Rats, Neuregulins and Radical Prostatectomy: A Conceptual Overview
Abstract
1. Introduction
2. Pathogenesis of Radical-Prostatectomy-Associated Morbidity
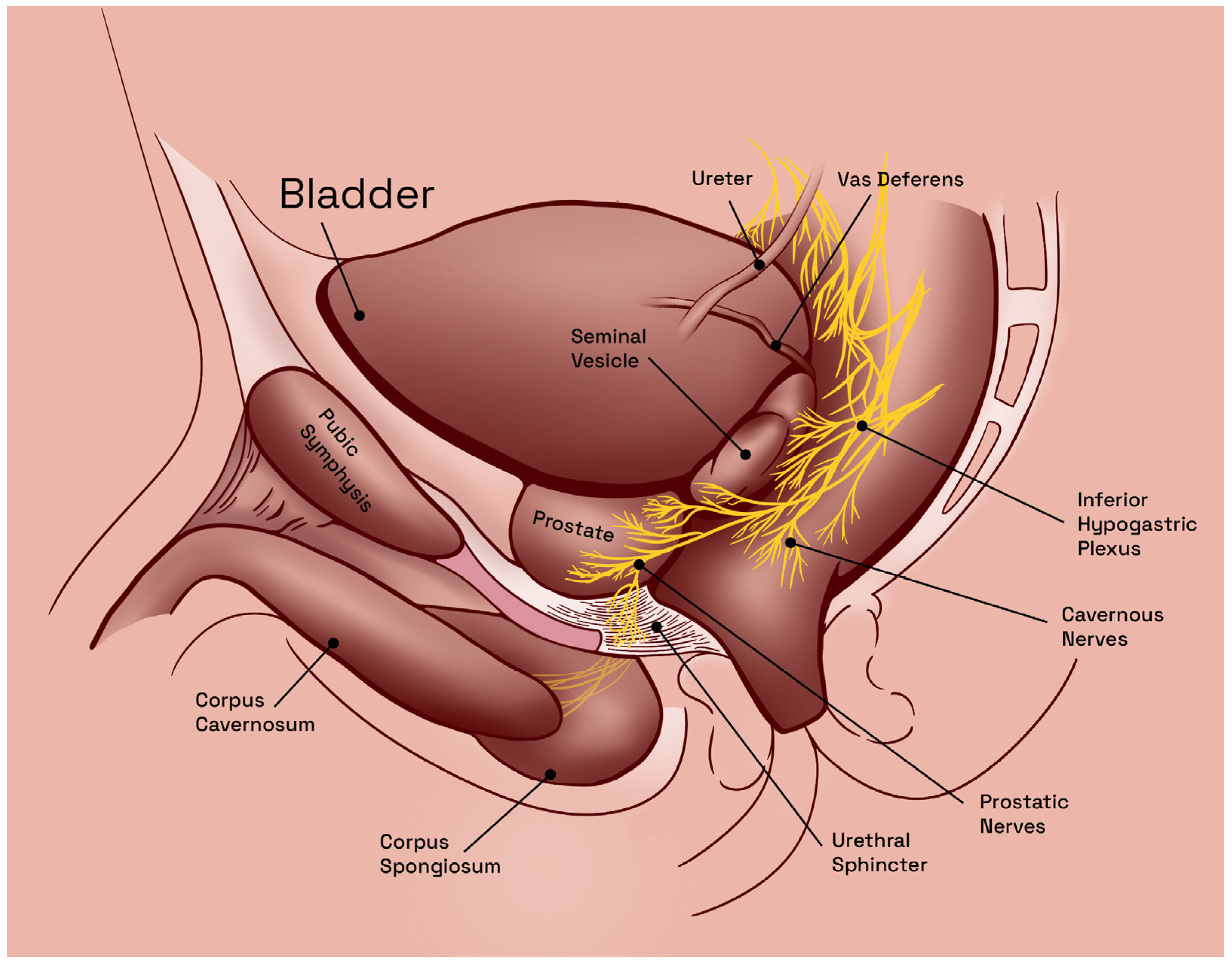
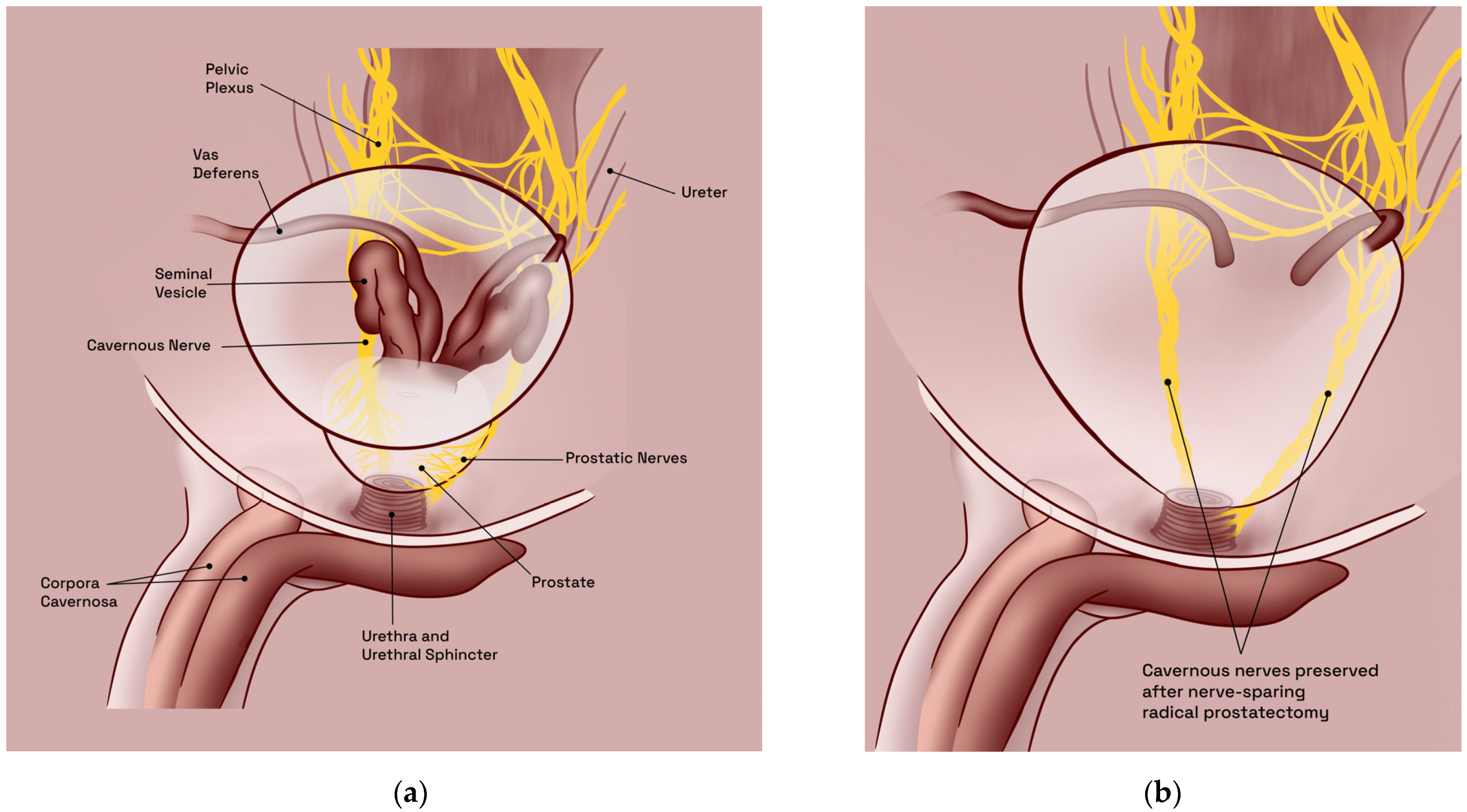
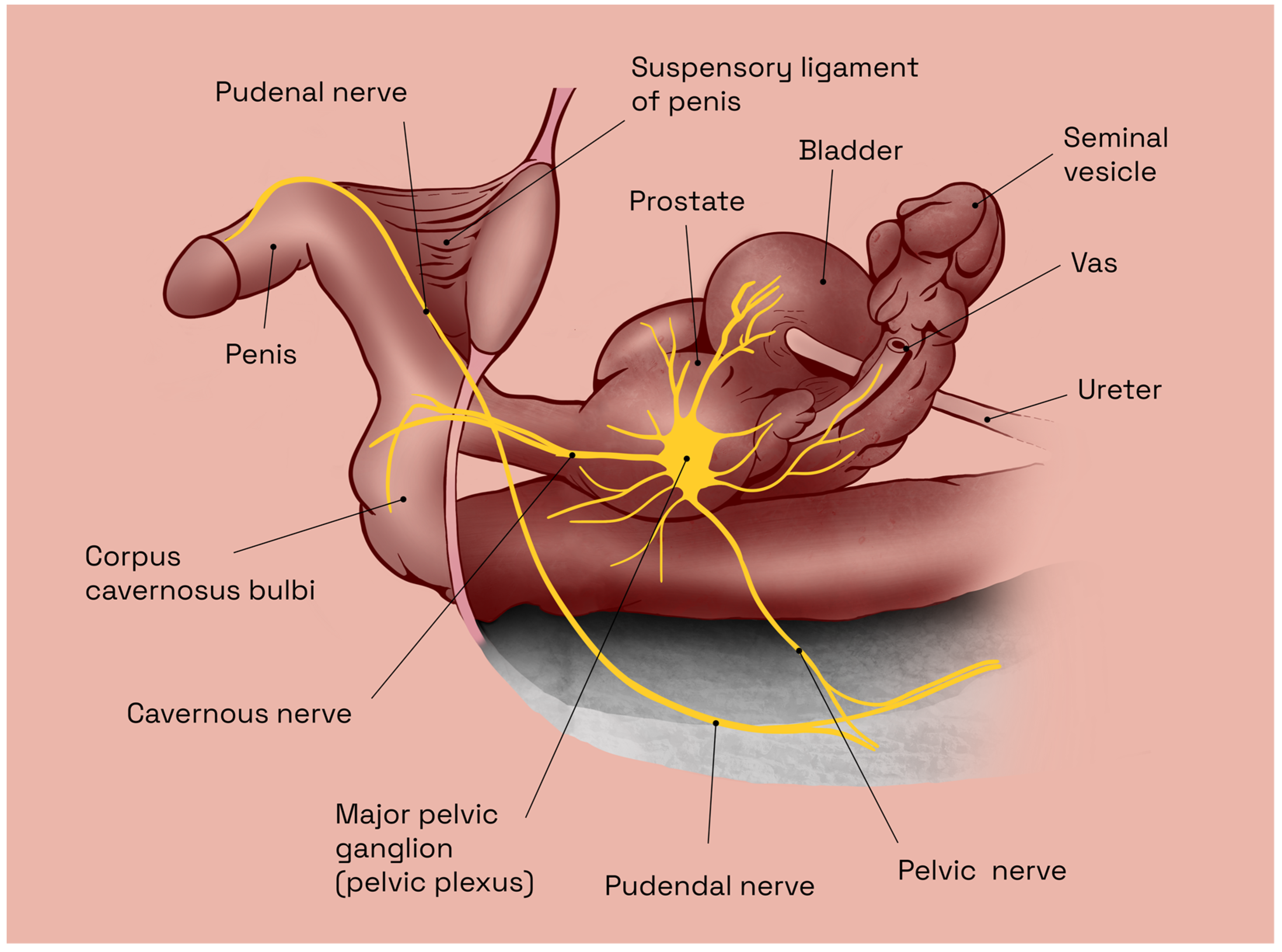
2.1. Predisposing Anatomic Considerations
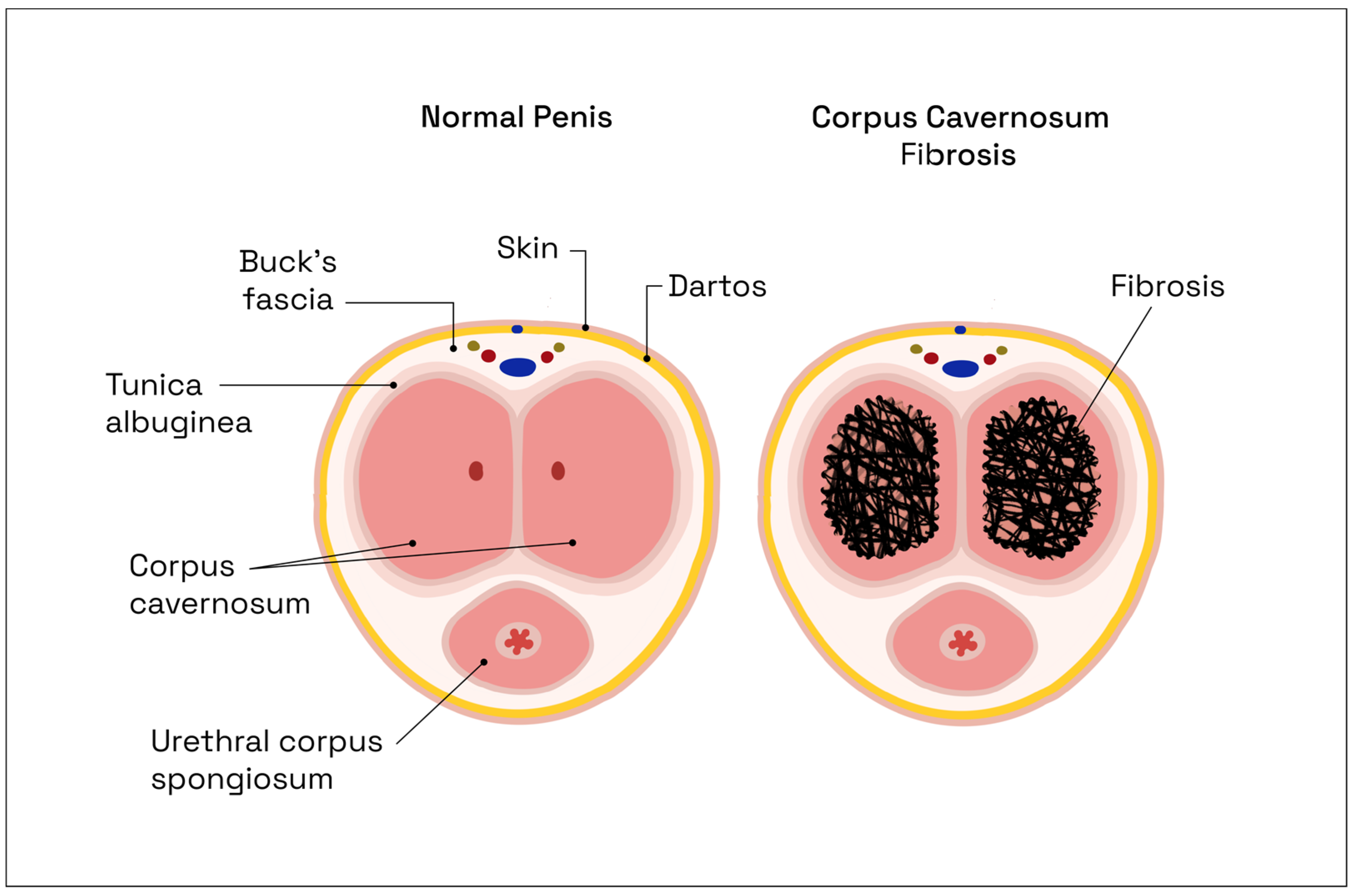
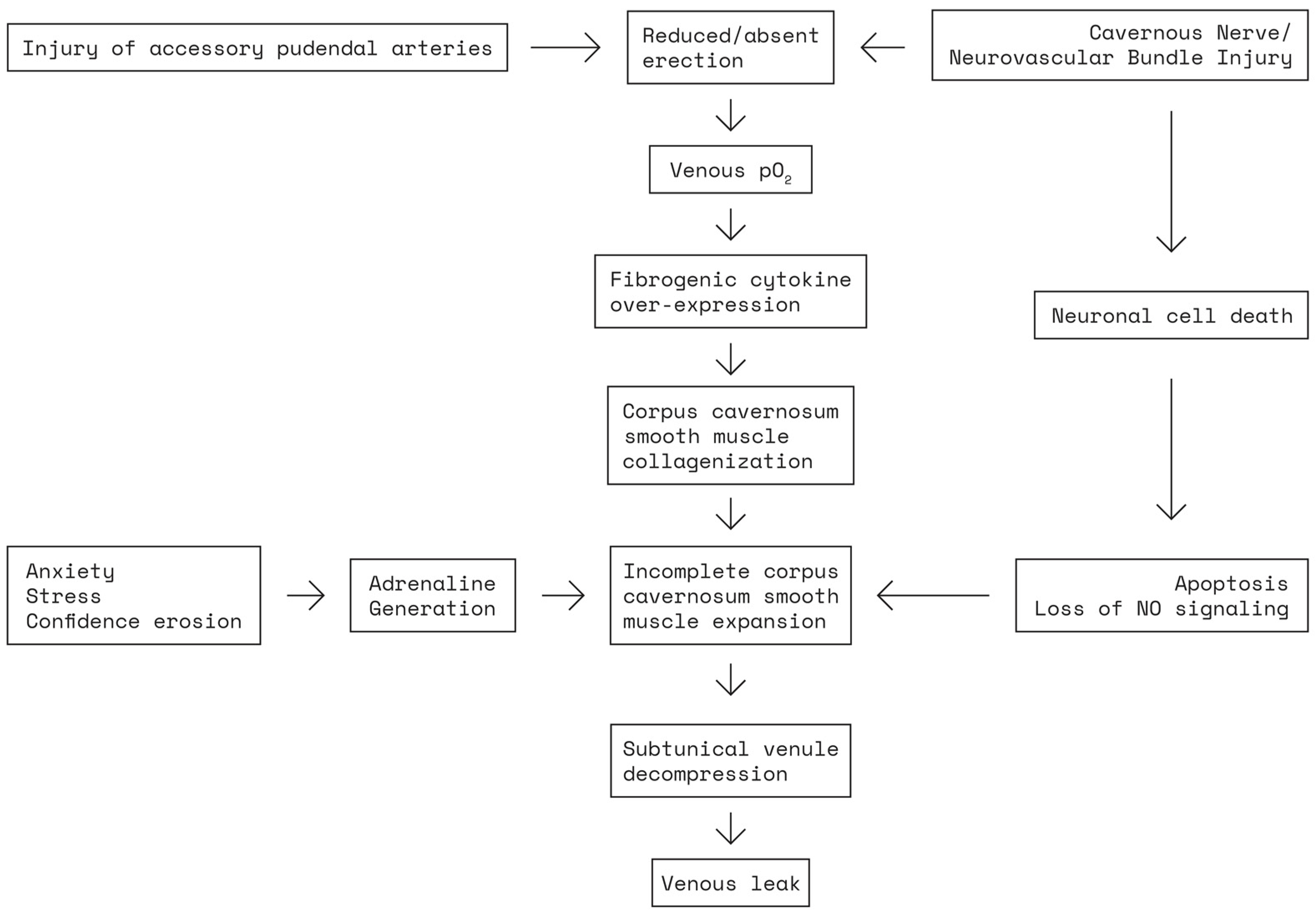
2.2. Pathophysiology of Erectile Dysfunction following Nerve-Sparing Radical Prostatectomy
3. Current Treatment Options for Post-Radical-Prostatectomy Erectile Dysfunction
4. The Cavernous Nerve Lesion Rat Model
4.1. Advantages and Limitations of the Cavernous Nerve Lesion Rat Model
4.2. Anatomy and Standard Preparation Protocols for the Cavernous Nerve Rat Model
4.3. Previous Applications of the Cavernous Nerve Lesion Rat Model in Post Radical Prostatectomy Erectile Dysfunction Experimental Research
5. Neuregulins, GGF2, and Future Therapeutic Perspectives for Post-Radical Prostatectomy Erectile Dysfunction
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef]
