Incidence and Predictors of Incidental Biochemical and Radiologic Pancreatic Alterations Following Uncomplicated ERCP
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Design and Setting
2.2. Patients, Variables, and Outcomes
2.3. Statistical Analyses
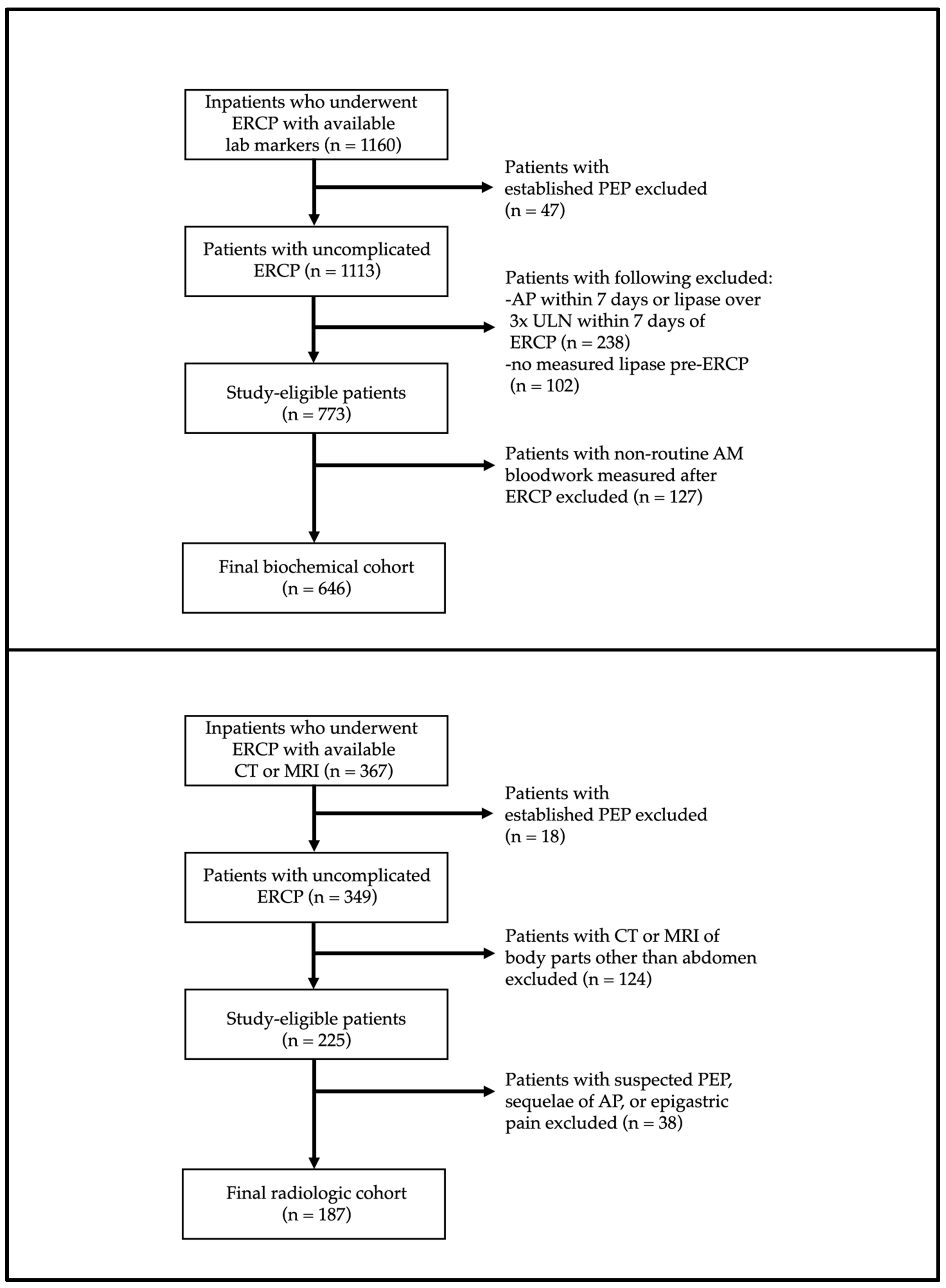
3. Results
3.1. Demographics and Descriptive Results
3.2. Predictors of Incidental Biochemical or Radiologic Alterations
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maple, J.T.; Ikenberry, S.O.; Anderson, M.A.; Appalaneni, V.; Decker, G.A.; Early, D.; Evans, J.A.; Fanelli, R.D.; Fisher, D.; Fisher, L.; et al. The role of endoscopy in the management of choledocholithiasis. Gastrointest. Endosc. 2011, 74, 731–744. [Google Scholar] [CrossRef]
- American Society for Gastrointestinal Endoscopy (ASGE) Standards of Practice Committee; Anderson, M.A.; Appalaneni, V.; Ben-Menachem, T.; Decker, G.A.; Early, D.S.; Evans, J.A.; Fanelli, R.D.; Fisher, D.A.; Fisher, L.R.; et al. The role of endoscopy in the evaluation and treatment of patients with biliary neoplasia. Gastrointest. Endosc. 2013, 77, 167–174. [Google Scholar] [CrossRef]
- Chandrasekhara, V.; Khashab, M.A.; Muthusamy, V.R.; Acosta, R.D.; Agrawal, D.; Bruining, D.H.; Eloubeidi, M.A.; Fanelli, R.D.; Faulx, A.L.; Gurudu, S.R.; et al. Adverse events associated with ERCP. Gastrointest. Endosc. 2017, 85, 32–47. [Google Scholar] [CrossRef]
- Forbes, N.; Elmunzer, B.J.; Keswani, R.N.; Hilsden, R.J.; Hall, M.; Anderson, J.; Arvanitakis, M.; Chen, Y.-I.; Duloy, A.; Elta, G.; et al. Consensus-based development of a causal attribution system for post-ERCP adverse events. Gut, 2022; online ahead of print. [Google Scholar] [CrossRef]
- Cotton, P.; Lehman, G.; Vennes, J.; Geenen, J.; Russell, R.; Meyers, W.; Liguory, C.; Nickl, N. Endoscopic sphincterotomy complications and their management: An attempt at consensus. Gastrointest. Endosc. 1991, 37, 383–393. [Google Scholar] [CrossRef]
- Cotton, P.B.; Eisen, G.M.; Aabakken, L.; Baron, T.H.; Hutter, M.M.; Jacobson, B.C.; Mergener, K.; Nemcek, A., Jr.; Petersen, B.T.; Petrini, J.L.; et al. A lexicon for endoscopic adverse events: Report of an ASGE workshop. Gastrointest. Endosc. 2010, 71, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Jeurnink, S.M.; Steyerberg, E.W.; Kuipers, E.J.; Siersema, P.D. The burden of endoscopic retrograde cholangiopancreatography (ERCP) performed with the patient under conscious sedation. Surg. Endosc. 2012, 26, 2213–2219. [Google Scholar] [CrossRef] [PubMed]
