Contemporary Management of Cardiogenic Shock Complicating Acute Myocardial Infarction
Abstract
1. Introduction
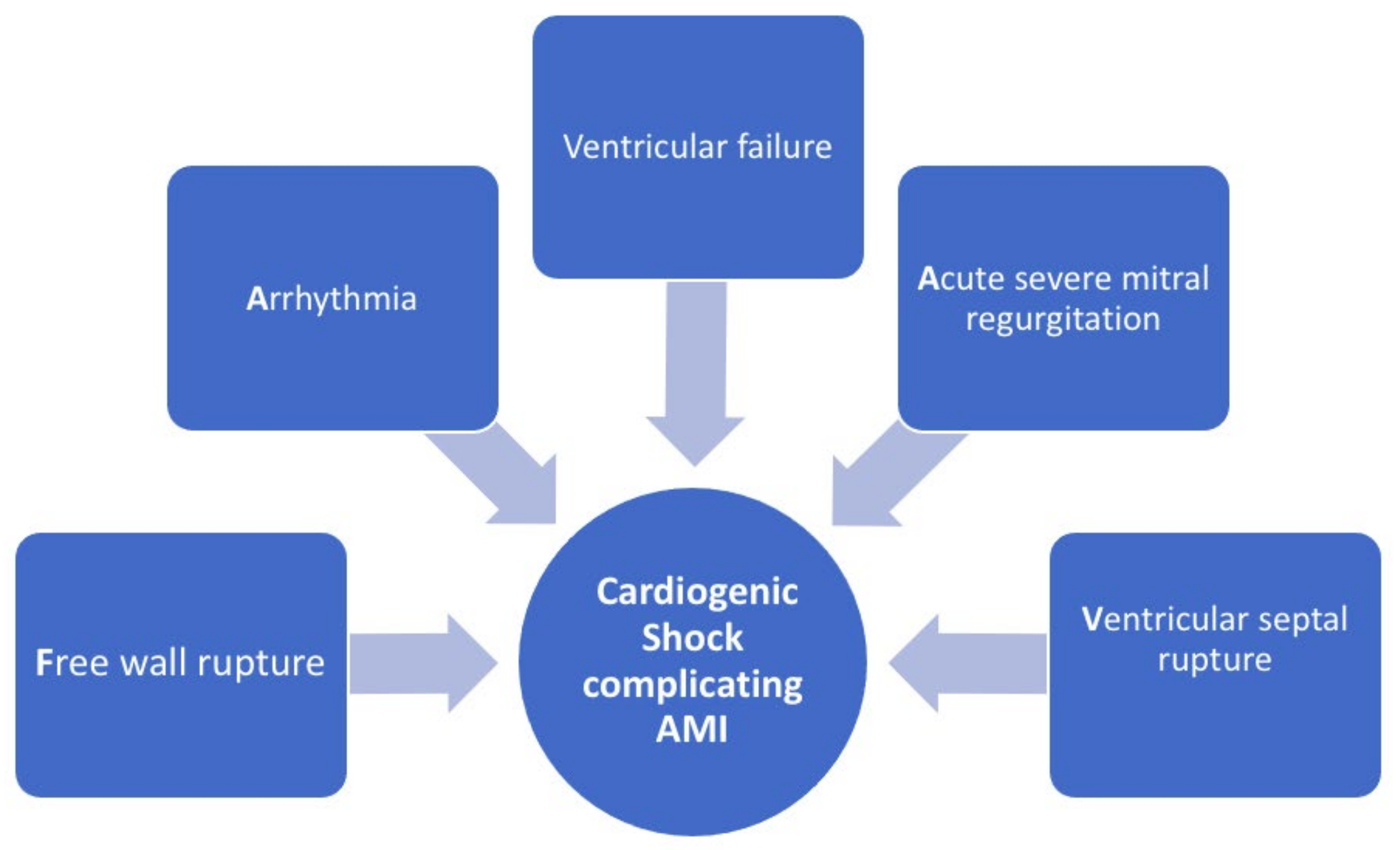