- Novacescu, D.; Feciche, B.; Cumpanas, A.; Bardan, R.; Rusmir, A.; Bitar, Y.; Barbos, V.; Cut, T.G.; Raica, M.; Latcu, S. Contemporary Clinical Definitions, Differential Diagnosis, and Novel Predictive Tools for Renal Cell Carcinoma. Biomedicines 2022, 10, 2926. [Google Scholar] [CrossRef] [PubMed]
- Novacescu, D.; Cut, T.G.; Cumpanas, A.; Bratosin, F.; Ceauşu, R.; Raica, M. Novel Expression of Thymine Dimers in Renal Cell Carcinoma, Demonstrated through Immunohistochemistry. Biomedicines 2022, 10, 2673. [Google Scholar] [CrossRef] [PubMed]
- Novacescu, D.; Cut, T.G.; Cumpanas, A.; Latcu, S.; Bardan, R.; Ferician, O.; Secasan, C.-C.; Rusmir, A.; Raica, M. Evaluating Established Roles, Future Perspectives and Methodological Heterogeneity for Wilms’ Tumor 1 (WT1) Antigen Detection in Adult Renal Cell Carcinoma, Using a Novel N-Terminus Targeted Antibody (Clone WT49). Biomedicines 2022, 10, 912. [Google Scholar] [CrossRef]
- Saltman, A.; Zegar, J.; Haj-Hamed, M.; Verma, S.; Sidana, A. Prostate Cancer Biomarkers and Multiparametric MRI: Is There a Role for Both in Prostate Cancer Management? Ther. Adv. Urol. 2021, 13, 1756287221997186. [Google Scholar] [CrossRef]
