Assessment of Low-Level Air Pollution and Cardiovascular Incidence in Gdansk, Poland: Time-Series Cross-Sectional Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Its Climate
2.2. Incidence Data
2.3. Environmental Data
2.4. Statistical Methods
| Total Count (% of All) | Daily | ||||
|---|---|---|---|---|---|
| Mean (SD) | Min. | Max. | IQR | ||
| Strokes (ICD-10: I63) | 7619 | 3.48 (1.99) | 0 | 13 | 3 |
| Women | 4047 (53%) | 1.85 (1.45) | 0 | 9 | 2 |
| Men | 3572 (47%) | 1.63 (1.35) | 0 | 8 | 1 |
| After 65 years | 5520 (72%) | 2.52 (1.68) | 0 | 11 | 3 |
| Before 65 years | 2099 (28%) | 0.96 (1.03) | 0 | 6 | 2 |
| Myocardial-infarctions (ICD-10: I21, I22) | 6910 | 3.15 (1.96) | 0 | 12 | 2 |
| Women | 2750 (40%) | 1.26 (1.18) | 0 | 7 | 2 |
| Men | 4160 (60%) | 1.90 (1.50) | 0 | 9 | 2 |
| After 65 years | 4061 (59%) | 1.85 (1.44) | 0 | 9 | 2 |
| Before 65 years | 2849 (41%) | 1.30 (1.27) | 0 | 8 | 2 |
| Missing Values (Total Count for Studied Period) | Daily | ||||
|---|---|---|---|---|---|
| Mean (SD) | Min | Max | IQR | ||
| Chemical compounds (μg/m3) | |||||
| SO2 | 0 | 6.31 (4.08) | 1.98 | 57.73 | 3.65 |
| NO | 0 | 22.83 (17.57) | 4.46 | 170.94 | 14.12 |
| NO2 | 0 | 23.55 (11.96) | 5.09 | 96.49 | 14.62 |
| NOx | 0 | 36.47 (29.65) | 7.05 | 294.11 | 22.52 |
| CO | 0 | 496.1 (203.26) | 244.3 | 2280.1 | 164.04 |
| PM10 | 0 | 26.87 (16.68) | 5.66 | 151.17 | 16.76 |
| PM2.5 | 393 | 20.07 (14.27) | 3.58 | 178.83 | 12.84 |
| O3 | 2 | 55.53 (22.8) | 2.36 | 130 | 31.7 |
| CO2 | 8 | 800.6 (60.64) | 681.9 | 1094 | 79.89 |
| Meteorological data | |||||
| Temperature (°C) | 0 | 10.32 (8.73) | −15.99 | 33.08 | 13.7 |
| Atmospheric pressure (hPa) | 0 | 1011.4 (8.51) | 978.2 | 1039.5 | 10.95 |
| Humidity (%) | 0 | 83.05 (7.14) | 46.18 | 96.84 | 9.46 |
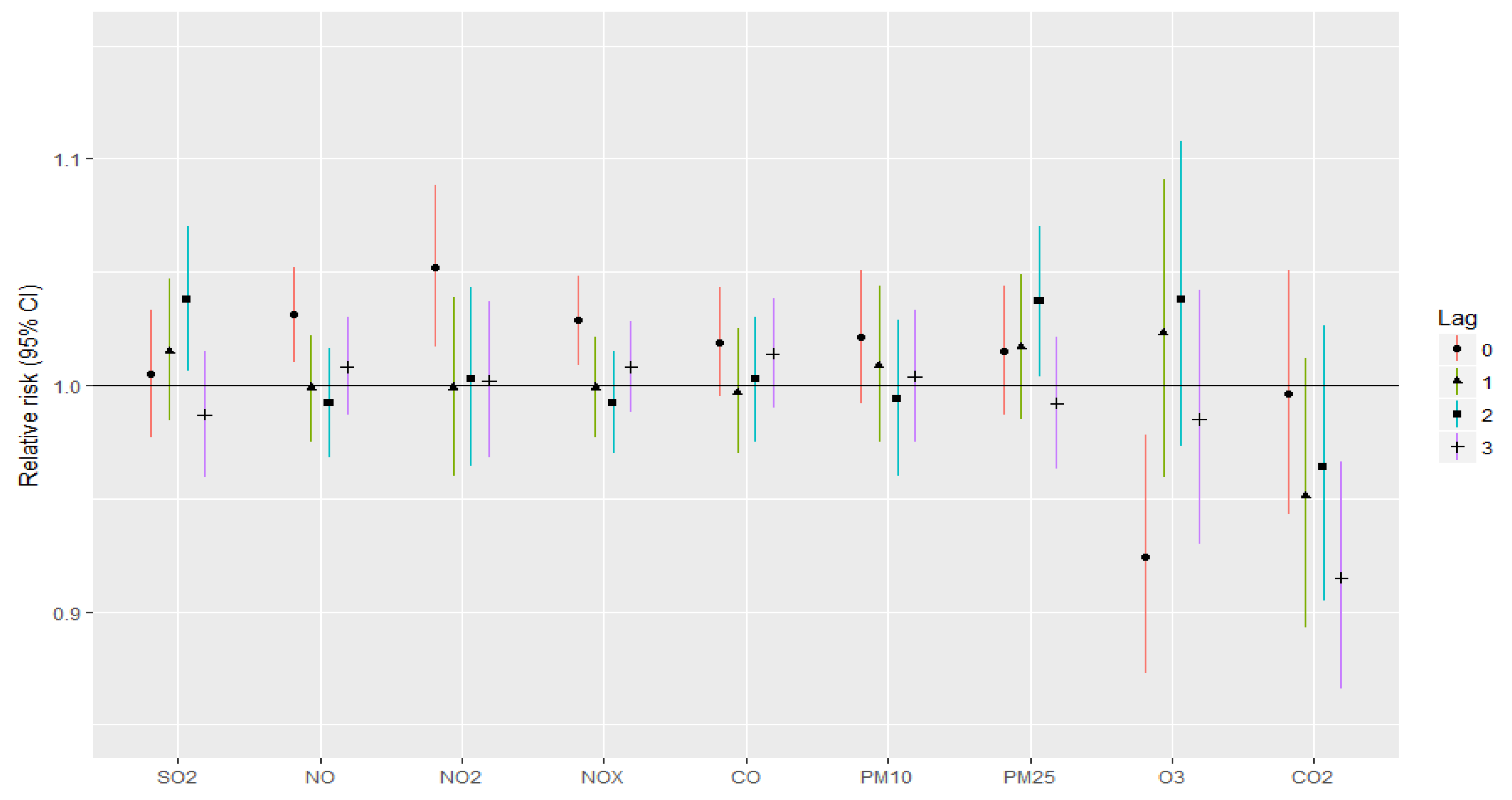
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lopez, E.O.; Ballard, B.D.; Jan, A. Cardiovascular Disease; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- The New EU Alliance Puts Cardiovascular Health in the Spotlight. Available online: https://www.escardio.org/The-ESC/Advocacy/the-new-eu-alliance-puts-cardiovascular-health-in-the-spotlight (accessed on 11 August 2022).