- Forbes, N.; Chau, M.; Koury, H.F.; Lethebe, B.C.; Smith, Z.L.; Wani, S.; Keswani, R.N.; Elmunzer, B.J.; Anderson, J.T.; Heitman, S.J.; et al. Development and validation of a patient-reported scale for tolerability of endoscopic procedures using conscious sedation. Gastrointest. Endosc. 2021, 94, 103–110.e2. [Google Scholar] [CrossRef]
- Friedrich, S.; Reis, S.; Meybohm, P.; Kranke, P. Preoperative anxiety. Curr. Opin. Anaesthesiol. 2022, 35, 674–678. [Google Scholar] [CrossRef]
- Goyal, H.; Sachdeva, S.; Sherazi, S.A.A.; Gupta, S.; Perisetti, A.; Ali, A.; Chandan, S.; Tharian, B.; Sharma, N.; Thosani, N. Early prediction of post-ERCP pancreatitis by post-procedure amylase and lipase levels: A systematic review and meta-analysis. Endosc. Int. Open. 2022, 10, E952–E970. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.P.; Nietert, P.J.; Bobo, J.F.; Haj, M.; Forbes, N.; Elmunzer, B.J. Serum Amylase as a Biomarker for Proof-of-Concept Studies in Post Endoscopic Retrograde Cholangiopancreatography Pancreatitis Prevention. Clin. Gastroenterol. Hepatol. 2022; in press. [Google Scholar] [CrossRef]
- Woźniak, B.; Wiśniewska-Jarosińska, M.; Drzewoski, J. Evaluation of selected parameters of the inflammatory response to endoscopic retrograde cholangiopancreatography. Pancreas 2001, 23, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Forbes, N.; Koury, H.F.; Bass, S.; Cole, M.; Mohamed, R.; Turbide, C.; Gonzalez-Moreno, E.; Kayal, A.; Chau, M.; Lethebe, B.C.; et al. Characteristics and Outcomes of ERCP at a Canadian Tertiary Centre: Initial Results from a Prospective High-Fidelity Biliary Endoscopy Registry. J. Can. Assoc. Gastroenterol. 2021, 4, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Dumonceau, J.-M.; Kapral, C.; Aabakken, L.; Papanikolaou, I.S.; Tringali, A.; Vanbiervliet, G.; Beyna, T.; Dinis-Ribeiro, M.; Hritz, I.; Mariani, A.; et al. ERCP-related adverse events: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2020, 52, 127–149. [Google Scholar] [CrossRef] [PubMed]
