Abstract
Little is known about the time-varying risk factors for fractures in kidney transplant recipients (KTRs). Using the Korea Organ Transplantation Registry, a nationwide cohort study of KTRs, the incidence, locations, and time-varying predictors of fractures were analyzed, including at baseline and post-transplant 6-month variables in KTRs who underwent KT between January 2014 and June 2019. Among 4134 KTRs, with a median follow-up of 2.94 years (12,441.04 person-years), 63 patients developed fractures. The cumulative 5-year incidence was 2.10%. The most frequent locations were leg (25.40%) and foot/ankle (22.22%). In multivariable analysis, older recipient age at baseline (hazard ratio [HR], 1.035; 95% confidence interval [CI], 1.007–1.064; p = 0.013) and higher tacrolimus trough level (HR, 1.112; 95% CI, 1.029–1.202; p = 0.029) were associated with higher risks for fractures. Pretransplant diabetes mellitus had a time-dependent impact on fractures, with increasing risk as time elapses (HR for diabetes mellitus 1.115; 95% CI, 0.439–2.832; HR for diabetes mellitus × time, 1.049; 95% CI, 1.007–1.094; p = 0.022). In conclusion, KTRs had a high risk of peripheral skeletal fractures in the first 5 years. At baseline recipient age, pretransplant diabetes mellitus and tacrolimus trough level after KT were responsible for the fractures in KTRs.
1. Introduction
Previous studies have described that the incidence of fractures increases in kidney transplant recipients (KTRs) versus the general population [1] and the risk exceeds that in dialysis patients in the first 1 to 3 years after transplantation [2]. These findings suggest that the bone remains fragile despite improvements in bone and mineral disturbances following kidney transplantation (KT). Chronic kidney disease (CKD) patients have chronic kidney disease–mineral bone disease (CKD-MBD), a disorder of mineral and bone metabolism. CKD-MBD encompasses biochemical abnormalities of calcium, phosphorus, parathyroid hormone (PTH), vitamin D, bone disease, and vascular calcification, which all contribute to fractures [3]. Preexisting CKD-MBD, consequences of KT-specific therapies including glucocorticoid use, and progressive graft dysfunction after KT contribute to high risks of fractures [4].
Several large-scale studies have examined factors that predispose KTRs to fractures [5,6]. However, the incidence of fractures varies widely from 3.3 to 99.6 fractures per 1000 person-years, with a 5-year cumulative incidence of 0.85–27% [5]. This is because the patient characteristics, study quality, the definition of fractures, and follow-up duration differed among studies. In addition, the changing patterns of treatments, such as the corticosteroid-free regimen adopted after 2000 and the increase in mean recipient age, were not reflected in recent studies. Thus, existing studies have poor consensus on general risk factors and transplant-specific risk factors for fractures in KTRs. Most of the previous studies included baseline variables when investigating predictors of fractures. However, the effect of a fixed baseline variable may change over time, or the risk factor itself may change over time [7]. After KT, laboratory variables change as the allograft function recovers and the early post-transplantation period accompanies the greatest change in immunosuppressants. The present study aimed to investigate the incidence of and assess the time-varying risk factors for fractures following KT by including baseline and post-transplant variables in the analysis.
2. Materials and Methods
2.1. Study Population and Data Collection
Data were obtained from the Korea Organ Transplantation Registry (KOTRY), a prospective multicenter nationwide cohort study of KTRs in South Korea. Forty-one transplantation centers participated in the KOTRY. In the KOTRY, pretransplant and follow-up data are collected by each center at baseline and post-transplantation after 6 months and 1 year, and then annually. Therefore, the collected dataset is complete for each participant. A total of 5403 KTRs aged ≥ 18 years who underwent KT between January 2014 and June 2019 were enrolled in the KOTRY. Among them, 1269 KTRs lacked post-transplantation 6-month data because their post-transplant periods were less than 6 months. Therefore, this study included 4134 KTRs in the analysis. The KOTRY provided patient demographics at the time of transplantation, including age, sex, height, weight, body mass index (BMI), smoking status, primary renal disease, date of dialysis initiation, dialysis modalities used before KT, dialysis vintage, KT date, donor type, other medical comorbidities, and individual patient treatments following KT. BMI was calculated as the patient’s weight in kilograms divided by height in meters squared (kg/m2). Baseline laboratory parameters included serum levels of hemoglobin, creatinine, albumin, corrected calcium (Ca), phosphorus (P), and intact PTH at the time of transplantation. Serum levels of hemoglobin, creatinine, albumin, corrected Ca, and P, and tacrolimus trough levels at 6 months after KT were collected. Data on medications taken at 6 months post-transplantation including vitamin D analogs, tacrolimus, cyclosporine, mycophenolic acid, mammalian target of rapamycin (mTOR) inhibitor, and corticosteroids were also collected. The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation [8]. Death-censored graft loss and rejection treatment within 6 months post-transplantation were also included.
The outcome of this study was fracture occurrence. The information on fracture occurrence was collected in the original registry, which was identified by medical records from patients’ charts. All incident fractures were identified regardless of cause, and traumatic fractures were not assessed separately according to trauma level, as previously described [9]. This is because judging the level of trauma is subjective, as fractures at all locations were included, and high trauma non-spine fractures were also associated with a low bone mineral density as low trauma non-spine fractures [10].
