Predicting Gross Motor Function in Children and Adolescents with Cerebral Palsy Applying Artificial Intelligence Using Data on Assistive Devices
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Gross Motor Function Measure-66 (GMFM-66)
- 0 = does not initiate
- 1 = initiates
- 2 = partially completes
- 3 = completes completely
- NT = Not tested
2.3. Gross Motor Function Classification System (GMFCS)
- The GMFCS levels for children with CP (6 to 12 years) are [11]:
- Level I: Walking without limitations; limitations of higher motor skills.
- Level II: Independent walking without walking aids in the community.
- Level III: Walking with aids, limitations in walking in the community.
- Level IV: Independent locomotion limited; children are pushed in a wheelchair or use powered mobility
- Level V: Independent locomotion is severely limited even with powered mobility.
2.4. Data Preparation
2.5. Generation and Evaluation of the Four Machine Learning Algorithms
- Feed forward neural net (FNN)
- Random forest (RF)
- Support vector machine (SVM)
- Extreme Gradient Boosting (XGBoost)
2.6. Evaluation of the Accuracy of MDSC in Group Analysis
3. Results
3.1. Descriptive Analysis
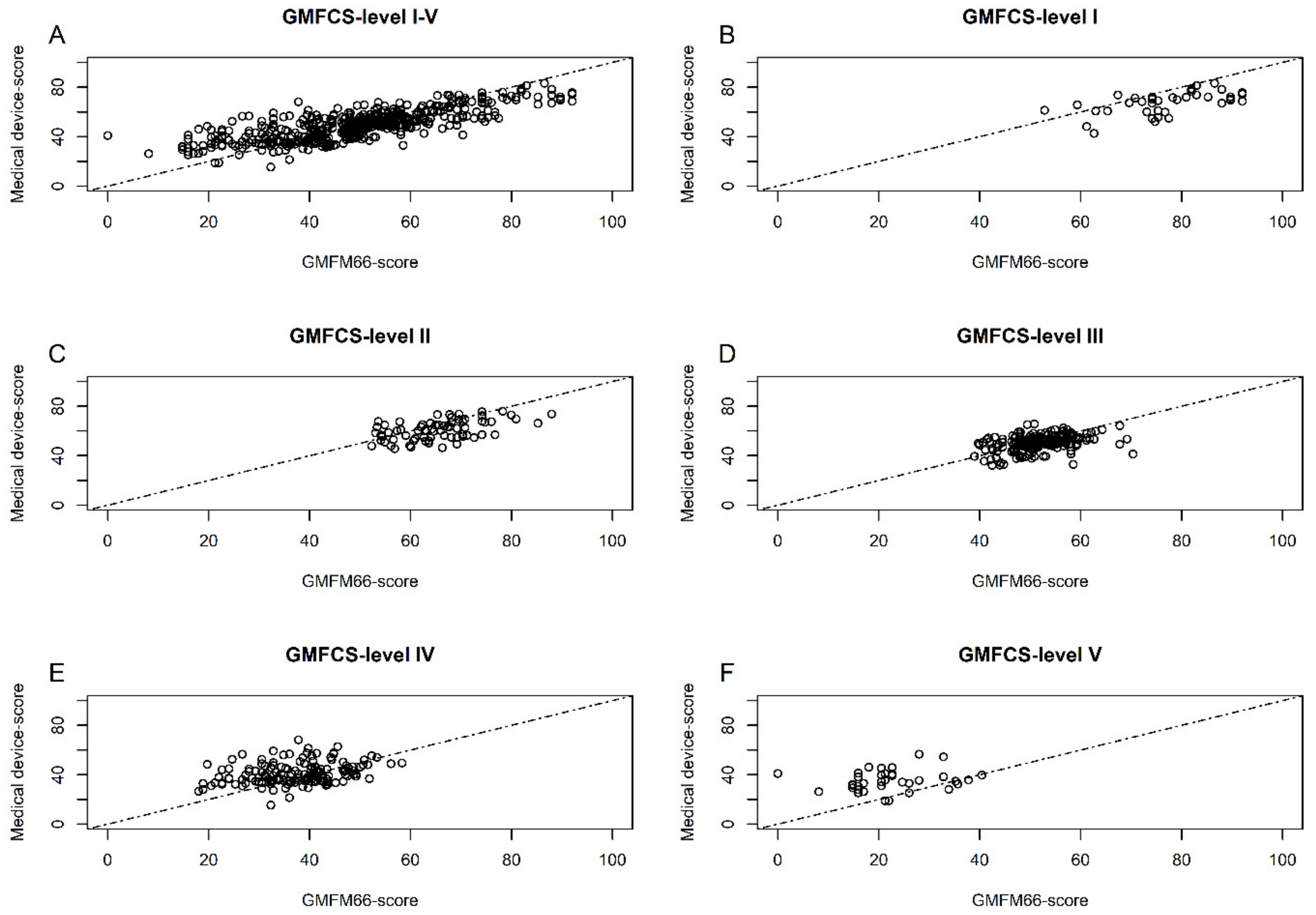
3.2. Evaluation of the Medical Device Score Calculator (MDSC)
3.3. Results of the Accuracy of MDSC in Group Analysis
4. Discussion
4.1. Related Works
4.2. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
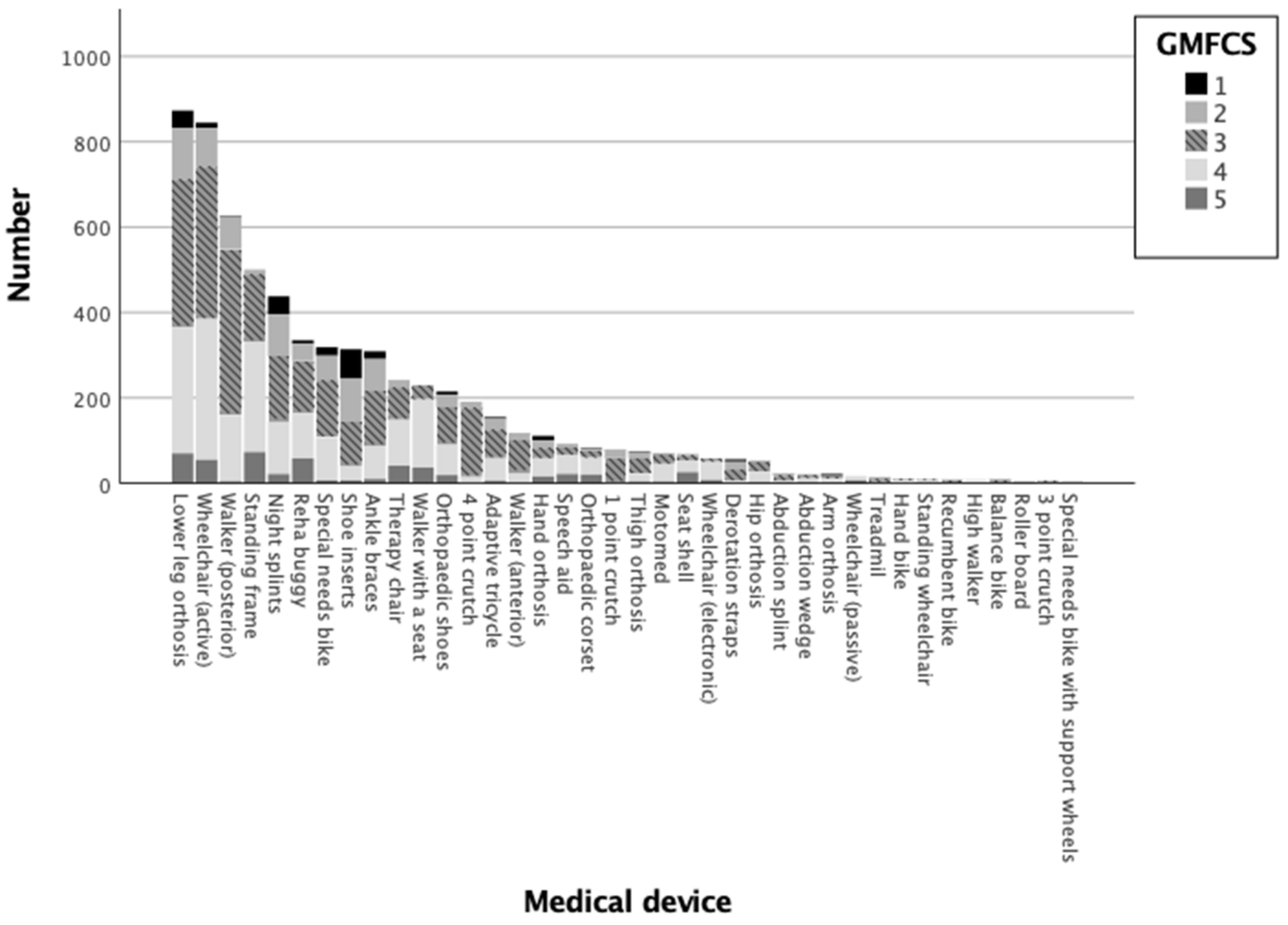
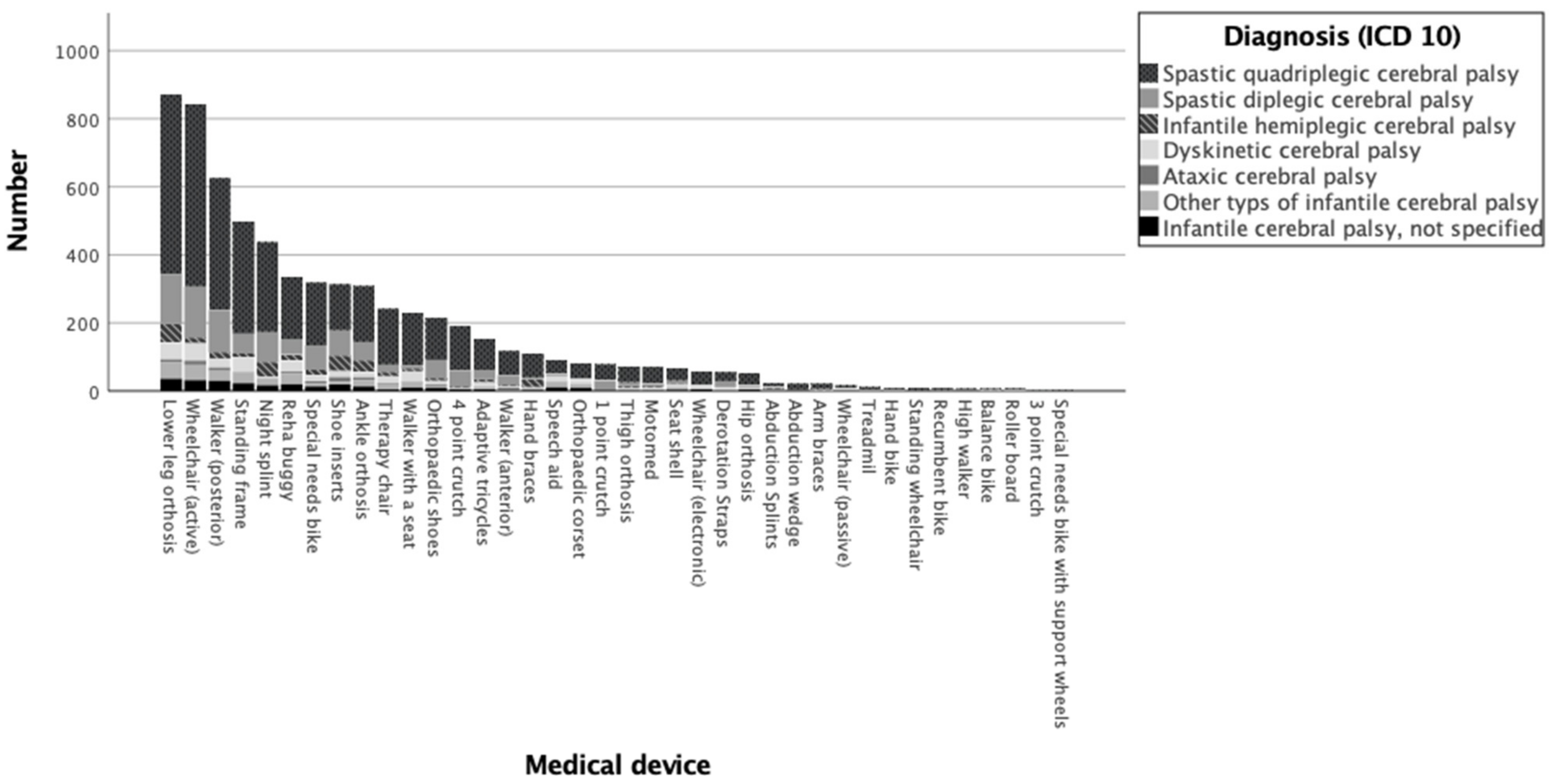
| Valid n | Column Valid n% | Mean GMFM-66 | 95.0% Lower CL for Mean | 95.0% Upper CL for Mean | Standard Deviation | ||
|---|---|---|---|---|---|---|---|
| Medical devices | 1 point crutch | 79 | 1.2% | 56.38 | 55.02 | 57.74 | 6.07 |
| 3 point crutch | 6 | 0.1% | 51.42 | 43.14 | 59.70 | 7.89 | |