- de-Madaria, E.; Buxbaum, J.L.; Maisonneuve, P.; de Paredes, A.G.G.; Zapater, P.; Guilabert, L.; Vaillo-Rocamora, A.; Rodríguez-Gandía, M.Á.; Donate-Ortega, J.; Lozada-Hernández, E.E.; et al. Aggressive or Moderate Fluid Resuscitation in Acute Pancreatitis. N. Engl. J. Med. 2022, 387, 989–1000. [Google Scholar] [CrossRef] [PubMed]
- Bishay, K.; Causada-Calo, N.; Scaffidi, M.A.; Walsh, C.M.; Anderson, J.T.; Rostom, A.; Dube, C.; Keswani, R.N.; Heitman, S.J.; Hilsden, R.J.; et al. Associations between endoscopist feedback and improvements in colonoscopy quality indicators: A systematic review and meta-analysis. Gastrointest. Endosc. 2020, 92, 1030–1040.e9. [Google Scholar] [CrossRef]
- Thaker, A.M.; Mosko, J.D.; Berzin, T.M. Post-endoscopic retrograde cholangiopancreatography pancreatitis. Gastroenterol. Rep. 2015, 3, 32–40. [Google Scholar] [CrossRef]
- Mäkelä, A.; Kuusi, T.; Schröder, T. Inhibition of serum phospholipase-A2 in acute pancreatitis by pharmacological agents in vitro. Scand. J. Clin. Lab. Investig. 1997, 57, 401–407. [Google Scholar] [CrossRef]
- Buxbaum, J.L.; Freeman, M.; Amateau, S.K.; Chalhoub, J.M.; Coelho-Prabhu, N.; Desai, M.; Elhanafi, S.E.; Forbes, N.; Fujii-Lau, L.L.; Kohli, D.R.; et al. American Society for Gastrointestinal Endoscopy guideline on post-ERCP pancreatitis prevention strategies: Summary and recommendations. Gastrointest. Endosc. 2023, 97, 153–162. [Google Scholar] [CrossRef]
- Buxbaum, J.L.; Freeman, M.; Amateau, S.K.; Chalhoub, J.M.; Chowdhury, A.; Coelho-Prabhu, N.; Das, R.; Desai, M.; Elhanafi, S.E.; Forbes, N.; et al. American Society for Gastrointestinal Endoscopy guideline on post-ERCP pancreatitis prevention strategies: Methodology and review of evidence. Gastrointest. Endosc. 2023, 97, 163–183.e40. [Google Scholar] [CrossRef] [PubMed]
- Smith, Z.L.; Elmunzer, B.J.; Cooper, G.S.; Chak, A. Real-World Practice Patterns in the Era of Rectal Indomethacin for Prophylaxis Against Post-ERCP Pancreatitis in a High-Risk Cohort. Am. J. Gastroenterol. 2020, 115, 934. [Google Scholar] [CrossRef] [PubMed]
