Promising Perinatal Outcome after Using a Simplified Low-Cost IVF Culture System Specifically Designed for Resource-Poor Countries
Abstract
1. Introduction
2. Materials and Methods
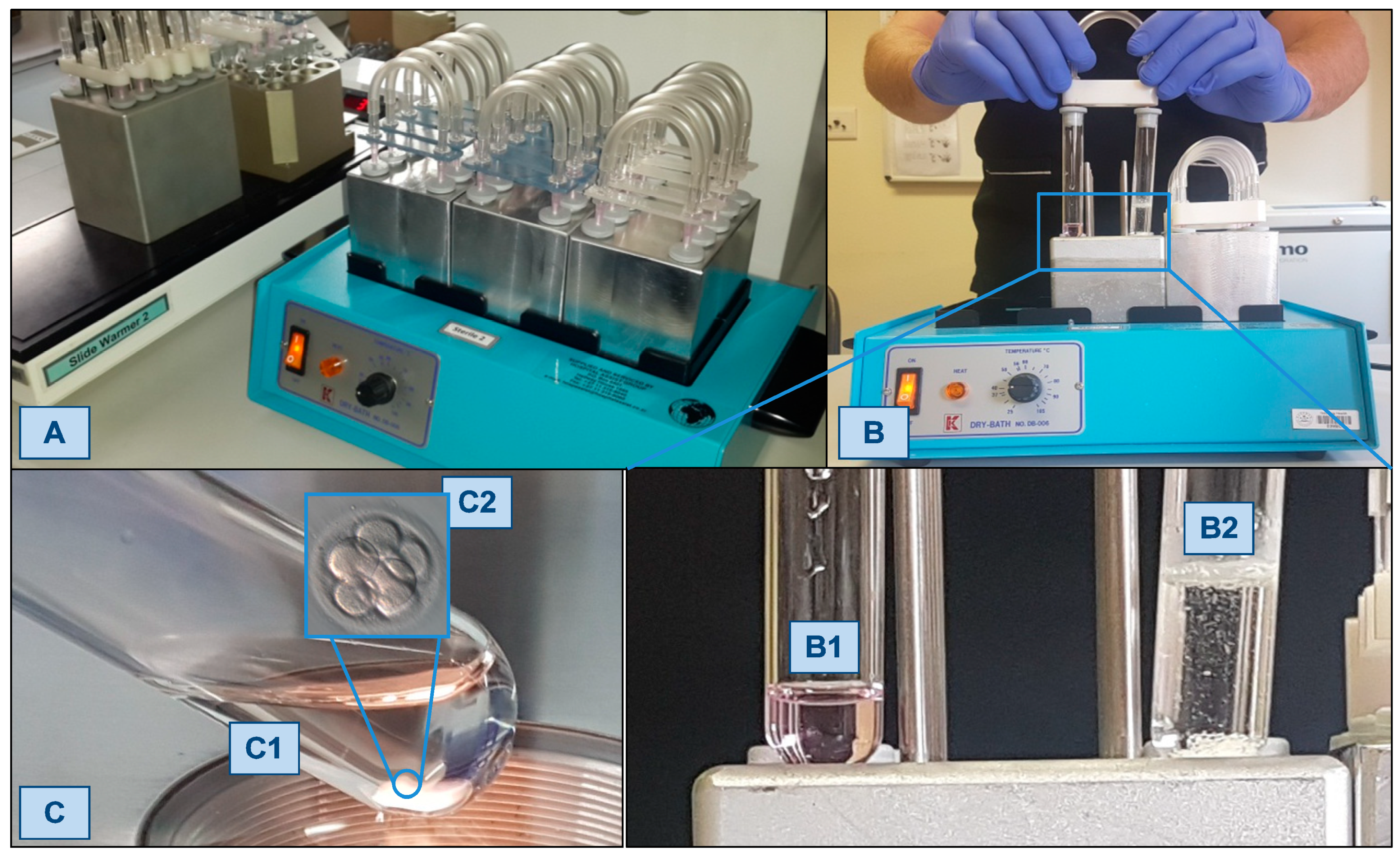
2.1. The Simplified Culture System (SCS)
2.2. Comparative Medical Economics of IVF Systems
2.3. The SCS Patient Cohort: Materials and Methods
2.4. The SCS Patient Cohort: Outcome Parameters
2.5. Comparison with BELRAP Data
2.6. Ethical Committee Approval
2.7. Statistics
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rutstein, S.O.; Iqbal, H.S. Infecundity, Infertility, and Childlessness in Developing Countries; DHS Comparative Reports; WHO: Geneva, Switzerland, 2004. [Google Scholar]
- Boivin, J.; Bunting, L.; Collins, J.A.; Nygren, K.G. International estimates of infertility prevalence and treatment-seeking: Potential need and demand for infertility medical care. Hum. Reprod. 2007, 22, 1506–1512. [Google Scholar] [CrossRef] [PubMed]
- Mascarenhas, M.; Flaxman, S.; Boerma, T.; Vanderpoel, S.; Stevens, G.A. National, regional, and global trends in infertility prevalence since 1990: A systematic analysis of 277 health surveys. PLoS Med. 2012, 9, e1001356. [Google Scholar] [CrossRef]
- Inhorn, M.C.; Patrizio, P. Infertility around the globe: New thinking on gender, reproductive technologies and global movements in the 21st century. Hum. Reprod. Update 2015, 21, 411–426. [Google Scholar] [CrossRef]
- Dyer, S.J.; Abrahams, N.; Mokoena, N.E.; van der Spuy, Z.M. “You are a man because you have children”: Experiences, reproductive health knowledge and treatment-seeking behaviour among men suffering from couple infertility in South Africa. Hum. Reprod. 2004, 19, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Ombelet, W.; Cooke, I.; Dyer, S.; Devroey, P. Infertility and the provision of infertility medical services in developing countries. Hum. Reprod. Update 2008, 14, 605–621. [Google Scholar] [CrossRef] [PubMed]
- Dyer, S.J.; Patel, M. The economic impact of infertility on women in developing countries—A systematic review. Facts Views Vis. Obgyn. 2012, 4, 102–109. [Google Scholar] [PubMed]
- Chiware, T.M.; Vermeulen, N.; Blondeel, K.; Farquharson, R.; Kiarie, J.; Lundin, K.; Matsaseng, T.C.; Ombelet, W.; Toskin, I. IVF and other ART in low- and middle-income countries: A systematic landscape analysis. Hum. Reprod. Update 2021, 27, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Afferri, A.; Allen, H.; Booth, A.; Dierickx, S.; Pacey, A.; Balen, J. Barriers and facilitators for the inclusion of fertility care in reproductive health policies in Africa: A qualitative evidence synthesis. Hum. Reprod. Update 2022, 28, 190–199. [Google Scholar] [CrossRef]
- WHO Fact Sheet. Available online: www.who.int/news-room/fact-sheets/detail/infertility (accessed on 14 September 2020).
- Ombelet, W. Is global access to infertility care realistic? The Walking Egg Project. Reprod. Biomed. Online 2014, 28, 267–272. [Google Scholar] [CrossRef]