- Choroby Sercowo-Naczyniowe—Instytut Mikroekologii. Available online: https://instytut-mikroekologii.pl/dolegliwosci/nadwaga-i-otylosc/ (accessed on 30 July 2022).
- Jankowski, P.; Kosior, D.A.; Sowa, P.; Szóstak-Janiak, K.; Kozieł, P.; Krzykwa, A.; Sawicka, E.; Haberka, M.; Setny, M.; Kamiński, K.; et al. Secondary Prevention of Coronary Artery Disease in Poland. Results from the POLASPIRE Survey. Cardiology Journal 2020, 27, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthi, R.V.; Feigin, V.L.; Forouzanfar, M.H.; Mensah, G.A.; Connor, M.; Bennett, D.A.; Moran, A.E.; Sacco, R.L.; Anderson, L.M.; Truelsen, T.; et al. Global and Regional Burden of First-Ever Ischaemic and Haemorrhagic Stroke during 1990-2010: Findings from the Global Burden of Disease Study 2010. Lancet Glob. Health 2013, 1, e259–e281. [Google Scholar] [CrossRef]
- Raciborski, F.; Gujski, M.; Gawinska, E.; Klak, A.; Slowik, A.; Wnuk, M.; Instytut Ochrony Zdrowia. Udary Mózgu: Rosnacy Problem w Starzejacym Sie Spoleczenstwie; Instytut Ochrony Zdrowia: Warszawa, Poland, 2016; ISBN 978-83-944863-3-4. [Google Scholar]
- Flora, G.D.; Nayak, M.K. A Brief Review of Cardiovascular Diseases, Associated Risk Factors and Current Treatment Regimes. Curr. Pharm. Des. 2019, 25, 4063–4084. [Google Scholar] [CrossRef]
- Nangia, R.; Singh, H.; Kaur, K. Prevalence of Cardiovascular Disease (CVD) Risk Factors. Med. J. Armed. Forces India 2016, 72, 315–319. [Google Scholar] [CrossRef]
- Ruan, Y.; Guo, Y.; Zheng, Y.; Huang, Z.; Sun, S.; Kowal, P.; Shi, Y.; Wu, F. Cardiovascular Disease (CVD) and Associated Risk Factors among Older Adults in Six Low-and Middle-Income Countries: Results from SAGE Wave 1. BMC Public Health 2018, 18, 778. [Google Scholar] [CrossRef]
- Frontiers. Monitoring Impacts of Urbanisation and Industrialisation on Air Quality in the Anthropocene Using Urban Pond Sediments. Available online: https://www.frontiersin.org/articles/10.3389/feart.2018.00131/full (accessed on 12 August 2022).
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Polivka, B.J. The Great London Smog of 1952. Am. J. Nurs. 2018, 118, 57–61. [Google Scholar] [CrossRef]
- Fisher, J.A.; Puett, R.C.; Laden, F.; Wellenius, G.A.; Sapkota, A.; Liao, D.; Yanosky, J.D.; Carter-Pokras, O.; He, X.; Hart, J.E. Case-Crossover Analysis of Short-Term Particulate Matter Exposures and Stroke in the Health Professionals Follow-up Study. Environ. Int. 2019, 124, 153–160. [Google Scholar] [CrossRef]
- Sun, S.; Stewart, J.D.; Eliot, M.N.; Yanosky, J.D.; Liao, D.; Tinker, L.F.; Eaton, C.B.; Whitsel, E.A.; Wellenius, G.A. Short-Term Exposure to Air Pollution and Incidence of Stroke in the Women’s Health Initiative. Environ. Int. 2019, 132, 105065. [Google Scholar] [CrossRef] [PubMed]
- Milojevic, A.; Wilkinson, P.; Armstrong, B.; Bhaskaran, K.; Smeeth, L.; Hajat, S. Short-Term Effects of Air Pollution on a Range of Cardiovascular Events in England and Wales: Case-Crossover Analysis of the MINAP Database, Hospital Admissions and Mortality. Heart 2014, 100, 1093–1098. [Google Scholar] [CrossRef]
- Vivanco-Hidalgo, R.M.; Wellenius, G.A.; Basagaña, X.; Cirach, M.; González, A.G.; de Ceballos, P.; Zabalza, A.; Jiménez-Conde, J.; Soriano-Tarraga, C.; Giralt-Steinhauer, E.; et al. Short-Term Exposure to Traffic-Related Air Pollution and Ischemic Stroke Onset in Barcelona, Spain. Environ. Res. 2018, 162, 160–165. [Google Scholar] [CrossRef]
- Gdańsk. Available online: https://en.wikipedia.org/w/index.php?title=Gda%C5%84sk&oldid=1104591935 (accessed on 18 August 2022).