- Avila, P.; Holmes, I.; Kouanda, A.; Arain, M.; Dai, S.-C. Practice patterns of post-ERCP pancreatitis prophylaxis techniques in the United States: A survey of advanced endoscopists. Gastrointest. Endosc. 2020, 91, 568–573.e2. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.; Addison, A.; De Rosa, A.; Brooks, A.; Cameron, I.C. Retrospective study of patients with acute pancreatitis: Is serum amylase still required? BMJ Open 2012, 2, e001471. [Google Scholar] [CrossRef] [PubMed]
| Parameters | No Lipasemia (n = 478) | Lipasemia 1–2× ULN (n = 81) | Lipasemia 2–3× ULN (n = 26) | Lipasemia >3× ULN (n = 61) | p-Value |
|---|---|---|---|---|---|
| Mean age (SD) | 59.0 (19.1) | 60.5 (18.5) | 56.3 (16.0) | 58.9 (19.4) | 0.65 |
| Sex, % female (n) | 47.3 (226) | 45.7 (37) | 50.0 (13) | 60.7 (37) | 0.34 |
| Mean Charlson Comorbidity Index (SD) | 3.1 (2.7) | 3.0 (2.5) | 2.7 (2.9) | 3.2 (2.8) | 0.88 |
| Indication CBD stones, % (n) Cholangitis, % (n) Malignant biliary obstruction, % (n) Pancreatic indication(s), % (n) All others, % (n) | 65.3 (312) 10.5 (50) 8.8 (42) 0.6 (3) 14.9 (71) | 60.5 (49) 11.1 (9) 12.3 (10) 0.0 (0) 16.0 (13) | 69.2 (18) 3.8 (1) 19.2 (5) 3.8 (1) 3.8 (1) | 67.2 (41) 14.8 (9) 4.9 (3) 1.6 (1) 11.5 (7) | 0.76 |
| Trainee endoscopist involved, % (n) | 71.6 (342) | 66.7 (54) | 61.5 (16) | 67.2 (41) | 0.56 |
| Prior ERCP, % (n) | 20.3 (97) | 18.5 (15) | 3.9 (1) | 16.4 (10) | 0.11 |
| Pancreatogram performed, % (n) | 2.3 (11) | 3.8 (3) | 19.3 (5) | 6.5 (4) | <0.001 |
| PD cannulation, % (n) | 18.4 (86) | 28.8 (23) | 50.0 (13) | 39.0 (23) | <0.001 |
| Double wire technique utilized, % (n) | 11.4 (53) | 28.8 (23) | 45.8 (11) | 27.6 (16) | <0.001 |
| Sphincterotomy performed, % (n) | 80.1 (375) | 81.0 (64) | 70.8 (17) | 73.7 (42) | 0.61 |
| Balloon sphincteroplasty performed, % (n) | 20.9 (99) | 21.3 (17) | 15.4 (4) | 33.9 (20) | 0.18 |
| Pre-cut sphincterotomy performed, % (n) | 5.7 (27) | 7.4 (6) | 20.0 (5) | 13.6 (8) | <0.001 |
| Needle-knife papillotomy performed, % (n) | 6.1 (29) | 6.2 (5) | 12.0 (3) | 15.3 (9) | <0.001 |
| Cannulation attempts 1 or 2, % (n) 3 to 5, % (n) 6 to 10, % (n) >10, % (n) | 56.4 (246) 27.1 (118) 9.4 (41) 7.1 (31) | 47.1 (33) 31.4 (22) 8.6 (6) 12.9 (9) | 40.0 (8) 20.0 (4) 25.0 (5) 15.0 (3) | 29.6 (16) 37.0 (20) 25.9 (14) 7.4 (4) | <0.001 |
| CBD stent(s) placed, % (n) | 21.4 (101) | 35.8 (29) | 36 (9) | 35.6 (21) | 0.002 |