| 4 point crutch | 191 | 2.9% | 52.35 | 51.62 | 53.08 | 5.15 | |
| Abduction splint | 23 | 0.3% | 47.38 | 40.31 | 54.45 | 16.35 | |
| Abduction wedge | 22 | 0.3% | 37.22 | 29.96 | 44.49 | 16.38 | |
| Adaptive tricycle | 155 | 2.3% | 49.19 | 47.25 | 51.13 | 12.23 | |
| Ankle orthosis | 309 | 4.7% | 50.79 | 49.02 | 52.56 | 15.81 | |
| Arm braces | 22 | 0.3% | 50.94 | 41.00 | 60.87 | 22.40 | |
| Balance bike | 8 | 0.1% | 51.95 | 40.01 | 63.89 | 14.29 | |
| Derotation straps | 56 | 0.8% | 57.15 | 53.53 | 60.77 | 13.51 | |
| Hand bike | 10 | 0.2% | 46.89 | 39.79 | 53.99 | 9.93 | |
| Hand orthosis | 111 | 1.7% | 43.62 | 40.12 | 47.12 | 18.61 | |
| High walker | 8 | 0.1% | 36.25 | 24.79 | 47.71 | 13.71 | |
| Hip orthosis | 54 | 0.8% | 40.01 | 36.38 | 43.65 | 13.32 | |
| Lower leg orthosis | 873 | 13.2% | 46.67 | 45.66 | 47.69 | 15.31 | |
| Motomed | 71 | 1.1% | 41.98 | 38.96 | 44.99 | 12.75 | |
| Night splints | 438 | 6.6% | 51.65 | 50.10 | 53.21 | 16.55 | |
| Orthopaedic shoes | 215 | 3.2% | 47.73 | 45.87 | 49.59 | 13.81 | |
| Orthopaedic corset | 82 | 1.2% | 34.58 | 31.24 | 37.92 | 15.19 | |
| Recumbent bike | 9 | 0.1% | 51.63 | 41.45 | 61.82 | 13.25 | |
| Reha buggy | 335 | 5.1% | 40.93 | 39.20 | 42.65 | 16.04 | |
| Roller board | 8 | 0.1% | 30.29 | 19.01 | 41.57 | 13.49 | |
| Seat shell | 69 | 1.0% | 32.59 | 28.97 | 36.21 | 15.07 | |
| Shoe inserts | 314 | 4.7% | 61.06 | 59.33 | 62.79 | 15.61 | |
| Special needs bike | 319 | 4.8% | 51.25 | 49.81 | 52.68 | 12.99 | |
| Special needs bike with support wheels | 5 | 0.1% | 71.19 | 56.30 | 86.09 | 12.00 | |
| Speech aid | 93 | 1.4% | 35.59 | 32.76 | 38.43 | 13.79 | |
| Standing frames | 500 | 7.6% | 38.13 | 37.03 | 39.23 | 12.53 | |
| Standing wheelchair | 10 | 0.2% | 39.16 | 35.84 | 42.48 | 4.65 | |