All patients provided written informed consent before KOTRY enrollment. The study was performed in line with the principles of the Declaration of Helsinki and approved by the Institutional Review Board of Incheon St. Mary′s Hospital (OC19OISI0172).
2.2. Comparison of Clinical Characteristics and Cumulative Incidence of Fractures
Patients were divided into two groups: those who developed fractures (fracture group) and those who did not develop fractures (no fracture group). We compared the clinical baseline characteristics between the two groups, including demographic characteristics, laboratory findings, use of vitamin D analogs, and transplant-specific factors such as percentage of panel reactive antibody, the presence of a human leukocyte antigen donor-specific antibody, the positivity of cross-match testing, type of induction therapy (interleukin [IL]-2 receptor antibody or anti-thymocyte globulin), and main immunosuppressant type (tacrolimus, cyclosporine, mycophenolic acid, mTOR inhibitor, and corticosteroid). Laboratory parameters and medication use at 6 months post-transplantation and death-censored graft loss and rejection treatment within 6 months of post-transplantation were compared between the two groups. The cumulative incidence of fractures was also compared between the two groups.
2.3. Statistical Analysis
Continuous data are expressed as mean ± standard deviation or median with interquartile range, and comparisons were made using Student′s t-test or the Mann–Whitney U-test as appropriate. Categorical data are expressed as numbers with percentages and were compared using the chi-square test. The time to the first fracture was modeled using the Kaplan–Meier method. The Cox proportional hazard (PH) model was used to identify risk factors for fractures. The univariable analysis was followed by multivariable analyses using the forward conditional method. To determine the variables to be included in the multivariable model, the univariable Cox PH regression analysis is applied first to identify the impact of individual variables. Variables are identified as significant using a 0.2 significance level in the univariable analysis. To test the assumption of proportionality after the construction of the multivariable model, the scaled Schoenfeld residuals have been used. If the model displayed non-proportionality for variables included, the stratified Cox PH model and time-dependent Cox PH model were used to identify baseline and 6-month post-transplantation risk factors for fractures [11]. The results are presented as hazard ratios (HR) with 95% confidence intervals (CI). In all analyses, a p-value < 0.05 (two-tailed) was considered to indicate statistical significance. The statistical analyses were performed using R statistical software (version 4.1.3; R Foundation for Statistical Computing, Vienna, Austria). In addition, all graphs were generated using Prism software (GraphPad, San Diego, CA, USA) and R statistical software.
3. Results
3.1. Patient Characteristics at Baseline
Table 1 shows the baseline characteristics of the patients by study group. The mean recipient age was 49.00 years and the mean BMI was 23.09 kg/m2. Among the 4134 KTRs, 59.19% were male, 22.62% were smokers, and 28.96% had diabetes mellitus. The median time on dialysis was 30.33 months [interquartile range (IQR), 3.87–85.25 months]; 14.85% of the patients underwent preemptive transplantation, while 7.89% of the patients underwent re-transplantation.

Table 1.
Baseline characteristics of the kidney transplant recipients.
The fracture group was older and had a higher prevalence of diabetes mellitus and diabetic nephropathy as the primary renal diseases than the no fracture group. The fracture group had lower baseline intact PTH levels, more frequently used IL-2 receptor antibody, and less frequently used anti-thymocyte globulin as an induction regimen than the no fracture group.
3.2. Patient Characteristics at 6 Months Post-Transplantation
Laboratory data at 6 months post-transplantation were compared between the two groups. The fracture group had a lower serum P level and Ca × P product value. Tacrolimus was used more frequently as the maintenance immunosuppressant than the no fracture group, although it was not statistically significant. There were no significant differences in serum levels of hemoglobin, albumin, and corrected Ca, eGFR, proportions of death-censored graft loss, and rejection treatment within 6 months, and medications including vitamin D analogs, cyclosporine, mycophenolic acid, mTOR inhibitor, and corticosteroids. Despite the tendency of a lower tacrolimus dose in the fracture group, the serum tacrolimus trough level was significantly higher in the fracture group (Table 2).

Table 2.
Clinical characteristics of the kidney transplant recipients at 6 months post-transplantation.
3.3. Fracture Incidence and Location
During a follow-up of 12,441.04 person-years (median, 2.94 years), 63 patients developed incident fractures. The cumulative incidence of fractures was 2.10% at 5 years (Figure 1). The most frequent fracture locations were the lower leg [n = 16 (25.40%)] and foot/ankle [n = 14 (22.22%)]. Less common fracture sites were the vertebra [n = 9 (14.29%)], upper arm/forearm [n = 7 (11.11%)], hand [n = 6 (9.52%)], skull/face [n = 4 (6.35%)], rib/thorax [n = 3 (4.76%)], hip/femur [n = 3 (4.76%)], and clavicle/scapula [n = 1 (1.59%)] (Figure 2).