- Sekhoacha, M.; Riet, K.; Motloung, P.; Gumenku, L.; Adegoke, A.; Mashele, S. Prostate Cancer Review: Genetics, Diagnosis, Treatment Options, and Alternative Approaches. Molecules 2022, 27, 5730. [Google Scholar] [CrossRef] [PubMed]
- Emanu, J.C.; Avildsen, I.K.; Nelson, C.J. Erectile Dysfunction after Radical Prostatectomy: Prevalence, Medical Treatments, and Psychosocial Interventions. Curr. Opin. Support. Palliat. Care 2016, 10, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Knipper, S.; Ott, S.; Schlemmer, H.-P.; Grimm, M.-O.; Graefen, M.; Wiegel, T. Options for Curative Treatment of Localized Prostate Cancer. Dtsch. Arztebl. Int. 2021, 118, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Capogrosso, P.; Vertosick, E.A.; Benfante, N.E.; Eastham, J.A.; Scardino, P.J.; Vickers, A.J.; Mulhall, J.P. Are We Improving Erectile Function Recovery After Radical Prostatectomy? Analysis of Patients Treated over the Last Decade. Eur. Urol. 2019, 75, 221–228. [Google Scholar] [CrossRef]
- Jiang, N.; Wu, C.; Zhou, X.; Zhai, G.; Wu, J. Cavernous Nerve Injury Resulted Erectile Dysfunction and Regeneration. J. Immunol. Res. 2021, 2021, 5353785. [Google Scholar] [CrossRef]