| Therapy chair | 242 | 3.7% | 38.52 | 36.84 | 40.21 | 13.30 | |
| Thigh orthosis | 73 | 1.1% | 48.19 | 44.91 | 51.48 | 14.09 | |
| Treadmill | 12 | 0.2% | 55.35 | 45.13 | 65.57 | 16.08 | |
| Walker (anterior) | 118 | 1.8% | 51.56 | 49.96 | 53.17 | 8.80 | |
| Walker (posterior) | 626 | 9.5% | 49.73 | 49.02 | 50.43 | 9.01 | |
| Walker with a seat | 230 | 3.5% | 34.37 | 33.14 | 35.60 | 9.45 | |
| Wheelchair (active) | 845 | 12.8% | 46.50 | 45.60 | 47.40 | 13.31 | |
| Wheelchair (electronic) | 58 | 0.9% | 34.66 | 31.86 | 37.46 | 10.64 | |
| Wheelchair (passive) | 17 | 0.3% | 26.20 | 20.20 | 32.20 | 11.67 | |
| Total | 6616 | 100.0% | 46.50 | 46.13 | 46.86 | 15.05 | |
References
- Bax, M.; Goldstein, M.; Rosenbaum, P.; Leviton, A.; Paneth, N.; Dan, B.; Jacobsson, B.; Damiano, D. Proposed definition and classification of cerebral palsy, April 2005. Dev. Med. Child Neurol. 2005, 47, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Shevell, M.I.; Dagenais, L.; Hall, N. The relationship of cerebral palsy subtype and functional motor impairment: A population-based study. Dev. Med. Child Neurol. 2009, 51, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Shevell, M. Cerebral palsy to cerebral palsy spectrum disorder: Time for a name change? Neurology 2018. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Verma, I. Cerebral palsy in children: An overview. J. Clin. Orthop. Trauma 2012, 3, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Cantero, M.J.P.; Medinilla, E.E.M.; Martínez, A.C.; Gutiérrez, S.G. Comprehensive approach to children with cerebral palsy. An. Pediatr. (Engl. Ed.) 2021, 95, 276.e1–276.e11. [Google Scholar] [CrossRef] [PubMed]
- Scholtes, F.; Kolh, P. Big data to analyze patterns of care and improve outcomes for children with cerebral palsy. Dev. Med. Child Neurol. 2021, 63, 1246. [Google Scholar] [CrossRef]