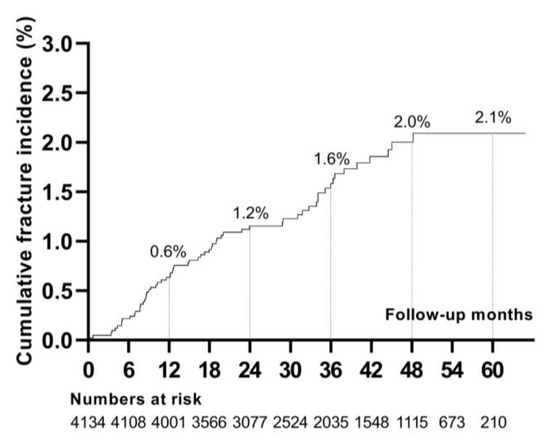
Figure 1.
Cumulative incidence of fractures after kidney transplantation.
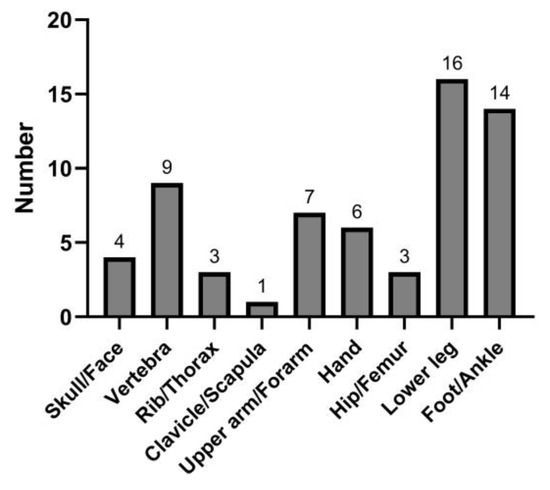
Figure 2.
Location of the incident fractures.
3.4. Predictors of Incident Fractures
Risk factors for incident fractures were analyzed (Table 3 and Table 4). In the univariable analysis, fracture risk was influenced by recipient age, pretransplant diabetes mellitus, preemptive KT, and the use of IL-2 receptor antibody and anti-thymocyte globulin as induction therapy. Among the variables at 6 months post-transplantation, serum P level, Ca × P product, and tacrolimus trough level were associated with incident fractures in the univariable analysis. In multivariable analysis, since pretransplant diabetes mellitus violated the proportional hazards assumption with a p-value of less than 0.05 by scaled Schoenfeld residuals, we applied the stratified Cox regression (Table 3). Recipient age (HR, 1.035; 95% CI, 1.007–1.064; p = 0.013) and tacrolimus trough level (HR, 1.112; 95% CI, 1.028–1.202; p = 0.008) were significantly associated with a higher risk of fractures. Furthermore, to identify the time-varying effect of pretransplant diabetes mellitus, we performed an extended Cox regression analysis with diabetes mellitus as a continuous time-varying coefficient (Table 4). In this multivariable model, recipient age (HR, 1.035; 95% CI, 1.007–1.064; p = 0.013) and tacrolimus trough level (HR, 1.112; 95% CI, 1.029–1.202; p = 0.008) were significantly associated with a higher risk of fractures. The fracture-free survival rate of KTRs with pretransplant diabetes mellitus was significantly lower than that of KTRs without pretransplant diabetes mellitus (p < 0.001), and the survival curve declined steeper as the time after KT elapsed (Figure 3A). Pretransplant diabetes mellitus showed an increased hazard ratio over the length of the follow-up period (HR, 1.049; 95% CI, 1.007–1.094; p = 0.022) (Table 4 and Figure 3B).

Table 3.
Risk factors for incident fractures of the kidney transplant recipients.

Table 4.
Multivariable Cox regression for risk factors for incident fractures of the kidney transplant recipients with diabetes mellitus as a continuous time-dependent coefficient.
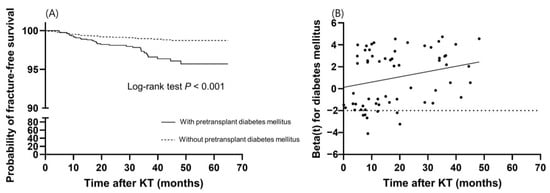
Figure 3.
Time-dependent effect of pretransplant diabetes mellitus on fractures. (A) Kaplan–Meier survival curve of fracture-free survival according to pretransplant diabetes mellitus. (B) The effect of pretransplant diabetes mellitus on fractures over time. The dotted line is the reference line for the null effect. The solid line shows a linear effect with a slope of 0.048.
4. Discussion
This study demonstrated the time-varying tacrolimus trough level and time-varying effect of baseline diabetes mellitus affected the risk of fractures in KTRs. In this nationwide cohort study of 4134 KTRs, the 5-year cumulative incidence of fractures after KT was 2.10%. The most common fracture sites were the leg and foot/ankle. Older recipient age at baseline and higher tacrolimus trough level at 6 months post-transplantation increased the risk of fractures, and pretransplant diabetes mellitus showed an increased risk of fractures as time elapsed.