- Gdansk Climate: Weather by Month, Temperature, Precipitation, When to Go. Available online: https://www.climatestotravel.com/climate/poland/gdansk (accessed on 10 August 2022).
- Raporty o Stanie Środowiska. Available online: https://www.gios.gov.pl/pl/stan-srodowiska/raporty-o-stanie-srodowiska (accessed on 18 August 2022).
- Andersen, Z.J.; Olsen, T.S.; Andersen, K.K.; Loft, S.; Ketzel, M.; Raaschou-Nielsen, O. Association between Short-Term Exposure to Ultrafine Particles and Hospital Admissions for Stroke in Copenhagen, Denmark. Eur. Heart J. 2010, 31, 2034–2040. [Google Scholar] [CrossRef]
- Dominici, F.; Peng, R.D.; Bell, M.L.; Pham, L.; McDermott, A.; Zeger, S.L.; Samet, J.M. Fine Particulate Air Pollution and Hospital Admission for Cardiovascular and Respiratory Diseases. JAMA 2006, 295, 1127–1134. [Google Scholar] [CrossRef]
- Wellenius, G.A.; Burger, M.R.; Coull, B.A.; Schwartz, J.; Suh, H.H.; Koutrakis, P.; Schlaug, G.; Gold, D.R.; Mittleman, M.A. Ambient Air Pollution and the Risk of Acute Ischemic Stroke. Arch. Intern. Med. 2012, 172, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Stockfelt, L.; Andersson, E.M.; Molnár, P.; Gidhagen, L.; Segersson, D.; Rosengren, A.; Barregard, L.; Sallsten, G. Long-Term Effects of Total and Source-Specific Particulate Air Pollution on Incident Cardiovascular Disease in Gothenburg, Sweden. Environ. Res. 2017, 158, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Kuźma, Ł.; Struniawski, K.; Pogorzelski, S.; Bachórzewska-Gajewska, H.; Dobrzycki, S. Gender Differences in Association between Air Pollution and Daily Mortality in the Capital of the Green Lungs of Poland–Population-Based Study with 2,953,000 Person-Years of Follow-Up. J. Clin. Med. 2020, 9, 2351. [Google Scholar] [CrossRef] [PubMed]
- Kuźma, Ł.; Pogorzelski, S.; Struniawski, K.; Dobrzycki, S.; Bachórzewska-Gajewska, H. Effect of Air Pollution on the Number of Hospital Admissions for Acute Coronary Syndrome in Elderly Patients. Pol. Arch. Intern. Med. 2020, 130, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.S.; Langrish, J.P.; Nair, H.; McAllister, D.A.; Hunter, A.L.; Donaldson, K.; Newby, D.E.; Mills, N.L. Global Association of Air Pollution and Heart Failure: A Systematic Review and Meta-Analysis. Lancet 2013, 382, 1039–1048. [Google Scholar] [CrossRef]
- Chan, C.-C.; Chuang, K.-J.; Chien, L.-C.; Chen, W.-J.; Chang, W.-T. Urban Air Pollution and Emergency Admissions for Cerebrovascular Diseases in Taipei, Taiwan. Eur. Heart J. 2006, 27, 1238–1244. [Google Scholar] [CrossRef]
- Crouse, D.L.; Peters, P.A.; Villeneuve, P.J.; Proux, M.-O.; Shin, H.H.; Goldberg, M.S.; Johnson, M.; Wheeler, A.J.; Allen, R.W.; Atari, D.O.; et al. Within- and between-City Contrasts in Nitrogen Dioxide and Mortality in 10 Canadian Cities; a Subset of the Canadian Census Health and Environment Cohort (CanCHEC). J. Expo. Sci. Environ. Epidemiol. 2015, 25, 482–489. [Google Scholar] [CrossRef]
- Butland, B.K.; Atkinson, R.W.; Crichton, S.; Barratt, B.; Beevers, S.; Spiridou, A.; Hoang, U.; Kelly, F.J.; Wolfe, C.D. Air Pollution and the Incidence of Ischaemic and Haemorrhagic Stroke in the South London Stroke Register: A Case–Cross-over Analysis. J. Epidemiol. Community Health 2017, 71, 707–712. [Google Scholar] [CrossRef]
- Mechtouff, L.; Canoui-Poitrine, F.; Schott, A.-M.; Nighoghossian, N.; Trouillas, P.; Termoz, A.; Porthault-Chatard, S.; David, J.-S.; Chasles, V.; Derex, L. Lack of Association between Air Pollutant Exposure and Short-Term Risk of Ischaemic Stroke in Lyon, France. Int. J. Stroke 2012, 7, 669–674. [Google Scholar] [CrossRef]