- Patel, D.R. Therapeutic interventions in cerebral palsy. Indian J. Pediatr. 2005, 72, 979–983. [Google Scholar] [CrossRef]
- Novak, I.; Morgan, C.; Fahey, M.; Finch-Edmondson, M.; Galea, C.; Hines, A.; Langdon, K.; Namara, M.M.; Paton, M.C.; Popat, H.; et al. State of the Evidence Traffic Lights 2019: Systematic Review of Interventions for Preventing and Treating Children with Cerebral Palsy. Curr. Neurol. Neurosci. Rep. 2020, 20, 3. [Google Scholar] [CrossRef]
- Novak, I.; McIntyre, S.; Morgan, C.; Campbell, L.; Dark, L.; Morton, N.; Stumbles, E.; Wilson, S.A.; Goldsmith, S. A systematic review of interventions for children with cerebral palsy: State of the evidence. Dev. Med. Child Neurol. 2013, 55, 885–910. [Google Scholar] [CrossRef]
- Russell, D.J.; Wright, M.; Rosenbaum, P.L.; Avery, L.M. Gross Motor Function Measure (GMFM-66 and GMFM-88) User’s Manual; Mac Keith Press: London, UK, 2021. [Google Scholar]
- Florian Heinen, J.K.; Mall, V.; Berweck, S.; Lindner, M.; Michaelis, U.; Stein, S. GMFM und GMFCS-Messung und Klassifikation Motorischer Funktionen Übersicht, Handbuch; Hans Huber Verlag: Bern, Switzerland, 2006. [Google Scholar]
- Duran, I.; Stark, C.; Saglam, A.; Semmelweis, A.; Lioba Wunram, H.; Spiess, K.; Schoenau, E. Artificial intelligence to improve efficiency of administration of gross motor function assessment in children with cerebral palsy. Dev. Med. Child Neurol. 2022, 64, 228–234. [Google Scholar] [CrossRef]
- Russell, D.J.; Rosenbaum, P.L.; Avery, L.M.; Lane, M. Gross Motor Function Measure (GMFM-66 & GMFM-88) User’s Manual; Mac Keith: London, UK, 2002. [Google Scholar]