Previous reports demonstrated that KTRs are more susceptible to fractures in the early rather than late post-transplantation period. KTRs had a 1.34-fold greater risk of hip fractures than dialysis patients in the first 3 years after transplantation, and the risk decreased to a level comparable to that of dialysis patients after 3 years [2]. Similarly, the fracture risk of KTRs was up to 4.6-fold higher than that of the general population in the first 3 years after KT [12]. These findings suggest that KT does not restore the bone fragility of preexisting CKD-MBD, despite the improvement in mineral metabolism disturbances [13]. As vertebral fractures were closely related to mortality in CKD patients [14] and KTRs with fractures were more likely to experience graft loss or mortality [15], fractures continue to be a significant problem after KT. The cumulative incidence of fractures ranged widely from 0.85% to 27% in previous studies [5]. A recent study reported a 3-year cumulative incidence of non-vertebral fractures of 1.6% and a 10-year cumulative incidence of 1.7% [16], consistent with the results of this study. Although fracture incidence of KTRs was reported in various studies, the relatively low incidence in this study can be explained by the emergence of corticosteroid-limiting or withdrawal protocols after 2000 [17] and advances in the management of CKD-MBD that persist after transplantation.
In the present study, the foot/ankle and lower leg were the most common fracture locations, a finding consistent with those of previous reports [9,18]. The high incidence of fractures in the peripheral skeleton, an atypical site of osteoporotic fractures, suggests that various factors are involved in post-transplantation bone fragility. Pre-transplant renal-specific bone factors, post-transplant changes caused by immunosuppressive therapy, gradual allograft dysfunction, and continued mineral metabolism disturbances may impact peripheral fractures [19]. Vitamin K deficiency is also known to affect vascular calcification and poor bone quality in CKD patients, and it was also associated with incident fractures in de novo KTRs [20,21]. Immunosuppressive treatments alter the bone structure, function, and formation in combination with persistent secondary or tertiary hyperparathyroidism [22]. Glucocorticoid-induced osteopenia occurs because of impaired osteoblastogenesis and early osteoblast apoptosis, affecting the trabecular bone of the axial skeleton in particular [23]. In addition, the cumulative dose of glucocorticoids negatively correlates with bone turnover and volume [22]. In murine models, cyclosporine stimulated osteoclast activity more than osteoblast activity, resulting in bone loss [22,24], while tacrolimus induced severe trabecular bone loss [25]. An in vitro study showed that sirolimus interfered with osteoblast proliferation and differentiation [26], while everolimus reduced cancellous bone loss in ovariectomized rats by decreasing osteoclast-mediated bone resorption [27]. The susceptibility of the peripheral skeleton to fractures was demonstrated in a study using an early corticosteroid withdrawal protocol [28]. Interestingly, the bone mineral density in the peripheral skeleton showed progressive deterioration despite early corticosteroid withdrawal, whereas cortical bone mass and strength in the central skeleton were preserved. This is due to persistent hyperparathyroidism and elevated remodeling rates, which result in cortical and trabecular losses and decreased bone strength in the peripheral skeleton [28].
In this study, older age and diabetes mellitus at baseline were risk factors for fractures, as reported by previous studies [5,6,12,29]. However, dialysis modality, dialysis vintage, deceased donor, and female sex were not associated with fractures, a finding that is inconsistent with those of other reports [5,12,30]. In South Korea, most end-stage kidney disease patients are receiving hemodialysis and the proportion of peritoneal dialysis accounts for less than 10% in the past 10 years [31]. The effect of dialysis modality on fractures may not be clearly determined because of the low number of peritoneal dialysis patients. Recent studies also reported that donor type is not significantly associated with fracture risk [6,15]. As this study lacks data on menopausal status, estrogen therapy, or specific osteoporosis treatments, the reason for the lack of an association between the female sex and fractures is unclear. Since this study included KTRs performed in 2014–2019, these patients might have been treated with different drugs for CKD-MBD and osteoporosis than those in the early 2000s. Advances in drugs for CKD-MBD and osteoporosis might have weakened the effect of the female sex on fractures. The reason for the insignificant effects of dialysis vintage on fractures in this study is also unclear. We speculate that the different populations and study periods may have affected these discrepant results.
Interestingly, pretransplant diabetes mellitus showed an increased hazard ratio over the follow-up period. Diabetes mellitus is a well-known risk factor for fractures, even in the absence of kidney disease. Proposed pathophysiologic mechanisms are the direct effects of chronic hyperglycemia on bone microarchitecture, inefficient distribution of bone mass, and insufficient repair and adaptation response of bone. Increased risk of falls because of visual impairment and neuropathy may all increase the risk of fractures in patients with diabetes mellitus [32]. In a recent study using the US Renal Data System, the risks for upper extremity and lower leg fractures were significantly higher in dialysis patients with diabetic nephropathy than those with other renal diseases [33]. Since hyperglycemia worsens with the use of calcineurin inhibitors and corticosteroids, the ongoing effect of hyperglycemia on bone health may increase the risk for peripheral skeleton fractures, as post-transplantation periods increase in KTRs with diabetes mellitus.