- Villeneuve, P.J.; Chen, L.; Stieb, D.; Rowe, B.H. Associations between Outdoor Air Pollution and Emergency Department Visits for Stroke in Edmonton, Canada. Eur. J. Epidemiol. 2006, 21, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Khaniabadi, Y.O.; Daryanoosh, S.M.; Hopke, P.K.; Ferrante, M.; De Marco, A.; Sicard, P.; Oliveri Conti, G.; Goudarzi, G.; Basiri, H.; Mohammadi, M.J.; et al. Acute Myocardial Infarction and COPD Attributed to Ambient SO2 in Iran. Environ. Res. 2017, 156, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Tuan, T.S.; Venâncio, T.S.; Nascimento, L.F.C. Effects of Air Pollutant Exposure on Acute Myocardial Infarction, According to Gender. Arq. Bras. Cardiol. 2016, 107, 216–222. [Google Scholar] [CrossRef]
- Pereira Filho, M.A.; Pereira, L.A.A.; Arbex, F.F.; Arbex, M.; Conceição, G.M.; Santos, U.P.; Lopes, A.C.; Saldiva, P.H.N.; Braga, A.L.F.; Cendon, S. Effect of Air Pollution on Diabetes and Cardiovascular Diseases in São Paulo, Brazil. Braz. J. Med. Biol. Res. 2008, 41, 526–532. [Google Scholar] [CrossRef]
- Zhang, C.; Ding, R.; Xiao, C.; Xu, Y.; Cheng, H.; Zhu, F.; Lei, R.; Di, D.; Zhao, Q.; Cao, J. Association between Air Pollution and Cardiovascular Mortality in Hefei, China: A Time-Series Analysis. Environ. Pollut. 2017, 229, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, Y.; Ma, R.; Liu, X.; Liang, J.; Lin, H.; Shen, P.; Zhang, J.; Lu, P.; Tang, X.; et al. Long-Term Exposure to Ozone and Cardiovascular Mortality in a Large Chinese Cohort. Environ. Int. 2022, 165, 107280. [Google Scholar] [CrossRef]
- Chen, R.; Pan, G.; Kan, H.; Tan, J.; Song, W.; Wu, Z.; Xu, X.; Xu, Q.; Jiang, C.; Chen, B. Ambient Air Pollution and Daily Mortality in Anshan, China: A Time-Stratified Case-Crossover Analysis. Sci. Total Environ. 2010, 408, 6086–6091. [Google Scholar] [CrossRef]
- Son, J.-Y.; Lee, J.-T.; Kim, H.; Yi, O.; Bell, M.L. Susceptibility to Air Pollution Effects on Mortality in Seoul, Korea: A Case-Crossover Analysis of Individual-Level Effect Modifiers. J. Expo. Sci. Environ. Epidemiol. 2012, 22, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Tsai, D.-H.; Wang, J.-L.; Chuang, K.-J.; Chan, C.-C. Traffic-Related Air Pollution and Cardiovascular Mortality in Central Taiwan. Sci. Total Environ. 2010, 408, 1818–1823. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.C.; Hayes, R.B.; Ahn, J.; Shao, Y.; Silverman, D.T.; Jones, R.R.; Garcia, C.; Bell, M.L.; Thurston, G.D. Long-Term Exposure to Ozone and Cause-Specific Mortality Risk in the United States. Am. J. Respir. Crit. Care Med. 2019, 200, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.C.; Jerrett, M.; Pope, C.A.; Krewski, D.; Gapstur, S.M.; Diver, W.R.; Beckerman, B.S.; Marshall, J.D.; Su, J.; Crouse, D.L.; et al. Long-Term Ozone Exposure and Mortality in a Large Prospective Study. Am. J. Respir. Crit. Care Med. 2016, 193, 1134–1142. [Google Scholar] [CrossRef]
- Weichenthal, S.; Pinault, L.L.; Burnett, R.T. Impact of Oxidant Gases on the Relationship between Outdoor Fine Particulate Air Pollution and Nonaccidental, Cardiovascular, and Respiratory Mortality. Sci. Rep. 2017, 7, 16401. [Google Scholar] [CrossRef] [PubMed]
- Hvidtfeldt, U.A.; Sørensen, M.; Geels, C.; Ketzel, M.; Khan, J.; Tjønneland, A.; Overvad, K.; Brandt, J.; Raaschou-Nielsen, O. Long-Term Residential Exposure to PM2.5, PM10, Black Carbon, NO2, and Ozone and Mortality in a Danish Cohort. Environ. Int. 2019, 123, 265–272. [Google Scholar] [CrossRef]
- Bentayeb, M.; Wagner, V.; Stempfelet, M.; Zins, M.; Goldberg, M.; Pascal, M.; Larrieu, S.; Beaudeau, P.; Cassadou, S.; Eilstein, D.; et al. Association between Long-Term Exposure to Air Pollution and Mortality in France: A 25-Year Follow-up Study. Environ. Int. 2015, 85, 5–14. [Google Scholar] [CrossRef]
- Carey, I.M.; Atkinson, R.W.; Kent, A.J.; van Staa, T.; Cook, D.G.; Anderson, H.R. Mortality Associations with Long-Term Exposure to Outdoor Air Pollution in a National English Cohort. Am. J. Respir. Crit. Care Med. 2013, 187, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Bhaskaran, K.; Hajat, S.; Haines, A.; Herrett, E.; Wilkinson, P.; Smeeth, L. Effects of Air Pollution on the Incidence of Myocardial Infarction. Heart 2009, 95, 1746–1759. [Google Scholar] [CrossRef]