- Adair, B.; Said, C.M.; Rodda, J.; Morris, M.E. Psychometric properties of functional mobility tools in hereditary spastic paraplegia and other childhood neurological conditions. Dev. Med. Child Neurol. 2012, 54, 596–605. [Google Scholar] [CrossRef] [PubMed]
- Towns, M.; Rosenbaum, P.; Palisano, R.; Wright, F.V. Should the Gross Motor Function Classification System be used for children who do not have cerebral palsy? Dev. Med. Child Neurol. 2018, 60, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Palisano, R.J.; Rosenbaum, P.; Bartlett, D.; Livingston, M.H. Content validity of the expanded and revised Gross Motor Function Classification System. Dev. Med. Child Neurol. 2008, 50, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Palisano, R.; Rosenbaum, P.; Walter, S.; Russell, D.; Wood, E.; Galuppi, B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev. Med. Child Neurol. 1997, 39, 214–223. [Google Scholar] [CrossRef]
- Mooney, S.J.; Pejaver, V. Big Data in Public Health: Terminology, Machine Learning, and Privacy. Annu. Rev. Public Health 2018, 39, 95–112. [Google Scholar] [CrossRef]
- Mayer-Schönberger, V.; Ingelsson, E. Big Data and medicine: A big deal? J. Intern. Med. 2018, 283, 418–429. [Google Scholar] [CrossRef]
- Deo, R.C. Machine Learning in Medicine. Circulation 2015, 132, 1920–1930. [Google Scholar] [CrossRef]
- Liaw, A.; Wiener, M. Classification and Regression by randomForest. R News 2002, 2, 18–22. [Google Scholar]
- Fritsch, S.; Guenther, F.; Wright, M.N. neuralnet: Training of Neural Networks: R Package version 1.44.2. Available online: https://github.com/bips-hb/neuralnet (accessed on 3 January 2023).
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, Fourth Edition ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Bischl, B.; Lang, M.; Kotthoff, L.; Schiffner, J.; Richter, J.; Studerus, E.; Casalicchio, G.; Jones, Z.M. mlr: Machine Learning in R. J. Mach. Learn. Res. 2016, 17, 5938–5942. [Google Scholar]