- Nicolai, M.; Urkmez, A.; Sarikaya, S.; Fode, M.; Falcone, M.; Albersen, M.; Gul, M.; Hatzichristodoulou, G.; Capogrosso, P.; Russo, G.I. Penile Rehabilitation and Treatment Options for Erectile Dysfunction Following Radical Prostatectomy and Radiotherapy: A Systematic Review. Front. Surg. 2021, 8, 636974. [Google Scholar] [CrossRef]
- Walter, J.; Hutu, I.; Tomancok, B.; Cumpanas, A.A.; Bardan, R.; Latcu, S.; Rusmir, A.; Novacescu, D.; Georgescu, O.; Pentea, M.; et al. A New Electrode Design for Direct Bladder Wall Stimulation: A Pilot Minipig Study with Chronic Testing. Appl. Sci. 2022, 12, 1149. [Google Scholar] [CrossRef]
- Mukherjee, P.; Roy, S.; Ghosh, D.; Nandi, S.K. Role of Animal Models in Biomedical Research: A Review. Lab. Anim. Res. 2022, 38, 18. [Google Scholar] [CrossRef] [PubMed]
- Burnett, A.L.; Sezen, S.F.; Hoke, A.; Caggiano, A.O.; Iaci, J.; Lagoda, G.; Musicki, B.; Bella, A.J. GGF2 Is Neuroprotective in a Rat Model of Cavernous Nerve Injury-Induced Erectile Dysfunction. J. Sex. Med. 2015, 12, 897–905. [Google Scholar] [CrossRef] [PubMed]
- EAU Guidelines on Prostate Cancer—TREATMENT—Uroweb. Uroweb—European Association of Urology. Available online: https://uroweb.org/guidelines/prostate-cancer/chapter/treatment (accessed on 12 February 2023).