| PD stent(s) placed, % (n) | 6.9 (33) | 18.5 (15) | 26.9 (7) | 14.8 (9) | <0.001 |
| Mean cannulation time in minutes (SD) | 4.7 (6.7) | 6.3 (7.7) | 6.7 (7.0) | 6.6 (6.8) | <0.001 |
| Overall procedure time in minutes (SD) | 20.6 (13.9) | 22.7 (13.9) | 25.6 (13.8) | 27.1 (15.2) | <0.001 |
| Pre-procedural indomethacin given, % (n) | 41.8 (200) | 33.3 (27) | 34.6 (9) | 49.2 (30) | 0.38 |
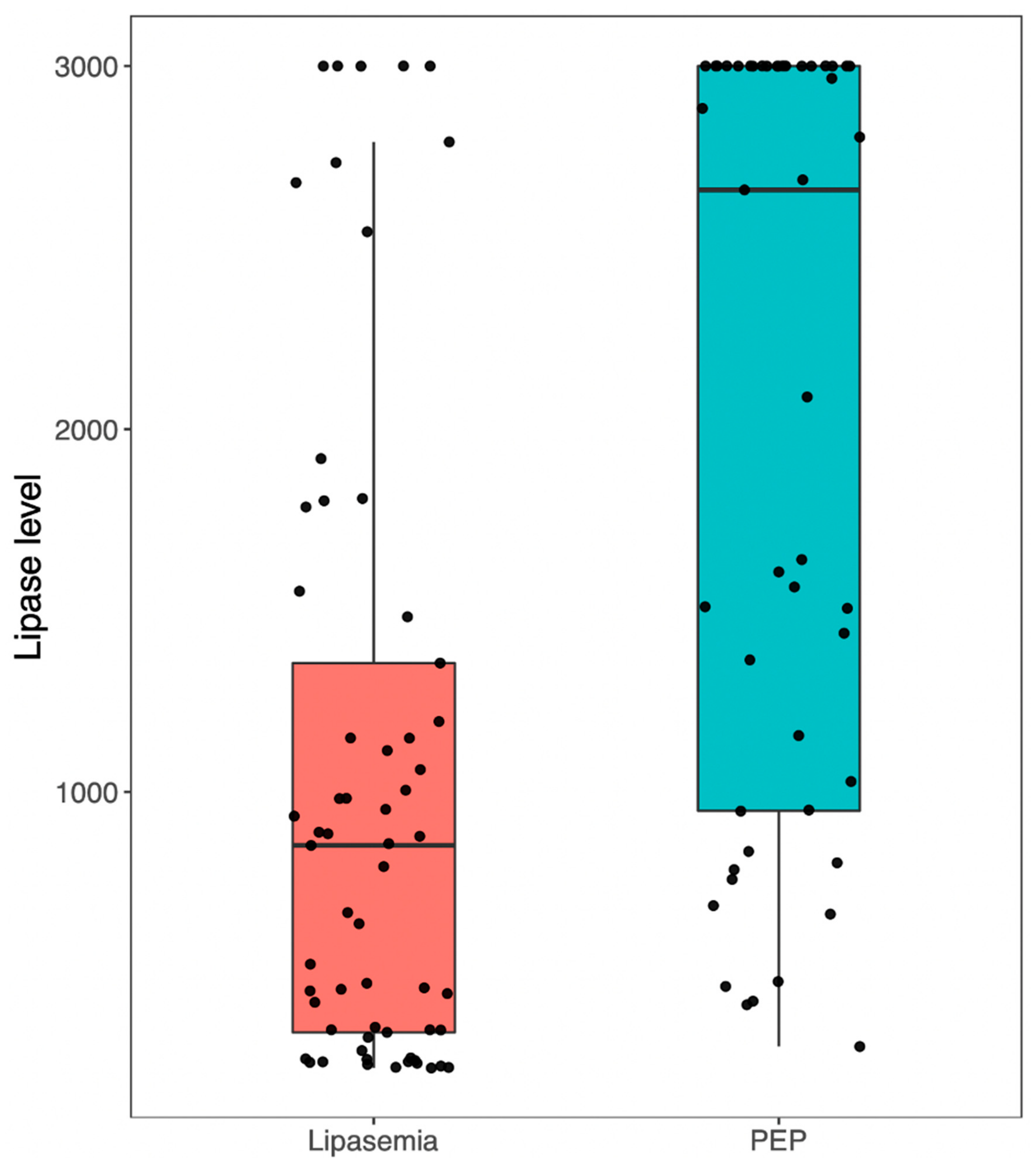
| Mean lipase level within 24 h of ERCP (SD) * | 36.4 (18.6) | 117.3 (23.5) | 196.4 (21) | 1037.8 (890.8) | <0.001 |
| Parameters | No Imaging Findings Consistent with AP (n = 148) | Imaging Findings Consistent with AP (n = 39) | p-Value |
|---|---|---|---|
| Mean age (SD) | 63.6 (16.4) | 60.4 (13.1) | 0.26 |
| Sex, % female (n) | 39.2 (58) | 25.6 (10) | 0.17 |
| Mean Charlson Comorbidity Index (SD) | 3.5 (2.8) | 3.1 (2.6) | 0.37 |
| Indication CBD stones, % (n) Cholangitis, % (n) Malignant biliary obstruction, % (n) Pancreatic indication(s), % (n) All others, % (n) | 31.8 (47) 13.5 (20) 28.4 (42) 0.0 (0) 26.4 (39) | 33.3 (13) 5.1 (2) 20.5 (8) 5.1 (2) 35.9 (14) | 0.08 |
| Trainee endoscopist involved, % (n) | 67.6 (100) | 66.7 (26) | >0.99 |
| Prior ERCP, % (n) | 29.9 (44) | 33.3 (13) | 0.83 |
| Pancreatogram performed, % (n) | 5.4 (8) | 15.8 (6) | 0.04 |
| PD cannulation, % (n) | 11.3 (15) | 16.1 (5) | 0.54 |
| Double wire technique utilized, % (n) | 66.2 (88) | 53.6 (15) | 0.30 |
| Sphincterotomy performed, % (n) | 19.4 (28) | 9.1 (3) | 0.21 |
| Balloon sphincteroplasty performed, % (n) | 9.0 (13) | 26.5 (9) | 0.01 |
| Pre-cut sphincterotomy performed, % (n) | 8.3 (12) | 8.8 (3) | >0.99 |
| Needle-knife papillotomy performed, % (n) | 11.3 (15) | 16.1 (5) | 0.54 |
| Cannulation attempts 1 or 2, % (n) 3 to 5, % (n) 6 to 10, % (n) >10, % (n) | 55.9 (62) 20.7 (23) 13.5 (15) 9.9 (11) | 26.9 (7) 26.9 (7) 7.7 (2) 38.5 (10) | 0.002 |
| CBD stent(s) placed, % (n) | 37.8 (54) | 50.0 (16) | 0.28 |
| Metal CBD stent placed, % (n) | |||
| PD stent(s) placed, % (n) | 6.8 (10) | 2.6 (1) | 0.46 |
| Mean cannulation time in minutes (SD) | 4.5 (5.6) | 8.7 (11.7) | 0.09 |
| Overall procedure time in minutes (SD) | 24.2 (14.7) | 34.0 (22.6) | 0.009 |