In this study, we aimed to evaluate the effect of time-varying factors on fractures since various factors change after KT, including allograft function, mineral metabolism, and immunosuppressants. Phosphate is a major factor in CKD-MBD, and hyperphosphatemia is closely related to fractures in CKD patients [34]. Conversely, in KTRs, hypophosphatemia was associated with increased fracture risk [35]. In this study, KTRs with fractures had a significantly lower serum phosphorus level at 6 months post-transplantation compared to KTRs without fractures. However, hypophosphatemia at 6 months post-transplantation did not predict future fractures in this study. Among the post-transplantation variables, a higher tacrolimus trough level at 6 months post-transplantation was associated with an increased risk of fractures. As tacrolimus is bound to plasma proteins in the circulation, especially albumin, circumstances affecting plasma protein concentration may change the tacrolimus level [36]. Since our data did not include long-term tacrolimus levels, the results are insufficient to conclude that long-term high tacrolimus levels directly impact bone health. However, there is evidence that tacrolimus affects bone structure in the current literature. In rat models, calcineurin inhibitors, cyclosporine, or tacrolimus, stimulate bone mass loss independent of corticosteroid treatment [37]. It was shown that tacrolimus induced trabecular bone loss and high-turnover osteoporosis in rats [25]. In comparison with cyclosporine, the reduction in trabecular bone mass was more marked in tacrolimus-treated rats [38]. In a study with corticosteroid withdrawal protocol, there was a progressive decline in cortical and trabecular bone density at the peripheral skeleton, despite of preservation of bone mineral density at the central skeleton [28]. Thus, higher tacrolimus trough level may have contributed to a more severe trabecular bone loss at the peripheral skeleton in KTRs.
This study has several limitations. First, confounders associated with fractures such as previous fractures, alcohol consumption, physical fitness, exercise, menopausal status, estrogen replacement therapy, intact PTH level at 6 months post-transplantation, treatment for hyperparathyroidism, including parathyroidectomy, and cumulative doses of corticosteroids were not analyzed, nor was dual X-ray absorptiometry used. Especially, the lack of data on cumulative doses of corticosteroids might have underreported the detrimental effects of steroids on bone fractures in this study. Second, the fracture events may have been underestimated; the incidence of vertebral fractures may have been missed because non-traumatic vertebral fractures can be undiagnosed unless actively searched for. Third, the long-term time-varying variables beyond 1 year after KT were not analyzed because of insufficient data. Fourth, defining fractures according to the level of trauma could not be conducted. However, the current study has strengths, including being a nationwide cohort study that included relatively recent KTRs and using complete data from comprehensive medical records. In addition, medications and laboratory parameters at 6 months post-transplantation were included to assess the time-varying effect of certain factors and the effects of time-varying variables, which were not evaluated in previous studies.
In conclusion, KTRs are susceptible to peripheral skeletal fractures in the first 5 years. Baseline recipient age and pretransplant diabetes mellitus were associated with fractures in KTRs, and the strength of association with diabetes mellitus increased over the follow-up period. A tacrolimus trough level after KT may be associated with the risk of fractures, which needs verification with tacrolimus levels in the long term. A better understanding of the incidence of and risk factors for fractures remains important, as a well-established fracture prediction tool can prevent and guide treatment decisions in KTRs.
Author Contributions
Conceptualization, H.E.Y.; data curation, J.-H.L., J.S.J., H.J., J.Y. and M.S.K.; formal analysis, S.H.E., D.W.K. and H.E.Y.; investigation, J.-H.L., J.S.J., H.J., J.Y. and M.S.K.; methodology, S.H.E. and H.E.Y.; supervision, H.E.Y.; writing—original draft, S.H.E. and D.W.K.; writing—review and editing, H.E.Y. The Korean Organ Transplantation Registry Study Group participated in collecting data. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Institute of Health research project (2014-ER6301-00, 2014-ER6301-01, 2014-ER6301-02, 2017-ER6301-00, 2017-ER6301-01, 2017-ER6301-02). This study was also supported by a cooperative research fund from the Korean Society of Nephrology (2022).
Institutional Review Board Statement
The study was performed in line with the principles of the Declaration of Helsinki and approved by the Institutional Review Board of Incheon St. Mary’s Hospital (OC19OISI0172).
Informed Consent Statement
All patients provided written informed consent before KOTRY enrollment. Written informed consent for publication must be obtained from participating patients who can be identified (including the patients themselves).
Data Availability Statement
The data used in this study are available from the KOTRY office upon reasonable request.