- Van Blerkom, J.; Ombelet, W.; Klerkx, E.; Janssen, M.; Dhont, N.; Nargund, G.; Campo, R. First Births with a Simplified Culture System for Clinical IVF and ET. Reprod. Biomed. Online 2014, 28, 310–320. [Google Scholar] [CrossRef]
- Ombelet, W.; Van Blerkom, J.; Nargund, G.; Van der Auwera, I.; Janssen, M.; Dhont, N.; Bosmans, E.; Boshoff, G.; Vertessen, V.J.; Campo, R. Multiyear outcomes using sibling oocytes demonstrates safety and efficacy of a simplified culture system consistent with use in a low-cost IVF setting. Reprod. Biomed. Online 2022, 45, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Pinborg, A.; Wennerholm, U.B.; Romundstad, L.B.; Loft, A.; Aittomaki, K.; Söderström-Anttila, V.; Nygren, K.G.; Hazekamp, J.; Bergh, C. Why do singletons conceived after assisted reproduction technology have adverse perinatal outcome? Systematic review and meta-analysis. Hum. Reprod. Update 2013, 19, 87–104. [Google Scholar] [CrossRef] [PubMed]
- Palomba, S.; Homburg, R.; Santagni, S.; La Sala, G.B.; Orvieto, R. Risk of adverse pregnancy and perinatal outcomes after high technology infertility treatment: A comprehensive systematic review. Reprod. Biol. Endocrinol. 2016, 14, 76. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Sheng, X.; Wu, D.; Gao, S.; You, Y.; Yang, T.; Wang, H. Adverse Obstetric Outcomes Associated With In Vitro Fertilization in Singleton Pregnancies. Reprod. Sci. 2017, 24, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.B.; Sheng, X.Q.; Wu, D.; Gao, S.Y.; You, Y.P.; Yang, T.B.; Wang, H. Worldwide prevalence of adverse pregnancy outcomes among singleton pregnancies after in vitro fertilization/intracytoplasmic sperm injection: A systematic review and meta-analysis. Arch. Gynecol. Obstet. 2017, 295, 285–301. [Google Scholar] [CrossRef]
- Sunderam, S.; Kissin, D.M.; Zhang, Y.; Jewett, A.; Boulet, S.L.; Warner, L.; Kroelinger, C.D.; Barfield, W.D. Assisted Reproductive Technology Surveillance—United States, 2018. MMWR Surveill. Summ. 2022, 71, 1–19. [Google Scholar] [CrossRef]
- Cavoretto, P.; Candiani, M.; Giorgione, V.; Inversetti, A.; Abu-Saba, M.M.; Tiberio, F.; Sigismondi, C.; Farina, A. Risk of spontaneous preterm birth in singleton pregnancies conceived after IVF/ICSI treatment: Meta-analysis of cohort studies. Ultrasound Obstet. Gynecol. 2018, 51, 43–53. [Google Scholar] [CrossRef]
- Ombelet, W.; Van Blerkom, J.; Klerkx, E.; Janssen, M.; Dhont, N.; Mestdagh, G.; Nargund, G.; Campo, R. The (t)WE lab Simplified IVF Procedure: First Births after freezing/thawing. Facts Views Vis. Obgyn. 2014, 6, 45–49. [Google Scholar]
- Ombelet, W.; Van Blerkom, J.; Nargund, G.; Janssen, M.; Jacobs, P.; Van der Auwera, I.; Dhont, N.; Bosmans, E.; Vertessen, V.J.; Campo, R. Perinatal outcome of babies born after using a simplified culture system for IVF versus ICSI followed by conventional culturing with sibling oocytes: A multi-year prospective cohort study. Reprod. Biomed. Online 2022, 45, 574–582. [Google Scholar] [CrossRef]
- BELRAP (Belgian Register for Assisted Reproduction). Available online: www.belrap.be (accessed on 1 December 2004).