- Atkinson, R.W.; Butland, B.K.; Dimitroulopoulou, C.; Heal, M.R.; Stedman, J.R.; Carslaw, N.; Jarvis, D.; Heaviside, C.; Vardoulakis, S.; Walton, H.; et al. Long-Term Exposure to Ambient Ozone and Mortality: A Quantitative Systematic Review and Meta-Analysis of Evidence from Cohort Studies. BMJ Open 2016, 6, e009493. [Google Scholar] [CrossRef]
- Di, Q.; Wang, Y.; Zanobetti, A.; Wang, Y.; Koutrakis, P.; Choirat, C.; Dominici, F.; Schwartz, J.D. Air Pollution and Mortality in the Medicare Population. N. Engl. J. Med. 2017, 376, 2513–2522. [Google Scholar] [CrossRef]
- Cakmak, S.; Hebbern, C.; Vanos, J.; Crouse, D.L.; Burnett, R. Ozone Exposure and Cardiovascular-Related Mortality in the Canadian Census Health and Environment Cohort (CANCHEC) by Spatial Synoptic Classification Zone. Environ. Pollut. 2016, 214, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Kazemiparkouhi, F.; Eum, K.-D.; Wang, B.; Manjourides, J.; Suh, H.H. Long-Term Ozone Exposures and Cause-Specific Mortality in a US Medicare Cohort. J. Expo. Sci. Environ. Epidemiol. 2020, 30, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Henrotin, J.B.; Besancenot, J.P.; Bejot, Y.; Giroud, M. Short-Term Effects of Ozone Air Pollution on Ischaemic Stroke Occurrence: A Case-Crossover Analysis from a 10-Year Population-Based Study in Dijon, France. Occup. Environ. Med. 2007, 64, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Zhou, Y.; Chen, R.; Yin, P.; Meng, X.; Wang, W.; Liu, C.; Ji, J.S.; Qiu, Y.; Kan, H.; et al. Long-Term Exposure to Ozone and Cardiovascular Mortality in China: A Nationwide Cohort Study. Lancet Planet. Health 2022, 6, e496–e503. [Google Scholar] [CrossRef]
- Talbott, E.O.; Rager, J.R.; Benson, S.; Brink, L.A.; Bilonick, R.A.; Wu, C. A Case-Crossover Analysis of the Impact of PM(2.5) on Cardiovascular Disease Hospitalizations for Selected CDC Tracking States. Environ. Res. 2014, 134, 455–465. [Google Scholar] [CrossRef]
- Amsalu, E.; Wang, T.; Li, H.; Liu, Y.; Wang, A.; Liu, X.; Tao, L.; Luo, Y.; Zhang, F.; Yang, X.; et al. Acute Effects of Fine Particulate Matter (PM2.5) on Hospital Admissions for Cardiovascular Disease in Beijing, China: A Time-Series Study. Environ. Health 2019, 18, 70. [Google Scholar] [CrossRef]
- Tian, Q.; Li, M.; Montgomery, S.; Fang, B.; Wang, C.; Xia, T.; Cao, Y. Short-Term Associations of Fine Particulate Matter and Synoptic Weather Types with Cardiovascular Mortality: An Ecological Time-Series Study in Shanghai, China. Int. J. Environ. Res. Public Health 2020, 17, 1111. [Google Scholar] [CrossRef]
- Shah, A.S.V.; Lee, K.K.; McAllister, D.A.; Hunter, A.; Nair, H.; Whiteley, W.; Langrish, J.P.; Newby, D.E.; Mills, N.L. Short Term Exposure to Air Pollution and Stroke: Systematic Review and Meta-Analysis. BMJ 2015, 350, h1295. [Google Scholar] [CrossRef]
- Wang, Z.; Peng, J.; Liu, P.; Duan, Y.; Huang, S.; Wen, Y.; Liao, Y.; Li, H.; Yan, S.; Cheng, J.; et al. Association between Short-Term Exposure to Air Pollution and Ischemic Stroke Onset: A Time-Stratified Case-Crossover Analysis Using a Distributed Lag Nonlinear Model in Shenzhen, China. Environ. Health 2020, 19, 1. [Google Scholar] [CrossRef]
- Yamaji, K.; Kohsaka, S.; Morimoto, T.; Fujii, K.; Amano, T.; Uemura, S.; Akasaka, T.; Kadota, K.; Nakamura, M.; Kimura, T. Relation of ST-Segment Elevation Myocardial Infarction to Daily Ambient Temperature and Air Pollutant Levels in a Japanese Nationwide Percutaneous Coronary Intervention Registry. Am. J. Cardiol. 2017, 119, 872–880. [Google Scholar] [CrossRef]
- Goldberg, M.S.; Burnett, R.T.; Stieb, D.M.; Brophy, J.M.; Daskalopoulou, S.S.; Valois, M.-F.; Brook, J.R. Associations between Ambient Air Pollution and Daily Mortality among Elderly Persons in Montreal, Quebec. Sci. Total Environ. 2013, 463–464, 931–942. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Song, W.; Bai, Y.; Liu, T.; Li, G.; Bian, Y.; Zeng, Q. Years of Life Lost (YLL) Due to Short-Term Exposure to Ambient Air Pollution in China: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 11467. [Google Scholar] [CrossRef] [PubMed]