- Benesty, M.; Khotilovich, V.; Tang, Y.; Cho, H.; Chen, K.; Mitchell, R.; Cano, I.; Zhou, T.; Li, M.; Xie, J.; et al. Extreme Gradient Boosting: R package version 1.6.0.1. Available online: https://github.com/dmlc/xgboost (accessed on 3 January 2023).
- Lin, L.I. A concordance correlation coefficient to evaluate reproducibility. Biometrics 1989, 45, 255–268. [Google Scholar] [CrossRef]
- Koch, R.; Spörl, E. Statistical methods for comparison of two measuring procedures and for calibration: Analysis of concordance, correlation and regression in the case of measuring intraocular pressure. Klin Monbl Augenheilkd 2007, 224, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Nicolini-Panisson, R.D.; Tedesco, A.P.; Folle, M.R.; Donadio, M.V.F. Selective dorsal rhizotomy in cerebral palsy: Selection criteria and postoperative physical therapy protocols. Rev. Paul Pediatr. 2018, 36, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, Y. Application of supervised machine learning algorithms in the classification of sagittal gait patterns of cerebral palsy children with spastic diplegia. Comput. Biol. Med. 2019, 106, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Reid, S.M.; Carlin, J.B.; Reddihough, D.S. Using the Gross Motor Function Classification System to describe patterns of motor severity in cerebral palsy. Dev. Med. Child Neurol. 2011, 53, 1007–1012. [Google Scholar] [CrossRef] [PubMed]

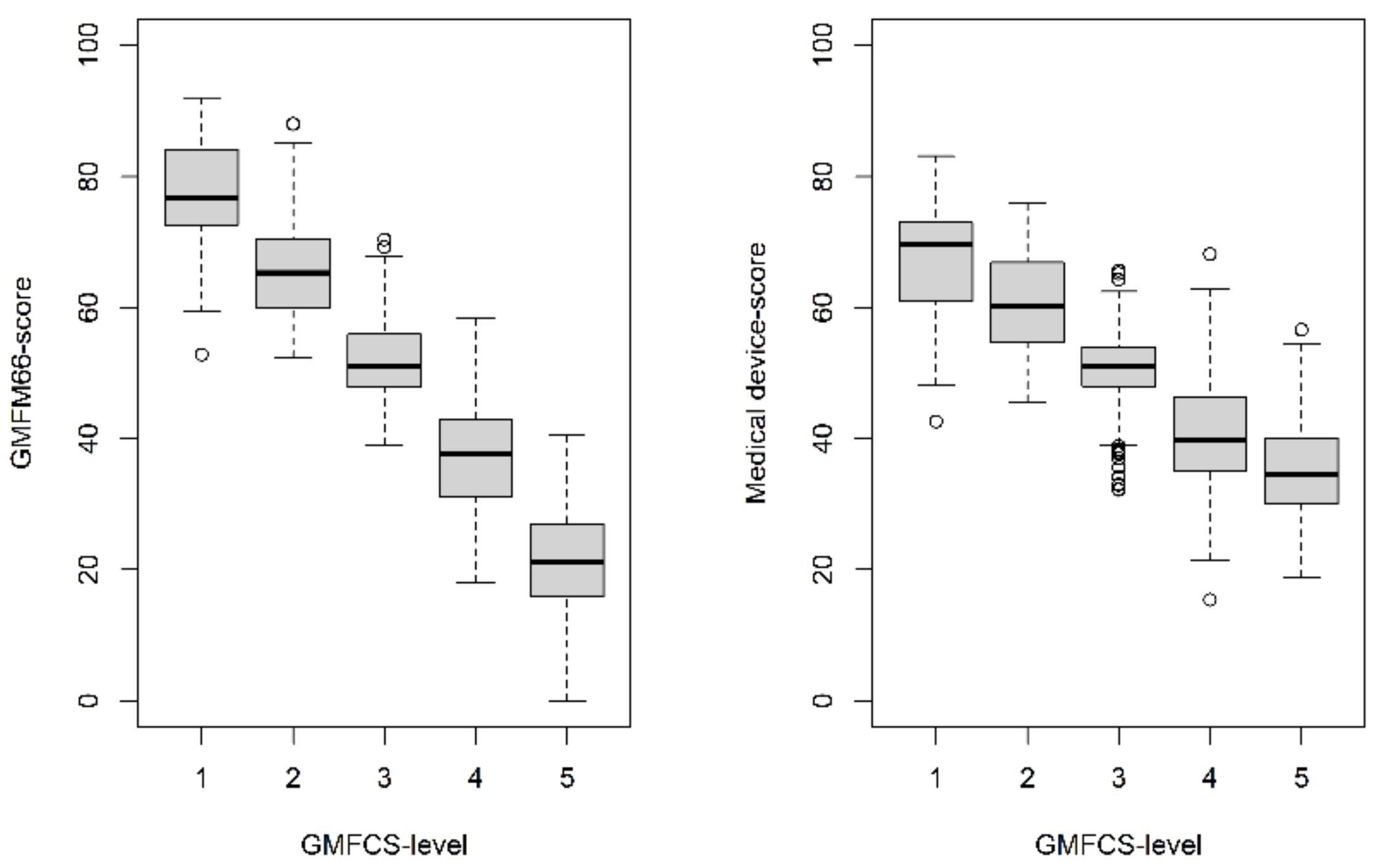
| GMFCS-level | ||||||
|---|---|---|---|---|---|---|
| I–V | I | II | III | IV | V | |
| n | 1581 | 118 | 289 | 592 | 470 | 112 |
| Female, n | 656 | 48 | 129 | 240 | 199 | 40 |
| Age, years M (SD) | 8.1 (4.3) | 9.0 (4.3) | 8.2 (4.2) | 7.8 (4.2) | 8.4 (4.5) | 7.2 (3.6) |