- Kesch, C.; Heidegger, I.; Kasivisvanathan, V.; Kretschmer, A.; Marra, G.; Preisser, F.; Tilki, D.; Tsaur, I.; Valerio, M.; van den Bergh, R.C.N.; et al. Radical Prostatectomy: Sequelae in the Course of Time. Front. Surg. 2021, 8, 684088. [Google Scholar] [CrossRef]
- Secasan, C.C.; Onchis, D.; Bardan, R.; Cumpanas, A.; Novacescu, D.; Botoca, C.; Dema, A.; Sporea, I. Artificial Intelligence System for Predicting Prostate Cancer Lesions from Shear Wave Elastography Measurements. Curr. Oncol. 2022, 29, 4212–4223. [Google Scholar] [CrossRef]
- Bass, E.J.; Pantovic, A.; Connor, M.J.; Loeb, S.; Rastinehad, A.R.; Winkler, M.; Gabe, R.; Ahmed, H.U. Diagnostic Accuracy of Magnetic Resonance Imaging Targeted Biopsy Techniques Compared to Transrectal Ultrasound Guided Biopsy of the Prostate: A Systematic Review and Meta-Analysis. Prostate Cancer Prostatic Dis. 2022, 25, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Delongchamps, N.B.; Peyromaure, M.; Schull, A.; Beuvon, F.; Bouazza, N.; Flam, T.; Zerbib, M.; Muradyan, N.; Legman, P.; Cornud, F. Prebiopsy Magnetic Resonance Imaging and Prostate Cancer Detection: Comparison of Random and Targeted Biopsies. J. Urol. 2013, 189, 493–499. [Google Scholar] [CrossRef]
- Haffner, J.; Lemaitre, L.; Puech, P.; Haber, G.-P.; Leroy, X.; Jones, J.S.; Villers, A. Role of Magnetic Resonance Imaging before Initial Biopsy: Comparison of Magnetic Resonance Imaging-Targeted and Systematic Biopsy for Significant Prostate Cancer Detection. BJU Int. 2011, 108 Pt 2, E171–E178. [Google Scholar] [CrossRef]
- Belas, O.; Klap, J.; Cornud, F.; Beuvon, F.; Peyromaure, M.; Zerbib, M.; Delongchamps, N.B. Prebiopsy multiparametric MRI of the prostate: The end of randomized biopsies? Prog. Urol. 2012, 22, 583–589. [Google Scholar] [CrossRef]
- Vargas, H.A.; Akin, O.; Franiel, T.; Mazaheri, Y.; Zheng, J.; Moskowitz, C.; Udo, K.; Eastham, J.; Hricak, H. Diffusion-Weighted Endorectal MR Imaging at 3 T for Prostate Cancer: Tumor Detection and Assessment of Aggressiveness. Radiology 2011, 259, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Panebianco, V.; Barchetti, F.; Sciarra, A.; Ciardi, A.; Indino, E.L.; Papalia, R.; Gallucci, M.; Tombolini, V.; Gentile, V.; Catalano, C. Multiparametric Magnetic Resonance Imaging vs. Standard Care in Men Being Evaluated for Prostate Cancer: A Randomized Study. Urol. Oncol. 2015, 33, 17.e1–17.e7. [Google Scholar] [CrossRef]
- Kyriazis, I.; Spinos, T.; Tsaturyan, A.; Kallidonis, P.; Stolzenburg, J.U.; Liatsikos, E. Different Nerve-Sparing Techniques during Radical Prostatectomy and Their Impact on Functional Outcomes. Cancers 2022, 14, 1601. [Google Scholar] [CrossRef]
- Yoham, A.L.; Bordoni, B. Anatomy, Abdomen and Pelvis, Inferior Hypogastric Plexus. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Burnett, A.L. Erectile Dysfunction Following Radical Prostatectomy. JAMA 2005, 293, 2648–2653. [Google Scholar] [CrossRef] [PubMed]
- Costello, A.J.; Brooks, M.; Cole, O.J. Anatomical Studies of the Neurovascular Bundle and Cavernosal Nerves. BJU Int. 2004, 94, 1071–1076. [Google Scholar] [CrossRef]
- Burnett, A.L. Erectile Function Outcomes in the Current Era of Anatomic Nerve-Sparing Radical Prostatectomy. Rev. Urol. 2006, 8, 47–53. [Google Scholar]
- Keast, J.R. Plasticity of Pelvic Autonomic Ganglia and Urogenital Innervation. Int. Rev. Cytol. 2006, 248, 141–208. [Google Scholar] [CrossRef]
- Bratu, O.; Oprea, I.; Marcu, D.; Spinu, D.; Niculae, A.; Geavlete, B.; Mischianu, D. Erectile Dysfunction Post-Radical Prostatectomy—A Challenge for Both Patient and Physician. J. Med. Life 2017, 10, 13–18. [Google Scholar]
- Schaumburg, H.H.; Zotova, E.; Cannella, B.; Raine, C.S.; Arezzo, J.; Tar, M.; Melman, A. Structural and Functional Investigations of the Murine Cavernosal Nerve: A Model System for Serial Spatio-Temporal Study of Autonomic Neuropathy. BJU Int. 2007, 99, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Burnett, A.L.; Lowenstein, C.J.; Bredt, D.S.; Chang, T.S.; Snyder, S.H. Nitric Oxide: A Physiologic Mediator of Penile Erection. Science 1992, 257, 401–403. [Google Scholar] [CrossRef]
- Panchatsharam, P.K.; Durland, J.; Zito, P.M. Physiology, Erection. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Saleh, A.; Abboudi, H.; Ghazal-Aswad, M.; Mayer, E.K.; Vale, J.A. Management of Erectile Dysfunction Post-Radical Prostatectomy. Res. Rep. Urol. 2015, 7, 19–33. [Google Scholar] [CrossRef]
- Montorsi, F.; Briganti, A.; Salonia, A.; Rigatti, P.; Burnett, A.L. Current and Future Strategies for Preventing and Managing Erectile Dysfunction Following Radical Prostatectomy. Eur. Urol. 2004, 45, 123–133. [Google Scholar] [CrossRef]