- Ombelet, W.; De Sutter, P.; Van der Elst, J.; Martens, G. Multiple gestation and infertility treatment: Registration, reflection and reaction: The Belgian project. Hum. Reprod. Update 2005, 11, 3–14. [Google Scholar] [CrossRef]
- De Neubourg, D.; Bogaerts, K.; Wyns, C.; Albert, A.; Camus, M.; Candeur, M.; Degueldre, M.; Delbaere, A.; Delvigne, A.; De Sutter, P.; et al. The history of Belgian assisted reproduction technology cycle registration and control: A case study in reducing the incidence of multiple pregnancy. Hum. Reprod. 2013, 28, 2709–2719. [Google Scholar] [CrossRef] [PubMed]
- Andersen, A.N.; Goossens, V.; Ferraretti, A.P.; Bhattacharya, S.; Felberbaum, R.; de Mouzon, J.; Nygren, K.G. European IVF-Monitoring Consortium, European Society of Human Reproduction and Embryology Assisted reproductive technology in Europe, 2004: Results generated from European registers by ESHRE. Hum. Reprod. 2008, 23, 756–771. [Google Scholar] [PubMed]
- de Mouzon, J.; Lancaster, P.; Nygren, K.G.; Sullivan, E.; Zegers-Hochschild, F.; Mansour, R.; Ishihara, O.; Adamson, D. World collaborative report on Assisted Reproductive Technology, 2002.International Committee for Monitoring Assisted Reproductive Technology. Hum. Reprod. 2009, 24, 2310–2320. [Google Scholar] [PubMed]
- Christiaens, I. Setting-Up a New IVF Centre: Is a “Walking Egg” Centre an Attractive Investment? Master’s Thesis, Biomedical Sciences Program at the Catholic University of Leuven, Leuven, Belgium, 2018. [Google Scholar]
- World Health Organization. Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction, 5th ed.; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Cooper, T.G.; Noonan, E.; von Eckardstein, S.; Auger, J.; Baker, H.W.; Behre, H.M.; Haugen, T.B.; Kruger, T.; Wang, C.; Mbizvo, M.T.; et al. World Health Organization reference values for human semen characteristics. Hum. Reprod. Update 2010, 16, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Ombelet, W.; Martens, G.; Bruckers, L. Pregnant after assisted reproduction: A risk pregnancy is born! 18-years perinatal outcome results from a population-based registry in Flanders, Belgium. Facts Views Vis. Obgyn. 2016, 8, 193–204. [Google Scholar]
- Zegers-Hochschild, F.; Crosby, J.A.; Musri, C.; de Souza, M.D.C.B.; Martinez, A.G.; Silva, A.A.; Mojarra, J.M.; Masoli, D.; Posada, N.; Latin American Network of Assisted Reproduction. Assisted reproductive technology in Latin America: The Latin American Registry, 2017. Reprod. Biomed. Online 2020, 41, 44–54. [Google Scholar] [CrossRef]
- Wyns, C.; De Geyter, C.; Calhaz-Jorge, C.; Kupka, M.S.; Motrenko, T.; Smeenk, J.; Bergh, C.; Tandler-Schneider, A.; Rugescu, I.A.; Goossens, V. European IVF-Monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE). ART in Europe, 2018: Results generated from European registries by ESHRE. Hum. Reprod. Open 2022, 3, hoac022. [Google Scholar]
- Kim, D.; Saada, A. The social determinants of infant mortality and birth outcomes in Western developed nations: A cross-country systematic review. Int. J. Environ. Res. Public Health 2013, 10, 2296–2335. [Google Scholar] [CrossRef]