- Simoni, M.; Baldacci, S.; Maio, S.; Cerrai, S.; Sarno, G.; Viegi, G. Adverse Effects of Outdoor Pollution in the Elderly. J. Thorac. Dis. 2015, 7, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Ohlwein, S.; Klümper, C.; Vossoughi, M.; Sugiri, D.; Stolz, S.; Vierkötter, A.; Schikowski, T.; Kara, K.; Germing, A.; Quass, U.; et al. Air Pollution and Diastolic Function in Elderly Women—Results from the SALIA Study Cohort. Int. J. Hyg. Environ. Health 2016, 219, 356–363. [Google Scholar] [CrossRef]
- Zanobetti, A.; Schwartz, J. The Effect of Particulate Air Pollution on Emergency Admissions for Myocardial Infarction: A Multicity Case-Crossover Analysis. Environ. Health Perspect. 2005, 113, 978–982. [Google Scholar] [CrossRef]
- Pope, C., 3rd; Muhlestein, J.; May, H.; Renlund, D.; Anderson, J.; Horne, B. Ischemic Heart Disease Events Triggered by Short-Term Exposure to Fine Particulate Air Pollution. Circulation 2006, 114, 2443–2448. [Google Scholar] [CrossRef]
- Cesaroni, G.; Forastiere, F.; Stafoggia, M.; Andersen, Z.J.; Badaloni, C.; Beelen, R.; Caracciolo, B.; de Faire, U.; Erbel, R.; Eriksen, K.T.; et al. Long Term Exposure to Ambient Air Pollution and Incidence of Acute Coronary Events: Prospective Cohort Study and Meta-Analysis in 11 European Cohorts from the ESCAPE Project. BMJ 2014, 348, f7412. [Google Scholar] [CrossRef]
- Kaufman, J.D.; Adar, S.D.; Barr, R.G.; Budoff, M.; Burke, G.L.; Curl, C.L.; Daviglus, M.L.; Roux, A.V.D.; Gassett, A.J.; Jacobs, D.R.; et al. Association between Air Pollution and Coronary Artery Calcification within Six Metropolitan Areas in the USA (the Multi-Ethnic Study of Atherosclerosis and Air Pollution): A Longitudinal Cohort Study. Lancet 2016, 388, 696–704. [Google Scholar] [CrossRef]
- Tibuakuu, M.; Michos, E.D.; Navas-Acien, A.; Jones, M.R. Air Pollution and Cardiovascular Disease: A Focus on Vulnerable Populations Worldwide. Curr. Epidemiol. Rep. 2018, 5, 370–378. [Google Scholar] [CrossRef]
- Pun, V.C.; Kazemiparkouhi, F.; Manjourides, J.; Suh, H.H. Long-Term PM2.5 Exposure and Respiratory, Cancer, and Cardiovascular Mortality in Older US Adults. Am. J. Epidemiol. 2017, 186, 961–969. [Google Scholar] [CrossRef]
- Katsoulis, M.; Dimakopoulou, K.; Pedeli, X.; Trichopoulos, D.; Gryparis, A.; Trichopoulou, A.; Katsouyanni, K. Long-Term Exposure to Traffic-Related Air Pollution and Cardiovascular Health in a Greek Cohort Study. Sci. Total Environ. 2014, 490, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Green, R.; Broadwin, R.; Malig, B.; Basu, R.; Gold, E.B.; Qi, L.; Sternfeld, B.; Bromberger, J.T.; Greendale, G.A.; Kravitz, H.M.; et al. Long- and Short-Term Exposure to Air Pollution and Inflammatory/Hemostatic Markers in Midlife Women. Epidemiology 2016, 27, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Kan, H.; Heiss, G.; Rose, K.M.; Whitsel, E.; Lurmann, F.; London, S.J. Traffic Exposure and Lung Function in Adults: The Atherosclerosis Risk in Communities Study. Thorax 2007, 62, 873–879. [Google Scholar] [CrossRef]
- Clougherty, J.E. A Growing Role for Gender Analysis in Air Pollution Epidemiology. Environ. Health Perspect. 2010, 118, 167–176. [Google Scholar] [CrossRef]
- Kan, H.; London, S.J.; Chen, G.; Zhang, Y.; Song, G.; Zhao, N.; Jiang, L.; Chen, B. Season, Sex, Age, and Education as Modifiers of the Effects of Outdoor Air Pollution on Daily Mortality in Shanghai, China: The Public Health and Air Pollution in Asia (PAPA) Study. Environ. Health Perspect. 2008, 116, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Abbey, D.E.; Nishino, N.; McDONNELL, W.F.; Burchette, R.J.; Knutsen, S.F.; Lawrence Beeson, W.; Yang, J.X. Long-Term Inhalable Particles and Other Air Pollutants Related to Mortality in Nonsmokers. Am. J. Respir. Crit. Care Med. 1999, 159, 373–382. [Google Scholar] [CrossRef]