| Pre-procedural indomethacin given, % (n) | 30.4 (45) | 28.2 (11) | 0.94 |
| Mean post-ERCP day of cross-sectional imaging, % (n) | 4.7 (3.5) | 3.5 (3.2) | 0.05 |
| Findings on cross-sectional imaging, % (n) Pancreatic enlargement, % (n) Pancreatic edema, inflammation, or fat stranding % (n) Pancreatic necrosis, % (n) Peri-pancreatic fluid collection, % (n) | N/A | 10.3 (4) 94.5 (37) 5.1 (2) 56.4 (22) | N/A |
| Parameters | AOR of Lipasemia 1–2× ULN | AOR of Lipasemia 2–3× ULN | AOR of Lipasemia >3× ULN | AOR of 1 or More Imaging Findings |
|---|---|---|---|---|
| Increasing age (each additional year) | 1.01 (0.99, 1.03) | 1.02 (0.98, 1.06) | 0.98 (0.96, 1.00) | 0.97 (0.93, 1.01) |
| Female sex (versus male sex) | 1.14 (0.64, 2.04) | 0.96 (0.30, 3.01) | 0.68 (0.33, 1.38) | 1.99 (0.46, 8.59) |
| Charlson Comorbidity Index 4 or higher (versus 3 or lower) | 0.95 (0.47, 1.94) | 0.36 (0.08, 1.58) | 1.56 (0.68, 3.57) | 1.43 (0.32, 6.45) |
| Indication for biliary obstruction (versus all others) | 0.96 (0.46, 2.02) | 4.52 (0.50, 40.93) | 1.17 (0.43, 3.20) | 0.52 (0.11, 2.33) |
| Trainee endoscopist involved (versus none) | 0.64 (0.36, 1.14) | 1.44 (0.37, 5.58) | 0.80 (0.38, 1.66) | 4.39 (0.84, 23.09) |
| Pancreatogram performed (versus none) | 0.78 (0.18, 3.45) | 7.22 (1.13, 46.02) | 1.07 (0.18, 6.26) | 4.74 (0.39, 57.81) |
| PD cannulation (versus none) | 0.76 (0.26, 2.19) | 0.33 (0.03, 3.23) | 0.75 (0.24, 2.35) | 1.07 (0.19, 6.20) |
| Double wire technique utilized (versus none) | 2.69 (0.69, 10.40) | 15.74 (1.15, 214.74) | 3.56 (0.89, 14.26) | 2.08 (0.13, 34.51) |
| Sphincterotomy performed (versus none) | 1.14 (0.51, 2.52) | 0.23 (0.06, 0.91) | 1.01 (0.40, 2.59) | 2.64 (0.50, 13.97) |
| Balloon sphincteroplasty performed (versus none) | 0.89 (0.43, 1.84) | 0.33 (0.06, 1.70) | 2.29 (1.08, 4.85) | 0.49 (0.05, 4.92) |
| Pre-cut sphincterotomy performed (versus none) | 0.95 (0.30, 3.04) | 0.96 (0.12, 7.90) | 0.94 (0.26, 3.38) | 2.53 (0.26, 24.96) |
| Needle-knife papillotomy performed (versus none) | 0.88 (0.17, 4.43) | 7.51 (0.54, 104.54) | 1.40 (0.25, 7.97) | 0.99 (0.02, 64.44) |
| >5 cannulation attempts (versus 5 or fewer) | 0.69 (0.26, 1.85) | 2.47 (0.43, 14.20) | 1.25 (0.42, 3.73) | 0.67 (0.07, 6.03) |