- Nouri, K.; Ott, J.; Stoegbauer, L.; Pietrowski, D.; Frantal, S.; Walch, K. Obstetric and perinatal outcomes in IVF versus ICSI-conceived pregnancies at a tertiary care center--a pilot study. Reprod. Biol. Endocrinol. 2013, 11, 84. [Google Scholar] [CrossRef]
- Liu, L.; Wang, H.; Li, Z.; Niu, J.; Tang, R. Obstetric and perinatal outcomes of intracytoplasmic sperm injection versus conventional invitro fertilization in couples with nonsevere male infertility. Fertil. Steril. 2020, 114, 792–800. [Google Scholar] [CrossRef]
- Tannus, S.; Son, W.Y.; Gilman, A.; Younes, G.; Shavit, T.; Dahan, M.H. The role of intracytoplasmic sperm injection in non-male factor infertility in advanced maternal age. Hum. Reprod. 2017, 32, 119–124. [Google Scholar] [CrossRef]
- Li, Z.; Wang, A.Y.; Bowman, M.; Hammarberg, K.; Farquhar, C.; Johnson, L.; Safi, N.; Sullivan, E.A. ICSI does not increase the cumulative live birth rate in non-male factor infertility. Hum. Reprod. 2018, 33, 1322–1330. [Google Scholar] [CrossRef] [PubMed]
- Dang, V.Q.; Vuong, L.N.; Luu, T.M.; Pham, T.D.; Ho, T.M.; Ha, A.N.; Truong, B.T.; Phan, A.K.; Nguyen, D.P.; Pham, T.N.; et al. Intracytoplasmic sperm injection versus conventional in-vitro fertilisation in couples with infertility in whom the male partner has normal total sperm count and motility: An open-label, randomised controlled trial. Lancet 2021, 397, 1554–1563. [Google Scholar] [CrossRef] [PubMed]
- Magnus, M.C.; Ghaderi, S.; Morken, N.H.; Magnus, P.; Bente Romundstad, L.; Skjærven, R.; Wilcox, A.J.; Eldevik Håberg, S. Vanishing twin syndrome among ART singletons and pregnancy outcomes. Hum. Reprod. 2017, 32, 2298–2304. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, Z.; Chen, L.; Liu, P. The late vanishing of a co-twin contributes to adverse perinatal outcomes in the surviving singleton. Hum. Reprod. 2020, 35, 1553–1561. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Zhang, Y.; Chen, N.; Gao, J.; Hu, J.; Cui, L.; Chen, Z.J. The Influence of the Vanishing Twin on the Perinatal Outcome of Surviving Singleton in IVF Pregnancy. Front. Endocrinol. 2022, 13, 832665. [Google Scholar] [CrossRef]
- Sullivan-Pyke, C.S.; Senapati, S.; Mainigi, M.A.; Barnhart, K.T. In Vitro fertilization and adverse obstetric and perinatal outcomes. Semin. Perinatol. 2017, 41, 345–353. [Google Scholar] [CrossRef]
- Sciorio, R.; El Hajj, N. Epigenetic Risks of Medically Assisted Reproduction. J. Clin. Med. 2022, 11, 2151. [Google Scholar] [CrossRef]
- Insua, M.F.; Cobo, A.C.; Larreategui, Z.; Ferrando, M.; Serra, V.; Meseguer, M. Obstetric and perinatal outcomes of pregnancies conceived with embryos cultured in a time-lapse monitoring system. Fertil. Steril. 2017, 108, 498–504. [Google Scholar] [CrossRef]
- Mascarenhas, M.; Fox, S.J.; Thompson, K.; Balen, A.H. Cumulative live birth rates and perinatal outcomes with the use of time-lapse imaging incubators for embryo culture: A retrospective cohort study of 1882 ART cycles. BJOG 2019, 126, 280–286. [Google Scholar] [CrossRef]