- Burnett, A.L. Rationale for Cavernous Nerve Restorative Therapy to Preserve Erectile Function after Radical Prostatectomy. Urology 2003, 61, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Walsh, P.C. The Discovery of the Cavernous Nerves and Development of Nerve Sparing Radical Retropubic Prostatectomy. J. Urol. 2007, 177, 1632–1635. [Google Scholar] [CrossRef] [PubMed]
- Milenkovic, U.; Albersen, M.; Castiglione, F. The Mechanisms and Potential of Stem Cell Therapy for Penile Fibrosis. Nat. Rev. Urol. 2019, 16, 79–97. [Google Scholar] [CrossRef]
- Moskovic, D.J.; Miles, B.J.; Lipshultz, L.I.; Khera, M. Emerging Concepts in Erectile Preservation Following Radical Prostatectomy: A Guide for Clinicians. Int. J. Impot. Res. 2011, 23, 181–192. [Google Scholar] [CrossRef]
- Mulhall, J.P.; Bivalacqua, T.J.; Becher, E.F. Standard Operating Procedure for the Preservation of Erectile Function Outcomes after Radical Prostatectomy. J. Sex. Med. 2013, 10, 195–203. [Google Scholar] [CrossRef]
- Müller, A.; Tal, R.; Donohue, J.F.; Akin-Olugbade, Y.; Kobylarz, K.; Paduch, D.; Cutter, S.C.; Mehrara, B.J.; Scardino, P.T.; Mulhall, J.P. The Effect of Hyperbaric Oxygen Therapy on Erectile Function Recovery in a Rat Cavernous Nerve Injury Model. J. Sex. Med. 2008, 5, 562–570. [Google Scholar] [CrossRef]
- Quinlan, D.M.; Nelson, R.J.; Partin, A.W.; Mostwin, J.L.; Walsh, P.C. The Rat as a Model for the Study of Penile Erection. J. Urol. 1989, 141, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Haney, N.M.; Nguyen, H.M.T.; Honda, M.; Abdel-Mageed, A.B.; Hellstrom, W.J.G. Bilateral Cavernous Nerve Crush Injury in the Rat Model: A Comparative Review of Pharmacologic Interventions. Sex. Med. Rev. 2018, 6, 234–241. [Google Scholar] [CrossRef]
- Canguven, O.; Burnett, A. Laboratory Forum: Cavernous Nerve Injury Using Rodent Animal Models. J. Sex. Med. 2008, 5, 1776–1785. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, Q.; Zheng, T.; Bian, J.; Sun, X.; Shi, Y.; Liang, X.; Gao, G.; Liu, G.; Deng, C. Neurotrophic Effect of Adipose Tissue-Derived Stem Cells on Erectile Function Recovery by Pigment Epithelium-Derived Factor Secretion in a Rat Model of Cavernous Nerve Injury. Stem Cells Int. 2015, 2016, e5161248. [Google Scholar] [CrossRef] [PubMed]
- Gajbhiye, S.V.; Jadhav, K.S.; Marathe, P.A.; Pawar, D.B. Animal Models of Erectile Dysfunction. Indian J. Urol. 2015, 31, 15. [Google Scholar] [CrossRef] [PubMed]
- Weyne, E.; Ilg, M.M.; Cakir, O.O.; Muneer, A.; Roussel, D.B.; Albersen, M.; Angulo, J.; Corona, G.; Bettocchi, C.; Reisman, Y.; et al. European Society for Sexual Medicine Consensus Statement on the Use of the Cavernous Nerve Injury Rodent Model to Study Postradical Prostatectomy Erectile Dysfunction. Sex. Med. 2020, 8, 327–337. [Google Scholar] [CrossRef] [PubMed]
- El-Sakka, A.I. Reversion of Penile Fibrosis: Current Information and a New Horizon. Arab J. Urol. 2011, 9, 49–55. [Google Scholar] [CrossRef]
- Sato, Y.; Rehman, J.; Santizo, C.; Melman, A.; Christ, G.J. Significant Physiological Roles of Ancillary Penile Nerves on Increase in Intracavernous Pressure in Rats: Experiments Using Electrical Stimulation of the Medial Preoptic Area. Int. J. Impot. Res. 2001, 13, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, H.Y.; Kim, S.Y.; Lee, S.H.; Lee, W.K.; Yang, D.Y. Spontaneous Recovery of Cavernous Nerve Crush Injury. Korean J. Urol. 2011, 52, 560–565. [Google Scholar] [CrossRef]
- Liu, K.; Sun, T.; Xu, W.; Song, J.; Chen, Y.; Ruan, Y.; Li, H.; Cui, K.; Zhang, Y.; Feng, Y.; et al. Relaxin-2 Prevents Erectile Dysfunction by Cavernous Nerve, Endothelial and Histopathological Protection Effects in Rats with Bilateral Cavernous Nerve Injury. World J. Mens. Health 2022, 40, e50. [Google Scholar] [CrossRef]
- Quinlan, A.; Hansen, S.; Zvara, P.; Lund, L. Validation of a Modified Rat Model for Erectile Function Evaluation. In Larner College of Medicine Fourth Year Advanced Integration Teaching/Scholarly Projects; UVM ScholarWorks: Burlington, VT, USA, 2022. [Google Scholar]
- Mulhall, J.P.; Müller, A.; Donohue, J.F.; Mullerad, M.; Kobylarz, K.; Paduch, D.A.; Tal, R.; Li, P.S.; Cohen-Gould, L.; Scardino, P.T. The Functional and Structural Consequences of Cavernous Nerve Injury Are Ameliorated by Sildenafil Citrate. J. Sex. Med. 2008, 5, 1126–1136. [Google Scholar] [CrossRef]
- Mullerad, M.; Donohue, J.F.; Li, P.S.; Scardino, P.T.; Mulhall, J.P. Functional Sequelae of Cavernous Nerve Injury in the Rat: Is There Model Dependency. J. Sex. Med. 2006, 3, 77–83. [Google Scholar] [CrossRef]
