20.7) | 0.1 |
| Beta blockers | 52 (40.0) | 56 (64.4) | <0.001 |
| MRA | 4 (3.1) | 2 (2.3) | 0.74 |
| iSLGT2 | 1 (0.8) | 3 (3.4) | 0.15 |
| Insulin | 27 (20.8) | 40 (46.0) | <0.001 |
| Other oral anti-diabetic drugs | 24 (18.5) | 23 (26.4) | 0.16 |
| Antiplatelet therapy | 43 (33.1) | 36 (41.1) | 0.21 |
| Statins | 98 (75.4) | 68 (78.2) | 0.64 |
| Loop diuretic | 66 (50.8) | 78 (89.6) | <0.001 |
| HCTZ/higrotone | 7 (5.4) | 14 (16.1) | 0.009 |
| Anti-vitamin K | 12 (9.2) | 21 (24.1) | 0.003 |
| Direct-acting anticoagulants | 1 (0.8) | 14 (16.1) | <0.001 |
| Intravenous iron | 21 (16.2) | 20 (23.0) | 0.21 |
| Oral iron | 75 (57.7) | 56 (64.4) | 0.32 |
| Erythropoietin | 52 (40.0) | 46 (52.9) | 0.062 |
| Laboratory results | |||
| Creatinine (mg/dl) | 3.5 ± 2.2 | 3.0 ± 1.0 | 0.005 |
| eGFR (mL/min/1.73 m2) | 17.7 ± 5.5 | 18.4 ± 5.5 | 0.18 |
| Potassium (mmol/L) | 4.8 ± 0.5 | 4.6 ± 0.6 | <0.001 |
| Creatinine/protein ratio (mg/g) | 1008.1 (362.0 − 2184.0) | 663.0 (286.1–1859.0) | 0.45 |
| HbA1c (%) | 6.4 ± 1.3 | 6.6 ± 1.4 | 0.15 |
| Hemoglobin (g/dl) | 12.1 ± 1.6 | 12.3 ± 1.9 | 0.16 |
| Transferrin saturation index (%) | 26.4 ± 10.4 | 24.7 ± 8.7 | 0.11 |
| LDL cholesterol (mg/dl) | 87.3 ± 27.1 | 78.7 ± 31.7 | 0.02 |
| NT-proBNP (n = 88) (ng/dl) | 1344 (393−3721) | 4480 (1417−7755) | <0.001 |
| HS-Troponin T (n = 63) (ng/L) | 47.0 (34.3−57.3) | 52.0 (31.7−73.8) | 0.61 |
| No Heart Failure (n = 130, 60%) | Heart Failure (n = 87, 40%) | p-Value | |
|---|---|---|---|
| Heart failure hospitalization, n (%) | 15 (11.5) | 35 (40.2) | <0.001 |
| Ambulatory intravenous diuretic, n (%) | 11 (8.5) | 39 (44.8) | <0.001 |
| Hospitalization due to renal cause, n (%) | 35 (26.9) | 20 (23.0) | 0.51 |
| Renal replacement therapies, n (%) | 42 (32.2) | 24 (27.6) | 0.46 |
| Hemodialysis, n (%) | 18 (42.9) | 19 (79.2) | 0.008 |
| Peritoneal dialysis, n (%) | 16 (38.1) | 5 (20.8) | |
| Kidney transplant, n (%) | 8 (19.0) | 0 (0) | |
| All-cause death, n (%) | 29 (22.3) | 35 (40.2) | 0.005 |
| All-cause death or renal replacement therapy, n (%) | 69 (53.1) | 58 (66.7) | 0.046 |
| Adjusted HF (97%CI for HR) | p-Value | |
|---|---|---|
| Female | 0.82 (0.56–1.21) | 0.32 |
| Age, per year | 1.003 (0.99–1.022) | 0.74 |
| Previous HF | 1.62 (1.04–2.52) | 0.034 |
| Diabetes mellitus | 0.72 (0.49–1.05) | 0.09 |
| Myocardial infarction | 1.72 (1.08–2.73) | 0.022 |
| Moderate-to-severe valve disease | 0.98 (0.51–1.88) | 0.95 |
| Atrial fibrillation | 1.03 (0.66–1.62) | 0.90 |
| eGFR, per mL/min/1.73 m2 | 0.87 (0.84–0.90) | <0.001 |
| RAAS inhibitors | 1.19 (0.76–1.87) | 0.44 |
| Beta-blockers | 0.89 (0.61–1.31) | 0.55 |
| Loop diuretics | 1.21 (0.78–1.86) | 0.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valdivielso Moré, S.; Vicente Elcano, M.; García Alonso, A.; Pascual Sanchez, S.; Galceran Herrera, I.; Barbosa Puig, F.; Belarte-Tornero, L.C.; Ruiz-Bustillo, S.; Morales Murillo, R.O.; Barrios, C.; et al. Characteristics of Patients with Heart Failure and Advanced Chronic Kidney Disease (Stages 4–5) Not Undergoing Renal Replacement Therapy (ERCA-IC Study). J. Clin. Med. 2023, 12, 2339. https://doi.org/10.3390/jcm12062339
Valdivielso Moré S, Vicente Elcano M, García Alonso A, Pascual Sanchez S, Galceran Herrera I, Barbosa Puig F, Belarte-Tornero LC, Ruiz-Bustillo S, Morales Murillo RO, Barrios C, et al. Characteristics of Patients with Heart Failure and Advanced Chronic Kidney Disease (Stages 4–5) Not Undergoing Renal Replacement Therapy (ERCA-IC Study). Journal of Clinical Medicine. 2023; 12(6):2339. https://doi.org/10.3390/jcm12062339
Chicago/Turabian StyleValdivielso Moré, Sandra, Miren Vicente Elcano, Anna García Alonso, Sergi Pascual Sanchez, Isabel Galceran Herrera, Francesc Barbosa Puig, Laia C. Belarte-Tornero, Sonia Ruiz-Bustillo, Ronald O. Morales Murillo, Clara Barrios, and et al. 2023. "Characteristics of Patients with Heart Failure and Advanced Chronic Kidney Disease (Stages 4–5) Not Undergoing Renal Replacement Therapy (ERCA-IC Study)" Journal of Clinical Medicine 12, no. 6: 2339. https://doi.org/10.3390/jcm12062339
APA StyleValdivielso Moré, S., Vicente Elcano, M., García Alonso, A., Pascual Sanchez, S., Galceran Herrera, I., Barbosa Puig, F., Belarte-Tornero, L. C., Ruiz-Bustillo, S., Morales Murillo, R. O., Barrios, C., Vime-Jubany, J., & Farre, N. (2023). Characteristics of Patients with Heart Failure and Advanced Chronic Kidney Disease (Stages 4–5) Not Undergoing Renal Replacement Therapy (ERCA-IC Study). Journal of Clinical Medicine, 12(6), 2339. https://doi.org/10.3390/jcm12062339