Acknowledgments
The Korean Organ Transplantation Registry Study Group: Myoung Soo Kim 1, Jaeseok Yang 2, Jin Min Kong 3, Oh Jung Kwon4, Deok Gie Kim 5, Cheol Woong Jung 6, Yeong Hoon Kim 7, Joong Kyung Kim 8, Chan-Duck Kim 9, Ji Won Min 10, Sung Kwang Park 11, Yeon Ho Park 12, Jae Berm Park 13, Jung Hwan Park 14, Jong-Won Park 15, Tae Hyun Ban 16, Sang Heon Song 17, Seung Hwan Song 18, Ho Sik Shin 19, Chul Woo Yang 20, Hye Eun Yoon 21, Kang Wook Lee 22, Dong Ryeol Lee 23, Dong Won Lee 24, Sam Yeol Lee 25, Sang-Ho Lee 26, Su Hyung Lee 27, Yu Ho Lee 28, Jung Pyo Lee 29, Jeong-Hoon Lee 30, Jin Seok Jeon 31, Heungman Jun 32, Kyunghwan Jeong 33, Ku Yong Chung 34, Hong Rae Cho 35, Ju Man Ki 36, Dong-Wan Chae 37, Soo Jin Na Choi 38, Sung Shin39, Seungyeup Han 40, Kyu Ha Huh 1. 1 Department of Surgery, Yonsei University College of Medicine; 2 Department of Internal Medicine, Yonsei University College of Medicine; 3 Department of Nephrology, BHS Hanseo Hospital; 4 Department of Surgery, College of Medicine, Han Yang University; 5 Department of Surgery, Yonsei University Wonju College of Medicine, Wonju Severance Christian Hospital; 6 Department of Surgery, Korea University Anam Hospital; 7 Department of Internal Medicine, Inje University Busan Paik Hospital; 8 Department of Internal Medicine, Bongseng Memorial Hospital; 9 Department of Internal Medicine, School of Medicine, Kyungpook National University Hospital; 10 Division of Nephrology, Department of Internal Medicine, Bucheon St. Mary′s Hospital; 11 Department of Internal Medicine, Chonbuk National University Medical School; 12 Department of Surgery, Gil Medical Center, Gachon University College of Medicine; 13 Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine; 14 Konkuk University School of Medicine, Department of Nephrology; 15 Department of Nephrology, Yeungnam University Hospital; 16 Division of Nephrology, Department of Internal Medicine, Eunpyeong St. Mary′s hospital; 17 Department of Internal Medicine, Pusan National University Hospital; 18 Department of Surgery, Ewha Womans University Seoul Hospital; 19 Division of Nephrology, Department of Internal Medicine, Kosin University College of Medicine; 20 Division of Nephrology, Department of Internal Medicine, Seoul St. Mary′s hospital; 21 Department of Internal Medicine, Incheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea; 22 Department of Nephrology, Chungnam National University Hospital; 23 Division of Nephrology, Department of Internal Medicine, Maryknoll Medical Center; 24 Division of Nephrology, Department of Internal Medicine, Pusan National University School of Medicine; 25 Department of Surgery, Kangdong Sacred Heart Hospital, Hallym University College of Medicine; 26 Department of Nephrology, Kyung Hee University Hospital at Gangdong; 27 Department of Surgery, Ajou University School of Medicine; 28 Division of Nephrology, Department of Internal Medicine, CHA Bundang Medical Center, CHA University, Seongnam, Korea; 29 Department of Nephrology, SMG-SNU Boramae Medical Center; 30 Department of Surgery, Myongji Hospital, Hanyang University; 31 Department of Internal Medicine, Soonchunhyang University Seoul Hospital; 32 Department of Surgery, Korea University Anam Hospital; 33 Department of Internal Medicine, Kyung Hee University College of Medicine; 34 Department of Surgery, Ewha Womans University Mokdong Hospital; 35 Department of Surgery, Ulsan University Hospital; 36 Department of Surgery, Gangnam Severance Hospital, Yonsei University College of Medicine; 37 Division of Nephrology, Seoul National University Bundang Hospital; 38 Department of Surgery, Chonnam National University Medical School; 39 Department of Surgery, Asan Medical Center; 40 Department of Internal Medicine, Keimyung University School of Medicine, Daegu, Korea.
Conflicts of Interest
All the authors declare that they have no financial/conflicting interests to disclose.
References
- Hansen, D.; Olesen, J.B.; Gislason, G.H.; Abrahamsen, B.; Hommel, K. Risk of fracture in adults on renal replacement therapy: A Danish national cohort study. Nephrol. Dial. Transpl. 2016, 31, 1654–1662. [Google Scholar] [CrossRef]
- Ball, A.M.; Gillen, D.L.; Sherrard, D.; Weiss, N.S.; Emerson, S.S.; Seliger, S.L.; Kestenbaum, B.R.; Stehman-Breen, C. Risk of hip fracture among dialysis and renal transplant recipients. JAMA 2002, 288, 3014–3018. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.J.; Hwang, S.Y.; Kim, D.W.; Kim, D.; Shin, S.J.; Yoon, H.E. Sex-specific relationship between vascular calcification and incident fracture in patients with end-stage renal disease. Kidney Res. Clin. Pract. 2020, 39, 344–355. [Google Scholar] [CrossRef]