| Height, cm M (SD) | 119.1 (21.7) | 130.2 (24.3) | 122.3 (21.8) | 117.5 (21.0) | 118.4 (21.3) | 110.6 (17.5) |
| BMI, kg/m2 M (SD) | 16.0 (3.4) | 16.7 (3.0) | 16.3 (3.3) | 16.3 (3.4) | 15.7 (3.4) | 14.3 (2.7) |
| CP subtype: | ||||||
| Bilateral spastic, % | 75.6 | 50.0 | 75.8 | 82.1 | 77.4 | 60.7 |
| Unilateral spastic, % | 7.0 | 39.8 | 12.1 | 3.5 | 1.5 | 0.0 |
| Dyskinetic, % | 5.8 | 2.5 | 1.7 | 3.7 | 7.7 | 22.3 |
| Ataxic, % | 2.0 | 2.5 | 4.2 | 1.7 | 1.1 | 0.9 |
| Mixed type, % | 9.7 | 5.1 | 6.2 | 9.0 | 12.3 | 16.1 |
| Algorithms | CCC | MAE | RMSE |
|---|---|---|---|
| RF | 0.75 (0.71; 0.78) | 7.74 (7.15; 8.33) | 10.1 (9.51; 10.8) |
| SVM | 0.72 (0.68; 0.76) | 8.27 (7.63; 8.89) | 10.8 (10.1; 11.5) |
| FNN | 0.75 (0.71; 0.79) | 7.86 (7.26; 8.46) | 10.3 (9.67; 11.0) |
| XGBoost | 0.75 (0.71; 0.78) | 8.10 (7.50; 8.70) | 10.5 (9.85; 11.2) |
| Power of GMFM66-Score | ||||||||
|---|---|---|---|---|---|---|---|---|
| GMFM66-Score Difference of the Compared Groups | ||||||||
| Size of the Samples, n | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 30 | 0.0 | 0.5 | 2.0 | 3.9 | 20.2 | 68.1 | 94.8 | 99.1 |
| 40 | 0.1 | 1.8 | 5.5 | 20.5 | 71.3 | 97.9 | 98.8 | 100.0 |
| 50 | 0.6 | 4.4 | 12.8 | 58.8 | 96.7 | 99.9 | 100.0 | 100.0 |
| 75 | 3.6 | 16.1 | 77.1 | 99.4 | 99.9 | 100.0 | 100.0 | 100.0 |
| 100 | 10.9 | 60.9 | 99.7 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| 150 | 53.3 | 99.6 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| 200 | 89.8 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| 250 | 99.8 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Power of Medical Device-Score | ||||||||
| GMFM66-Score difference of the compared groups | ||||||||
| Size of the samples, n | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 30 | 0.7 | 2.3 | 6.7 | 10.6 | 21.0 | 40.8 | 55.0 | 80.6 |