- Gan, W.Q.; Koehoorn, M.; Davies, H.W.; Demers, P.A.; Tamburic, L.; Brauer, M. Long-Term Exposure to Traffic-Related Air Pollution and the Risk of Coronary Heart Disease Hospitalization and Mortality. Environ. Health Perspect. 2011, 119, 501–507. [Google Scholar] [CrossRef]
- Beelen, R.; Raaschou-Nielsen, O.; Stafoggia, M.; Andersen, Z.J.; Weinmayr, G.; Hoffmann, B.; Wolf, K.; Samoli, E.; Fischer, P.; Nieuwenhuijsen, M.; et al. Effects of Long-Term Exposure to Air Pollution on Natural-Cause Mortality: An Analysis of 22 European Cohorts within the Multicentre ESCAPE Project. Lancet 2014, 383, 785–795. [Google Scholar] [CrossRef]
- de Bont, J.; Jaganathan, S.; Dahlquist, M.; Persson, Å.; Stafoggia, M.; Ljungman, P. Ambient Air Pollution and Cardiovascular Diseases: An Umbrella Review of Systematic Reviews and Meta-Analyses. J. Intern. Med. 2022, 291, 779–800. [Google Scholar] [CrossRef]
- Azuma, K.; Kagi, N.; Yanagi, U.; Osawa, H. Effects of Low-Level Inhalation Exposure to Carbon Dioxide in Indoor Environments: A Short Review on Human Health and Psychomotor Performance. Environ. Int. 2018, 121, 51–56. [Google Scholar] [CrossRef]
- Tsai, D.-H.; Lin, J.-S.; Chan, C.-C. Office Workers’ Sick Building Syndrome and Indoor Carbon Dioxide Concentrations. J. Occup. Environ. Hyg. 2012, 9, 345–351. [Google Scholar] [CrossRef]
- MacNaughton, P.; Spengler, J.; Vallarino, J.; Santanam, S.; Satish, U.; Allen, J. Environmental Perceptions and Health before and after Relocation to a Green Building. Build. Environ. 2016, 104, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wargocki, P.; Lian, Z. Human Responses to Carbon Dioxide, a Follow-up Study at Recommended Exposure Limits in Non-Industrial Environments. Build. Environ. 2016, 100, 162–171. [Google Scholar] [CrossRef]
- Minhas, J.S.; Robinson, T.; Panerai, R. PaCO2 Measurement in Cerebral Haemodynamics: Face Mask or Nasal Cannulae? Physiol. Meas. 2017, 38, N101–N106. [Google Scholar] [CrossRef] [PubMed]
- Minhas, J.S.; Panerai, R.B.; Robinson, T.G. Modelling the Cerebral Haemodynamic Response in the Physiological Range of PaCO2. Physiol. Meas. 2018, 39, 065001. [Google Scholar] [CrossRef]
- Minhas, J.S.; Panerai, R.B.; Swienton, D.; Robinson, T.G. Feasibility of Improving Cerebral Autoregulation in Acute Intracerebral Hemorrhage (BREATHE-ICH) Study: Results from an Experimental Interventional Study. Int. J. Stroke 2020, 15, 627–637. [Google Scholar] [CrossRef]
- Salinet, A.S.M.; Minhas, J.S.; Panerai, R.B.; Bor-Seng-Shu, E.; Robinson, T.G. Do Acute Stroke Patients Develop Hypocapnia? A Systematic Review and Meta-Analysis. J. Neurol. Sci. 2019, 402, 30–39. [Google Scholar] [CrossRef]
- Biose, I.J.; Oremosu, J.; Bhatnagar, S.; Bix, G.J. Promising Cerebral Blood Flow Enhancers in Acute Ischemic Stroke. Transl. Stroke Res. 2022. Online ahead of print. [Google Scholar] [CrossRef]

| SO2 | NO | NO2 | NOx | CO | PM10 | PM2.5 | O3 | CO2 | Temp. | Humid | Pres | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SO2 | 1 | |||||||||||
| NO | 0.47 * | 1 | ||||||||||
| NO2 | 0.56 * | 0.88 * | 1 | |||||||||
| NOx | 0.46 * | 1 * | 0.86 * | 1 | ||||||||
| CO | 0.61 * | 0.86 * | 0.79 * | 0.85 * | 1 | |||||||
| PM10 | 0.62 * | 0.73 * | 0.75 * | 0.76 * | 0.79 * | 1 | ||||||
| PM2.5 | 0.64 * | 0.75 * | 0.75 * | 0.74 * | 0.85 * | 0.96 * | 1 | |||||
| O3 | −0.21 * | −0.37 * | −0.26 * | −0.38 * | −0.45 * | −0.20 * | −0.33 * | 1 | ||||
| CO2 | 0.03 | 0.32 * | 0.29 * | 0.32 * | 0.34 * | 0.30 * | 0.23 * | −0.09 * | 1 | |||
| Temp. | −0.49 * | −0.29 * | −0.29 * | −0.29 * | −0.49 * | −0.21 * | −0.33 * | 0.54 * | 0.01 | 1 | ||
| Humid | −0.01 | 0.21 * | 0.13 * | 0.21 * | 0.22 * | 0.02 | 0.16 * | −0.48 * | −0.02 | −0.13 * | 1 | |
| Pres | 0.13 * | 0.15 * | 0.16 * | 0.15 * | 0.15 * | 0.20 * | 0.18 * | 0.04 * | 0.15 * | −0.08 * | −0.19 * | 1 |