- Bouquegneau, A.; Salam, S.; Delanaye, P.; Eastell, R.; Khwaja, A. Bone Disease after Kidney Transplantation. Clin. J. Am. Soc. Nephrol. 2016, 11, 1282–1296. [Google Scholar] [CrossRef]
- Naylor, K.L.; Li, A.H.; Lam, N.N.; Hodsman, A.B.; Jamal, S.A.; Garg, A.X. Fracture risk in kidney transplant recipients: A systematic review. Transplantation 2013, 95, 1461–1470. [Google Scholar] [CrossRef] [PubMed]
- Iseri, K.; Carrero, J.J.; Evans, M.; Felländer-Tsai, L.; Berg, H.E.; Runesson, B.; Stenvinkel, P.; Lindholm, B.; Qureshi, A.R. Fractures after kidney transplantation: Incidence, predictors, and association with mortality. Bone 2020, 140, 115554. [Google Scholar] [CrossRef] [PubMed]
- Dekker, F.W.; de Mutsert, R.; van Dijk, P.C.; Zoccali, C.; Jager, K.J. Survival analysis: Time-dependent effects and time-varying risk factors. Kidney Int. 2008, 74, 994–997. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Nikkel, L.E.; Hollenbeak, C.S.; Fox, E.J.; Uemura, T.; Ghahramani, N. Risk of fractures after renal transplantation in the United States. Transplantation 2009, 87, 1846–1851. [Google Scholar] [CrossRef]
- Mackey, D.C.; Lui, L.Y.; Cawthon, P.M.; Bauer, D.C.; Nevitt, M.C.; Cauley, J.A.; Hillier, T.A.; Lewis, C.E.; Barrett-Connor, E.; Cummings, S.R. High-trauma fractures and low bone mineral density in older women and men. JAMA 2007, 298, 2381–2388. [Google Scholar] [CrossRef]
- Zhang, Z.; Reinikainen, J.; Adeleke, K.A.; Pieterse, M.E.; Groothuis-Oudshoorn, C.G.M. Time-varying covariates and coefficients in Cox regression models. Ann. Transl. Med. 2018, 6, 121. [Google Scholar] [CrossRef] [PubMed]
- Abbott, K.C.; Oglesby, R.J.; Hypolite, I.O.; Kirk, A.D.; Ko, C.W.; Welch, P.G.; Agodoa, L.Y.; Duncan, W.E. Hospitalizations for fractures after renal transplantation in the United States. Ann. Epidemiol. 2001, 11, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.S.; Lenihan, C.R.; Montez-Rath, M.E.; Lowenberg, D.W.; Chertow, G.M.; Winkelmayer, W.C. Temporal trends in the incidence, treatment and outcomes of hip fracture after first kidney transplantation in the United States. Am. J. Transpl. 2014, 14, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Castro-Alonso, C.; D’Marco, L.; Pomes, J.; Del Amo Conill, M.; García-Diez, A.I.; Molina, P.; Puchades, M.J.; Valdivielso, J.M.; Escudero, V.; Bover, J.; et al. Prevalence of Vertebral Fractures and Their Prognostic Significance in the Survival in Patients with Chronic Kidney Disease Stages 3–5 Not on Dialysis. J. Clin. Med. 2020, 9, 1604. [Google Scholar] [CrossRef] [PubMed]
- Salter, M.L.; Liu, X.; Bae, S.; Chu, N.M.; Miller Dunham, A.; Humbyrd, C.; Segev, D.L.; McAdams-DeMarco, M.A. Fractures and Subsequent Graft Loss and Mortality among Older Kidney Transplant Recipients. J. Am. Geriatr. Soc. 2019, 67, 1680–1688. [Google Scholar] [CrossRef]
- Naylor, K.L.; Jamal, S.A.; Zou, G.; McArthur, E.; Lam, N.N.; Leslie, W.D.; Hodsman, A.B.; Kim, S.J.; Knoll, G.A.; Fraser, L.A.; et al. Fracture Incidence in Adult Kidney Transplant Recipients. Transplantation 2016, 100, 167–175. [Google Scholar] [CrossRef]
- Nikkel, L.E.; Mohan, S.; Zhang, A.; McMahon, D.J.; Boutroy, S.; Dube, G.; Tanriover, B.; Cohen, D.; Ratner, L.; Hollenbeak, C.S.; et al. Reduced fracture risk with early corticosteroid withdrawal after kidney transplant. Am. J. Transpl. 2012, 12, 649–659. [Google Scholar] [CrossRef]
- Ramsey-Goldman, R.; Dunn, J.E.; Dunlop, D.D.; Stuart, F.P.; Abecassis, M.M.; Kaufman, D.B.; Langman, C.B.; Salinger, M.H.; Sprague, S.M. Increased risk of fracture in patients receiving solid organ transplants. J. Bone Miner. Res. 1999, 14, 456–463. [Google Scholar] [CrossRef]
- Sprague, S.M.; Belozeroff, V.; Danese, M.D.; Martin, L.P.; Olgaard, K. Abnormal bone and mineral metabolism in kidney transplant patients-a review. Am. J. Nephrol. 2008, 28, 246–253. [Google Scholar] [CrossRef]
- Fusaro, M.; Cosmai, L.; Evenepoel, P.; Nickolas, T.L.; Cheung, A.M.; Aghi, A.; Tripepi, G.; Plebani, M.; Iervasi, G.; Vettor, R.; et al. Vitamin K and Kidney Transplantation. Nutrients 2020, 12, 2717. [Google Scholar] [CrossRef]
- Evenepoel, P.; Claes, K.; Meijers, B.; Laurent, M.; Bammens, B.; Naesens, M.; Sprangers, B.; Pottel, H.; Cavalier, E.; Kuypers, D. Poor Vitamin K Status is Associated with Low Bone Mineral Density and Increased Fracture Risk in End-Stage Renal Disease. J. Bone Miner. Res. 2019, 34, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Malluche, H.H.; Monier-Faugere, M.C.; Herberth, J. Bone disease after renal transplantation. Nat. Rev. Nephrol. 2010, 6, 32–40. [Google Scholar] [CrossRef]