- Matsuda, Y.; Sasaki, M.; Kataoka-Sasaki, Y.; Takayanagi, A.; Kobayashi, K.; Oka, S.; Masahito, N.; Masumori, N.; Kocsis, J.; Honmou, O. Intravenous Infusion of Bone Marrow–Derived Mesenchymal Stem Cells Reduces Erectile Dysfunction Following Cavernous Nerve Injury in Rats. Sex. Med. 2017, 6, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Stanarius, A.; Uckert, S.; Machtens, S.A.; Stief, C.G.; Wolf, G.; Jonas, U. Immunocytochemical Distribution of Nitric Oxide Synthase in the Human Corpus Cavernosum: An Electron Microscopical Study Using the Tyramide Signal Amplification Technique. Urol. Res. 2001, 29, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Karakus, S.; Musicki, B.; La Favor, J.D.; Burnett, A.L. Cyclic AMP-Dependent Post-Translational Modification of Neuronal Nitric Oxide Synthase Neuroprotects Penile Erection in Rats. BJU Int. 2017, 120, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Sant, D.; Andersen, N.; Silvera, R.; Camarena, V.; Piñero, G.; Graham, R.; Khan, A.; Xu, X.-M.; Wang, G.; et al. Magnetic Separation of Peripheral Nerve-Resident Cells Underscores Key Molecular Features of Human Schwann Cells and Fibroblasts: An Immunochemical and Transcriptomics Approach. Sci. Rep. 2020, 10, 18433. [Google Scholar] [CrossRef]
- Yamashita, S.; Kato, R.; Kobayashi, K.; Hisasue, S.-I.; Arai, Y.; Tsukamoto, T. Inhibition of Interleukin-6 Attenuates Erectile Dysfunction in a Rat Model of Nerve-Sparing Radical Prostatectomy. J. Sex. Med. 2011, 8, 1957–1964. [Google Scholar] [CrossRef]
- Albersen, M.; Fandel, T.M.; Zhang, H.; Banie, L.; Lin, G.; De Ridder, D.; Lin, C.-S.; Lue, T.F. Pentoxifylline Promotes Recovery of Erectile Function in a Rat Model of Postprostatectomy Erectile Dysfunction. Eur. Urol. 2011, 59, 286–296. [Google Scholar] [CrossRef]
- Mulhall, J.P.; Müller, A.; Donohue, J.F.; Golijanin, D.; Tal, R.; Akin-Olugbade, Y.; Kobylarz, K.; Cohen-Gould, L.; Bennett, N.E.; Scardino, P. FK506 and Erectile Function Preservation in the Cavernous Nerve Injury Model: Optimal Dosing and Timing. J. Sex. Med. 2008, 5, 1334–1344. [Google Scholar] [CrossRef]
- Mulhall, J.P.; Klein, E.A.; Slawin, K.; Henning, A.K.; Scardino, P.T. A Randomized, Double-Blind, Placebo-Controlled Trial to Assess the Utility of Tacrolimus (FK506) for the Prevention of Erectile Dysfunction Following Bilateral Nerve-Sparing Radical Prostatectomy. J. Sex. Med. 2018, 15, 1293–1299. [Google Scholar] [CrossRef]
- Facio, F.; Burnett, A.L. Penile Rehabilitation and Neuromodulation. ScientificWorldJournal 2009, 9, 652–664. [Google Scholar] [CrossRef]
- Bella, A.J.; Lin, G.; Cagiannos, I.; Lue, T.F. Emerging Neuromodulatory Molecules for the Treatment of Neurogenic Erectile Dysfunction Caused by Cavernous Nerve Injury. Asian J. Androl. 2008, 10, 54–59. [Google Scholar] [CrossRef]
- Podlasek, C.A. Sonic Hedgehog, Apoptosis and the Penis. J. Sex. Med. 2009, 6 (Suppl. 3), 334–339. [Google Scholar] [CrossRef] [PubMed]
- Burnett, A.L.; Lue, T.F. Neuromodulatory Therapy to Improve Erectile Function Recovery Outcomes after Pelvic Surgery. J. Urol. 2006, 176, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Mulhall, J.P. Exploring the Potential Role of Neuromodulatory Drugs in Radical Prostatectomy Patients. J. Androl. 2009, 30, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Britsch, S. The Neuregulin-I/ErbB Signaling System in Development and Disease. In Advances in Anatomy Embryology and Cell Biology; Springer: Berlin/Heidelberg, Germany, 2007; Volume 190, pp. 1–65. [Google Scholar]
- Wieduwilt, M.J.; Moasser, M.M. The Epidermal Growth Factor Receptor Family: Biology Driving Targeted Therapeutics. Cell Mol. Life Sci. 2008, 65, 1566–1584. [Google Scholar] [CrossRef]
- Falls, D.L. Neuregulins: Functions, Forms, and Signaling Strategies. Exp. Cell Res. 2003, 284, 14–30. [Google Scholar] [CrossRef]
- Gambarotta, G.; Fregnan, F.; Gnavi, S.; Perroteau, I. Neuregulin 1 Role in Schwann Cell Regulation and Potential Applications to Promote Peripheral Nerve Regeneration. Int. Rev. Neurobiol. 2013, 108, 223–256. [Google Scholar] [CrossRef]
- Vullhorst, D.; Neddens, J.; Karavanova, I.; Tricoire, L.; Petralia, R.S.; McBain, C.J.; Buonanno, A. Selective Expression of ErbB4 in Interneurons, But Not Pyramidal Cells, of the Rodent Hippocampus. J. Neurosci. 2009, 29, 12255–12264. [Google Scholar] [CrossRef]
- Russell, K.S.; Stern, D.F.; Polverini, P.J.; Bender, J.R. Neuregulin Activation of ErbB Receptors in Vascular Endothelium Leads to Angiogenesis. Am. J. Physiol. 1999, 277, H2205–H2211. [Google Scholar] [CrossRef]
- Clement, C.M.; Thomas, L.K.; Mou, Y.; Croslan, D.R.; Gibbons, G.H.; Ford, B.D. Neuregulin-1 Attenuates Neointimal Formation Following Vascular Injury and Inhibits the Proliferation of Vascular Smooth Muscle Cells. J. Vasc. Res. 2007, 44, 303–312. [Google Scholar] [CrossRef]
- Hill, M.F.; Patel, A.V.; Murphy, A.; Smith, H.M.; Galindo, C.L.; Pentassuglia, L.; Peng, X.; Lenneman, C.G.; Odiete, O.; Friedman, D.B.; et al. Intravenous Glial Growth Factor 2 (GGF2) Isoform of Neuregulin-1β Improves Left Ventricular Function, Gene and Protein Expression in Rats after Myocardial Infarction. PLoS ONE 2013, 8, e55741. [Google Scholar] [CrossRef]