| 40 | 2.3 | 4.7 | 13.8 | 22.1 | 38.3 | 63.6 | 82.0 | 94.8 |
| 50 | 3.0 | 9.2 | 24.3 | 34.9 | 57.5 | 80.4 | 93.9 | 99.1 |
| 75 | 10.3 | 26.0 | 53.6 | 66.7 | 88.2 | 98.1 | 99.6 | 100.0 |
| 100 | 20.3 | 47.0 | 79.5 | 88.4 | 98.1 | 100.0 | 100.0 | 100.0 |
| 150 | 50.5 | 82.1 | 97.9 | 99.5 | 100.0 | 100.0 | 100.0 | 100.0 |
| 200 | 75.0 | 96.3 | 99.9 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| 250 | 91.0 | 99.5 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
von Elling-Tammen, L.; Stark, C.; Wloka, K.R.; Alberg, E.; Schoenau, E.; Duran, I. Predicting Gross Motor Function in Children and Adolescents with Cerebral Palsy Applying Artificial Intelligence Using Data on Assistive Devices. J. Clin. Med. 2023, 12, 2228. https://doi.org/10.3390/jcm12062228
von Elling-Tammen L, Stark C, Wloka KR, Alberg E, Schoenau E, Duran I. Predicting Gross Motor Function in Children and Adolescents with Cerebral Palsy Applying Artificial Intelligence Using Data on Assistive Devices. Journal of Clinical Medicine. 2023; 12(6):2228. https://doi.org/10.3390/jcm12062228
Chicago/Turabian Stylevon Elling-Tammen, Lisa, Christina Stark, Kim Ramona Wloka, Evelyn Alberg, Eckhard Schoenau, and Ibrahim Duran. 2023. "Predicting Gross Motor Function in Children and Adolescents with Cerebral Palsy Applying Artificial Intelligence Using Data on Assistive Devices" Journal of Clinical Medicine 12, no. 6: 2228. https://doi.org/10.3390/jcm12062228
APA Stylevon Elling-Tammen, L., Stark, C., Wloka, K. R., Alberg, E., Schoenau, E., & Duran, I. (2023). Predicting Gross Motor Function in Children and Adolescents with Cerebral Palsy Applying Artificial Intelligence Using Data on Assistive Devices. Journal of Clinical Medicine, 12(6), 2228. https://doi.org/10.3390/jcm12062228