- Rojas, E.; Carlini, R.G.; Clesca, P.; Arminio, A.; Suniaga, O.; De Elguezabal, K.; Weisinger, J.R.; Hruska, K.A.; Bellorin-Font, E. The pathogenesis of osteodystrophy after renal transplantation as detected by early alterations in bone remodeling. Kidney Int. 2003, 63, 1915–1923. [Google Scholar] [CrossRef] [PubMed]
- Schlosberg, M.; Movsowitz, C.; Epstein, S.; Ismail, F.; Fallon, M.D.; Thomas, S. The effect of cyclosporin A administration and its withdrawal on bone mineral metabolism in the rat. Endocrinology 1989, 124, 2179–2184. [Google Scholar] [CrossRef] [PubMed]
- Kirino, S.; Fukunaga, J.; Ikegami, S.; Tsuboi, H.; Kimata, M.; Nakata, N.; Nakano, M.; Ueno, T.; Mizukawa, N.; Sugahara, T. Regulation of bone metabolism in immunosuppressant (FK506)-treated rats. J. Bone Miner. Metab. 2004, 22, 554–560. [Google Scholar] [CrossRef]
- Singha, U.K.; Jiang, Y.; Yu, S.; Luo, M.; Lu, Y.; Zhang, J.; Xiao, G. Rapamycin inhibits osteoblast proliferation and differentiation in MC3T3-E1 cells and primary mouse bone marrow stromal cells. J. Cell. Biochem. 2008, 103, 434–446. [Google Scholar] [CrossRef]
- Kneissel, M.; Luong-Nguyen, N.H.; Baptist, M.; Cortesi, R.; Zumstein-Mecker, S.; Kossida, S.; O’Reilly, T.; Lane, H.; Susa, M. Everolimus suppresses cancellous bone loss, bone resorption, and cathepsin K expression by osteoclasts. Bone 2004, 35, 1144–1156. [Google Scholar] [CrossRef]
- Iyer, S.P.; Nikkel, L.E.; Nishiyama, K.K.; Dworakowski, E.; Cremers, S.; Zhang, C.; McMahon, D.J.; Boutroy, S.; Liu, X.S.; Ratner, L.E.; et al. Kidney transplantation with early corticosteroid withdrawal: Paradoxical effects at the central and peripheral skeleton. J. Am. Soc. Nephrol. 2014, 25, 1331–1341. [Google Scholar] [CrossRef]
- Vautour, L.M.; Melton, L.J., 3rd; Clarke, B.L.; Achenbach, S.J.; Oberg, A.L.; McCarthy, J.T. Long-term fracture risk following renal transplantation: A population-based study. Osteoporos Int. 2004, 15, 160–167. [Google Scholar] [CrossRef]
- Naylor, K.L.; Zou, G.; Leslie, W.D.; Hodsman, A.B.; Lam, N.N.; McArthur, E.; Fraser, L.A.; Knoll, G.A.; Adachi, J.D.; Kim, S.J.; et al. Risk factors for fracture in adult kidney transplant recipients. World J. Transpl. 2016, 6, 370–379. [Google Scholar] [CrossRef]
- Hong, Y.A.; Ban, T.H.; Kang, C.Y.; Hwang, S.D.; Choi, S.R.; Lee, H.; Jung, H.Y.; Kim, K.; Kwon, Y.E.; Kim, S.H.; et al. Trends in epidemiologic characteristics of end-stage renal disease from 2019 Korean Renal Data System (KORDS). Kidney Res. Clin. Pract. 2021, 40, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Fusaro, M.; Holden, R.; Lok, C.; Iervasi, G.; Plebani, M.; Aghi, A.; Gallieni, M.; Cozzolino, M. Phosphate and bone fracture risk in chronic kidney disease patients. Nephrol. Dial. Transpl. 2021, 36, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Oei, L.; Rivadeneira, F.; Zillikens, M.C.; Oei, E.H. Diabetes, diabetic complications, and fracture risk. Curr. Osteoporos Rep. 2015, 13, 106–115. [Google Scholar] [CrossRef]
- Ziolkowski, S.; Liu, S.; Montez-Rath, M.E.; Denburg, M.; Winkelmayer, W.C.; Chertow, G.M.; O’Shaughnessy, M.M. Association between cause of kidney failure and fracture incidence in a national US dialysis population cohort study. Clin. Kidney J. 2022, 15, 2245–2257. [Google Scholar] [CrossRef]
- Aleksova, J.; Wong, P.; Mulley, W.R.; Choy, K.W.; McLachlan, R.; Ebeling, P.R.; Kerr, P.G.; Milat, F. Serum phosphorus levels and fracture following renal transplantation. Clin. Endocrinol. 2017, 87, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Yuksel, Y.; Yuksel, D.; Yucetin, L.; Erbis, H.; Sarier, M.; Karatas, O.; Emek, M.; Erdogan, M.; Yavuz, A.; Demirbas, A. Use of Tacrolimus During Pregnancy After Kidney Transplantaion. Transpl. Proc. 2019, 51, 2361–2366. [Google Scholar] [CrossRef]
- Edwards, B.J.; Desai, A.; Tsai, J.; Du, H.; Edwards, G.R.; Bunta, A.D.; Hahr, A.; Abecassis, M.; Sprague, S. Elevated incidence of fractures in solid-organ transplant recipients on glucocorticoid-sparing immunosuppressive regimens. J. Osteoporos. 2011, 2011, 591793. [Google Scholar] [CrossRef]
- Cvetkovic, M.; Mann, G.N.; Romero, D.F.; Liang, X.G.; Ma, Y.; Jee, W.S.; Epstein, S. The deleterious effects of long-term cyclosporine A, cyclosporine G, and FK506 on bone mineral metabolism in vivo. Transplantation 1994, 57, 1231–1237. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).