| >10 cannulation attempts (versus 10 or fewer) | 1.49 (0.35, 6.28) | 0.98 (0.08, 12.01) | 0.11 (0.01, 0.88) | 33.95 (1.64, 704.13) |
| CBD stent(s) placed (versus none) | 1.29 (0.40, 4.16) | 0.99 (0.09, 10.64) | 4.19 (1.37, 12.77) | 8.10 (0.95, 68.95) |
| PD stent(s) placed (versus none) | 2.39 (0.74, 7.74) | 0.81 (0.13, 5.18) | 0.86 (0.23, 3.26) | 0.09 (0.00, 2.96) |
| Increasing cannulation time (per additional minute) | 1.01 (0.94, 1.09) | 0.86 (0.71, 1.03) | 0.98 (0.90, 1.06) | 1.03 (0.86, 1.25) |
| Increasing procedure timeleft (per additional minute) | 1.01 (0.98, 1.04) | 1.01 (0.96, 1.08) | 1.03 (1.00, 1.06) | 1.02 (0.95, 1.09) |
| No pre-procedural indomethacin given (versus all others) | 1.16 (0.61, 2.22) | 0.93 (0.28, 3.05) | 0.98 (0.45, 2.14) | 1.78 (0.30, 10.59) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chau, M.; Samnani, S.; Bazerbachi, F.; Mirakhur, A.; Ruan, Y.; Howarth, M.; Bass, S.; Cole, M.J.; Lei, Y.; Li, S.; et al. Incidence and Predictors of Incidental Biochemical and Radiologic Pancreatic Alterations Following Uncomplicated ERCP. J. Clin. Med. 2023, 12, 2230. https://doi.org/10.3390/jcm12062230
Chau M, Samnani S, Bazerbachi F, Mirakhur A, Ruan Y, Howarth M, Bass S, Cole MJ, Lei Y, Li S, et al. Incidence and Predictors of Incidental Biochemical and Radiologic Pancreatic Alterations Following Uncomplicated ERCP. Journal of Clinical Medicine. 2023; 12(6):2230. https://doi.org/10.3390/jcm12062230
Chicago/Turabian StyleChau, Millie, Sunil Samnani, Fateh Bazerbachi, Anirudh Mirakhur, Yibing Ruan, Megan Howarth, Sydney Bass, Martin J. Cole, Yang Lei, Suqing Li, and et al. 2023. "Incidence and Predictors of Incidental Biochemical and Radiologic Pancreatic Alterations Following Uncomplicated ERCP" Journal of Clinical Medicine 12, no. 6: 2230. https://doi.org/10.3390/jcm12062230
APA StyleChau, M., Samnani, S., Bazerbachi, F., Mirakhur, A., Ruan, Y., Howarth, M., Bass, S., Cole, M. J., Lei, Y., Li, S., Turbide, C., Mohamed, R., Brenner, D. R., Heitman, S. J., Elmunzer, B. J., & Forbes, N. (2023). Incidence and Predictors of Incidental Biochemical and Radiologic Pancreatic Alterations Following Uncomplicated ERCP. Journal of Clinical Medicine, 12(6), 2230. https://doi.org/10.3390/jcm12062230