- Alhelou, Y.; Mat Adenan, N.A.; Ali, J. Embryo culture conditions are significantly improved during uninterrupted incubation: A randomized controlled trial. Reprod. Biol. 2018, 18, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Dumoulin, J.C.; Land, J.A.; Van Montfoort, A.P.; Nelissen, E.C.; Coonen, E.; Derhaag, J.G.; Schreurs, I.L.; Dunselman, G.A.; Kester, A.D.; Geraedts, J.P.; et al. Effect of in vitro culture of human embryos on birthweight of newborns. Hum. Reprod. 2010, 25, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Nelissen, E.C.; Van Montfoort, A.P.; Coonen, E.; Derhaag, J.G.; Geraedts, J.P.; Smits, L.J.; Land, J.A.; Evers, J.L.; Dumoulin, J.C. Further evidence that culture media affect perinatal outcome: Findings after transfer of fresh and cryopreserved embryos. Hum. Reprod. 2012, 27, 1966–1976. [Google Scholar] [CrossRef] [PubMed]
- Berntsen, S.; Pinborg, A. Large for gestational age and macrosomia in singletons born after frozen/thawed embryo transfer (FET) in assisted reproductive technology (ART). Birth Defects Res. 2018, 110, 630–643. [Google Scholar] [CrossRef]
- Terho, A.M.; Pelkonen, S.; Opdahl, S.; Romundstad, L.B.; Bergh, C.; Wennerholm, U.B.; Henningsen, A.A.; Pinborg, A.; Gissler, M.; Tiitinen, A. High birth weight and large-for-gestational-age in singletons born after frozen compared to fresh embryo transfer, by gestational week: A Nordic register study from the CoNARTaS group. Hum. Reprod. 2021, 36, 1083–1092. [Google Scholar] [CrossRef]
- Elias, F.T.S.; Weber-Adrian, D.; Pudwell, J.; Carter, J.; Walker, M.; Gaudet, L.; Smith, G.; Velez, M.P. Neonatal outcomes in singleton pregnancies conceived by fresh or frozen embryo transfer compared to spontaneous conceptions: A systematic review and meta-analysis. Arch. Gynecol. Obstet. 2020, 302, 31–45. [Google Scholar] [CrossRef]
- Westvik-Johari, K.; Romundstad, L.B.; Lawlor, D.A.; Bergh, C.; Gissler, M.; Henningsen, A.A.; Håberg, S.E.; Wennerholm, U.B.; Tiitinen, A.; Pinborg, A.; et al. Separating parental and treatment contributions to perinatal health after fresh and frozen embryo transfer in assisted reproduction: A cohort study with within-sibship analysis. PLoS Med. 2021, 18, e1003683. [Google Scholar] [CrossRef]

| All IVF/ICSI (BELRAP) | SCS | p Value (Fisher’s Exact Test) | ||
|---|---|---|---|---|
| Gestational age at delivery | 0.0549 | |||
| <37 weeks | 1505 (9.2) | 4 (3.8) | ||
| ≥37 weeks | 14,783 (91.8) | 101 (96.2) | ||
| Birthweight | 0.0399 | |||
| < 2.5 kg | 1338 (8.4) | 3 (2.9) | ||
| ≥2.5 kg | 14,521 (91.6) | 102 (97.1) |
| All IVF/ICSI (BELRAP) | SCS | p Value (Fisher’s Exact Test) | ||
|---|---|---|---|---|
| Gestational age at delivery | 0.6454 | |||
| <37 weeks | 1154 (10.1) | 6 (8.4) | ||
| ≥37 weeks | 10,264 (89.9) | 65 (91.6) | ||
| Birthweight | 0.7210 | |||
| <2.5 kg | 573 (5.2) | 3 (4.2) | ||
| ≥2.5 kg | 10,514 (94.8) | 68 (95.8) |
| IVF Only (BELRAP) | SCS | p Value (Fisher’s Exact Test) | ||
|---|---|---|---|---|
| Gestational age at delivery | 0.0296 | |||