| SEX | AGE | ALL | |||
|---|---|---|---|---|---|
| WOMEN | MEN | ≥65 YEARS | <65 YEARS | ||
| STROKE | |||||
| SO2 | 1.00 (0.97–1.03) | 0.99 (0.96–1.02) | 1.01 (0.99–1.03) | 0.95 (0.91–0.99) * | 0.99 (0.97–1.01) |
| NO | 1.02 (1.00–1.05) * | 1.01 (0.99–1.04) | 1.02 (1.00–1.04) * | 1.06 (0.97–1.04) | 1.02 (1.00–1.04) * |
| NO2 | 1.05 (1.01–1.09) * | 1.02 (0.98–1.06) | 1.05 (1.06–1.08) * | 1.00 (0.95–1.06) | 1.04 (1.01–1.06) * |
| NOx | 1.02 (1.00–1.04) | 1.01 (0.99–1.04) | 1.02 (1.00–1.04) * | 1.01 (0.97–1.04) | 1.02 (1.00–1.03) * |
| CO | 1.01 (0.99–1.04) | 0.99 (0.97–1.02) | 1.01 (0.99–1.03) | 0.98 (0.94–1.01) | 1.00 (0.98–1.02) |
| PM10 | 1.02 (0.99–1.05) | 1.00 (0.97–1.04) | 1.02 (0.99–1.05) | 0.99 (0.94–1.03) | 1.01 (0.99–1.03) |
| PM2.5 | 1.02 (0.99–1.05) | 1.01 (0.98–1.04) | 1.03 (1.00–1.05) * | 0.99 (0.94–1.03) | 1.02 (0.99–1.04) |
| O3 | 0.98 (0.94–1.03) | 1.02 (0.97–1.07) | 0.98 (0.96–1.04) | 1.01 (0.95–1.07) | 1.00 (0.97–1.03) |
| CO2 | 0.95 (0.91–0.99) * | 0.96 (0.92–0.99) * | 0.96 (0.92–0.99) * | 0.94 (0.89–0.99) * | 0.95 (0.92–0.98) * |
| Temp. | 0.98 (0.95–1.02) | 1.02 (0.98–1.06) | 0.98 (0.95–1.01) | 1.06 (1.01–1.11) * | 0.99 (0.97–1.02) |
| Humid. | 1.00 (0.98–1.02) | 0.98 (0.96–1.01) | 0.98 (0.96–1.00) | 1.02 (0.99–1.05) | 0.99 (0.98–1.01) |
| Pres. | 1.00 (0.99–1.02) | 0.99 (0.98–1.01) | 1.01 (0.99–1.02) | 0.98 (0.95–1.00) | 1.00 (0.99–1.01) |
| MYOCARDIAL INFARCTION | |||||
| SO2 | 1.04 (1.00–1.07) * | 1.03 (1.00–1.05) * | 1.04 (1.01–1.07) * | 1.02 (0.99–1.05) | 1.03 (1.01–1.05) * |
| NO | 1.03 (0.99–1.06) | 1.03 (1.01–1.06) * | 1.04 (1.01–1.07) * | 1.02 (1.00–1.05) * | 1.03 (1.01–1.05) * |
| NO2 | 1.06 (1.01–1.11) * | 1.05 (1.01–1.09) * | 1.07 (1.02–1.16) * | 1.04 (1.00–1.08) * | 1.05 (1.02–1.08) * |
| NOx | 1.02 (0.99–1.05) | 1.03 (1.01–1.05) * | 1.03 (1.01–1.06) * | 1.02 (1.00–1.04) * | 1.03 (1.01–1.04) * |
| CO | 1.00 (0.97–1.03) | 1.04 (1.01–1.06) * | 1.03 (0.99–1.06) | 1.02 (0.99–1.04) | 1.02 (1.00–1.04) * |
| PM10 | 1.00 (0.96–1.04) | 1.04 (1.01–1.07) * | 1.04 (1.00–1.07) * | 1.02 (0.99–1.05) | 1.02 (1.00–1.05) * |
| PM2.5 | 1.03 (0.99–1.07) | 1.04 (1.01–1.07) * | 1.04 (1.00–1.08) * | 1.03 (0.99–1.06) | 1.03 (1.01–1.06) * |
| O3 | 0.94 (0.89–0.99) * | 0.97 (0.93–1.01) | 0.96 (0.91–1.01) | 0.95 (0.91–0.99) * | 0.96 (0.92–0.99) * |
| CO2 | 0.85 (0.81–0.90) * | 0.90 (0.86–0.93) * | 0.84 (0.80–0.88) * | 0.91 (0.87–0.94) * | 0.88 (0.85–0.91) * |
| Temp. | 0.94 (0.90–0.98) * | 0.96 (0.93–0.99) * | 0.97 (0.93–1.01) | 0.95 (0.91–0.98) * | 0.95 (0.93–0.98) * |
| Humid. | 1.02 (0.99–1.05) | 1.03 (1.01–1.05) * | 1.04 (1.01–1.06) * | 1.02 (0.99–1.04) | 1.07 (1.01–1.04) * |
| Pres. | 0.98 (0.96–1.01) | 0.99 (0.97–1.01) | 0.99 (0.97–1.01) | 0.98 (0.98–1.00) | 0.98 (0.97–0.99) * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czernych, R.; Badyda, A.J.; Kozera, G.; Zagożdżon, P. Assessment of Low-Level Air Pollution and Cardiovascular Incidence in Gdansk, Poland: Time-Series Cross-Sectional Analysis. J. Clin. Med. 2023, 12, 2206. https://doi.org/10.3390/jcm12062206
Czernych R, Badyda AJ, Kozera G, Zagożdżon P. Assessment of Low-Level Air Pollution and Cardiovascular Incidence in Gdansk, Poland: Time-Series Cross-Sectional Analysis. Journal of Clinical Medicine. 2023; 12(6):2206. https://doi.org/10.3390/jcm12062206
Chicago/Turabian StyleCzernych, Radosław, Artur Jerzy Badyda, Grzegorz Kozera, and Paweł Zagożdżon. 2023. "Assessment of Low-Level Air Pollution and Cardiovascular Incidence in Gdansk, Poland: Time-Series Cross-Sectional Analysis" Journal of Clinical Medicine 12, no. 6: 2206. https://doi.org/10.3390/jcm12062206
APA StyleCzernych, R., Badyda, A. J., Kozera, G., & Zagożdżon, P. (2023). Assessment of Low-Level Air Pollution and Cardiovascular Incidence in Gdansk, Poland: Time-Series Cross-Sectional Analysis. Journal of Clinical Medicine, 12(6), 2206. https://doi.org/10.3390/jcm12062206