- Moises, H.W.; Zoega, T.; Gottesman, I.I. The Glial Growth Factors Deficiency and Synaptic Destabilization Hypothesis of Schizophrenia. BMC Psychiatry 2002, 2, 8. [Google Scholar] [CrossRef]
- Buonanno, A.; Fischbach, G.D. Neuregulin and ErbB Receptor Signaling Pathways in the Nervous System. Curr. Opin. Neurobiol. 2001, 11, 287–296. [Google Scholar] [CrossRef]
- Esper, R.M.; Pankonin, M.S.; Loeb, J.A. Neuregulins: Versatile Growth and Differentiation Factors in Nervous System Development and Human Disease. Brain Res. Rev. 2006, 51, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Stefansson, H.; Sigurdsson, E.; Steinthorsdottir, V.; Bjornsdottir, S.; Sigmundsson, T.; Ghosh, S.; Brynjolfsson, J.; Gunnarsdottir, S.; Ivarsson, O.; Chou, T.T.; et al. Neuregulin 1 and Susceptibility to Schizophrenia. Am. J. Hum. Genet. 2002, 71, 877–892. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Xiong, W.-C. Neuregulin 1 in Neural Development, Synaptic Plasticity and Schizophrenia. Nat. Rev. Neurosci. 2008, 9, 437–452. [Google Scholar] [CrossRef]
- Ieguchi, K.; Fujita, M.; Ma, Z.; Davari, P.; Taniguchi, Y.; Sekiguchi, K.; Wang, B.; Takada, Y.K.; Takada, Y. Direct Binding of the EGF-like Domain of Neuregulin-1 to Integrins (Avβ3 and A6β4) Is Involved in Neuregulin-1/ErbB Signaling. J. Biol. Chem. 2010, 285, 31388–31398. [Google Scholar] [CrossRef]
- Seroogy, K.B.; Dickerson, J.W.; Cassella, S.N.; Zhang-Auberson, L. Neuregulins. In Handbook of Biologically Active Peptides; Elsevier: Amsterdam, The Netherland, 2013; pp. 1633–1638. [Google Scholar] [CrossRef]
- Ou, G.; Lin, W.; Zhao, W. Neuregulins in Neurodegenerative Diseases. Front. Aging Neurosci. 2021, 13, 662474. [Google Scholar] [CrossRef] [PubMed]
- Bhatheja, K.; Field, J. Schwann Cells: Origins and Role in Axonal Maintenance and Regeneration. Int. J. Biochem. Cell Biol. 2006, 38, 1995–1999. [Google Scholar] [CrossRef]
- Scheib, J.; Höke, A. Advances in Peripheral Nerve Regeneration. Nat. Rev. Neurol. 2013, 9, 668–676. [Google Scholar] [CrossRef]
- Bella, A.J.; Hayashi, N.; Carrion, R.E.; Price, R.; Lue, T.F. FK1706 Enhances the Recovery of Erectile Function Following Bilateral Cavernous Nerve Crush Injury in the Rat. J. Sex. Med. 2007, 4, 341–346. [Google Scholar] [CrossRef]
- Lagoda, G.; Sezen, S.F.; Burnett, A.L. FK506 and Rapamycin Neuroprotect Erection and Involve Different Immunophilins in a Rat Model of Cavernous Nerve Injury. J. Sex. Med. 2009, 6, 1914–1923. [Google Scholar] [CrossRef]
- Chen, L.E.; Liu, K.; Seaber, A.V.; Katragadda, S.; Kirk, C.; Urbaniak, J.R. Recombinant Human Glial Growth Factor 2 (RhGGF2) Improves Functional Recovery of Crushed Peripheral Nerve (a Double-Blind Study). Neurochem. Int. 1998, 33, 341–351. [Google Scholar] [CrossRef] [PubMed]
- ter Laak, M.P.; Hamers, F.P.; Kirk, C.J.; Gispen, W.H. RhGGF2 Protects against Cisplatin-Induced Neuropathy in the Rat. J. Neurosci. Res. 2000, 60, 237–244. [Google Scholar] [CrossRef]
- Fricker, F.R.; Bennett, D.L. The Role of Neuregulin-1 in the Response to Nerve Injury. Future Neurol. 2011, 6, 809–822. [Google Scholar] [CrossRef]
- Wakatsuki, S.; Araki, T.; Sehara-Fujisawa, A. Neuregulin-1/Glial Growth Factor Stimulates Schwann Cell Migration by Inducing A5 Β1 Integrin-ErbB2-Focal Adhesion Kinase Complex Formation. Genes Cells 2014, 19, 66–77. [Google Scholar] [CrossRef] [PubMed]
| Severe BCNCI * (8–10/Group) | Moderate BCNCI (10–12/Group) | Sham Surgery (8–12/Group) | |
|---|---|---|---|
| Treatment regiment | 0.5 mg/kg GGF2 ** | 0.5 mg/kg GGF2 | Vehicle only |
| 5 mg/kg GGF2 | 5 mg/kg GGF2 | Vehicle only | |
| Vehicle only | Vehicle only | 15 mg/kg GGF2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novacescu, D.; Nesiu, A.; Bardan, R.; Latcu, S.C.; Dema, V.F.; Croitor, A.; Raica, M.; Cut, T.G.; Walter, J.; Cumpanas, A.A. Rats, Neuregulins and Radical Prostatectomy: A Conceptual Overview. J. Clin. Med. 2023, 12, 2208. https://doi.org/10.3390/jcm12062208
Novacescu D, Nesiu A, Bardan R, Latcu SC, Dema VF, Croitor A, Raica M, Cut TG, Walter J, Cumpanas AA. Rats, Neuregulins and Radical Prostatectomy: A Conceptual Overview. Journal of Clinical Medicine. 2023; 12(6):2208. https://doi.org/10.3390/jcm12062208
Chicago/Turabian StyleNovacescu, Dorin, Alexandru Nesiu, Razvan Bardan, Silviu Constantin Latcu, Vlad Filodel Dema, Alexei Croitor, Marius Raica, Talida Georgiana Cut, James Walter, and Alin Adrian Cumpanas. 2023. "Rats, Neuregulins and Radical Prostatectomy: A Conceptual Overview" Journal of Clinical Medicine 12, no. 6: 2208. https://doi.org/10.3390/jcm12062208
APA StyleNovacescu, D., Nesiu, A., Bardan, R., Latcu, S. C., Dema, V. F., Croitor, A., Raica, M., Cut, T. G., Walter, J., & Cumpanas, A. A. (2023). Rats, Neuregulins and Radical Prostatectomy: A Conceptual Overview. Journal of Clinical Medicine, 12(6), 2208. https://doi.org/10.3390/jcm12062208