| <37 weeks | 272 (10.2) | 4 (3.8) | ||
| ≥37 weeks | 2406 (89.8) | 101 (96.2) | ||
| Birthweight | 0.0161 | |||
| <2.5 kg | 264 (9.8) | 3 (2.9) | ||
| ≥2.5 kg | 2431 (90.2) | 102 (97.1) |
| OR (95% CI) BELRAP vs. SCS | p Value (Logistic Model) | |
|---|---|---|
| Gestational age at delivery < 37 w | ||
| No correction for risk factors | 2.852 [1.042; 7.803] | 0.0413 |
| Correction for risk factors | 2.627 [1.013; 6.816] | 0.0471 |
| Birth weight < 2.5 kg | ||
| No correction for risk factors Correction for risk factors | 3.692 [1.163; 11.721] 3.267 [1.118; 9.549] | 0.0267 0.0305 |
| IVF only (BELRAP) | SCS | p Value | ||
|---|---|---|---|---|
| Age of mother (years) | 0.7615 | |||
| <25 | 64 (2.4) | 3 (2.9) | ||
| 25–29 | 539 (20.0) | 21 (20.0) | ||
| 30–34 | 1116 (41.4) | 42 (40.0) | ||
| 35–39 | 742 (27.5) | 33 (31.4) | ||
| ≥40 | 234 (8.7) | 6 (5.7) | ||
| Day of transfer | 0.0001 | |||
| 2–3 | 1751 (65.0) | 33 (31.4) | ||
| 4–5 | 943 (35.0) | 72 (68.6) | ||
| Pituitary inhibition | 0.0001 | |||
| Agonist long | 1016 (37.8) | 14 (13.3) | ||
| Agonist short | 408 (15.2) | 11 (10.5) | ||
| Antagonist | 1265 (47.0) | 80 (76.2) | ||
| Rank of fresh cycle | 0.1691 | |||
| 1 | 1445 (53.7) | 49 (46.7) | ||
| 2 | 696 (25.9) | 30 (28.6) | ||
| 3 | 292 (10.9) | 18 (17.1) | ||
| >3 | 257 (9.6) | 8 (7.6) | ||
| Number of oocytes retrieved | 0.0001 | |||
| <6 | 761 (28.2) | 4 (3.8) | ||
| 6–10 | 1083 (40.2) | 50 (47.6) | ||
| 11–15 | 609 (22.6) | 36 (34.3) | ||
| 16–20 | 190 (7.1) | 11 (10.5) | ||
| >20 | 52 (1.9) | 4 (3.8) | ||
| Number of embryos transferred | 0.0029 | |||
| 1 | 1970 (73.2) | 92 (87.6) | ||
| 2 | 650 (24.1) | 12 (11.4) | ||
| ≥3 | 73 (2.7) | 1 (1.0) | ||
| Gender of baby | 0.1347 | |||
| Male | 1429 (53.3) | 64 (61.0) | ||
| Female | 1254 (46.7) | 41 (39.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ombelet, W.; Van Blerkom, J.; Bruckers, L.; Dhont, N.; Nargund, G.; Campo, R. Promising Perinatal Outcome after Using a Simplified Low-Cost IVF Culture System Specifically Designed for Resource-Poor Countries. J. Clin. Med. 2023, 12, 2264. https://doi.org/10.3390/jcm12062264
Ombelet W, Van Blerkom J, Bruckers L, Dhont N, Nargund G, Campo R. Promising Perinatal Outcome after Using a Simplified Low-Cost IVF Culture System Specifically Designed for Resource-Poor Countries. Journal of Clinical Medicine. 2023; 12(6):2264. https://doi.org/10.3390/jcm12062264
Chicago/Turabian StyleOmbelet, Willem, Jonathan Van Blerkom, Liesbeth Bruckers, Nathalie Dhont, Geeta Nargund, and Rudi Campo. 2023. "Promising Perinatal Outcome after Using a Simplified Low-Cost IVF Culture System Specifically Designed for Resource-Poor Countries" Journal of Clinical Medicine 12, no. 6: 2264. https://doi.org/10.3390/jcm12062264
APA StyleOmbelet, W., Van Blerkom, J., Bruckers, L., Dhont, N., Nargund, G., & Campo, R. (2023). Promising Perinatal Outcome after Using a Simplified Low-Cost IVF Culture System Specifically Designed for Resource-Poor Countries. Journal of Clinical Medicine, 12(6), 2264. https://doi.org/10.3390/jcm12062264