2. Definition and Pathophysiology
3. Clinical Assessment and Diagnosis
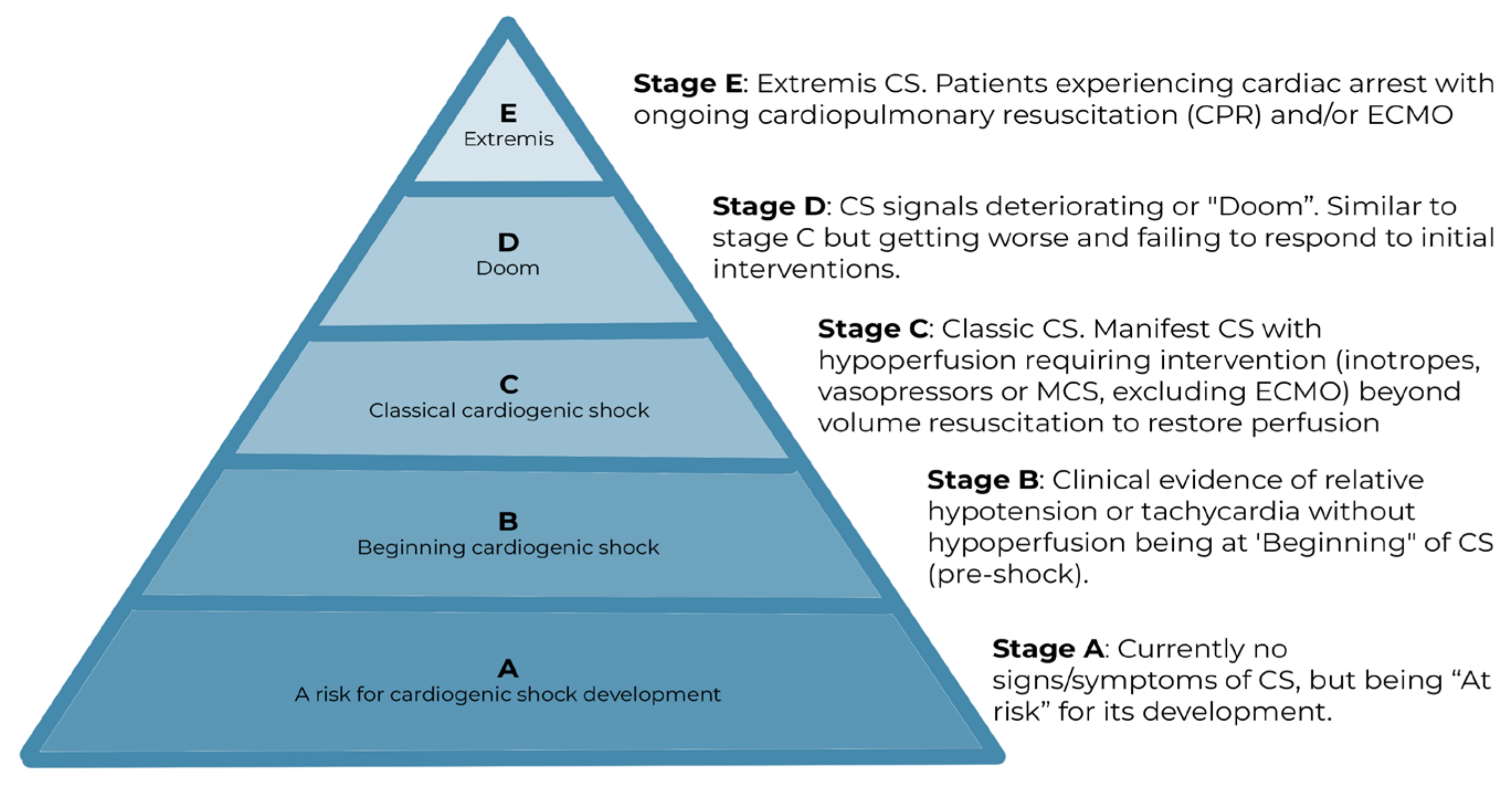
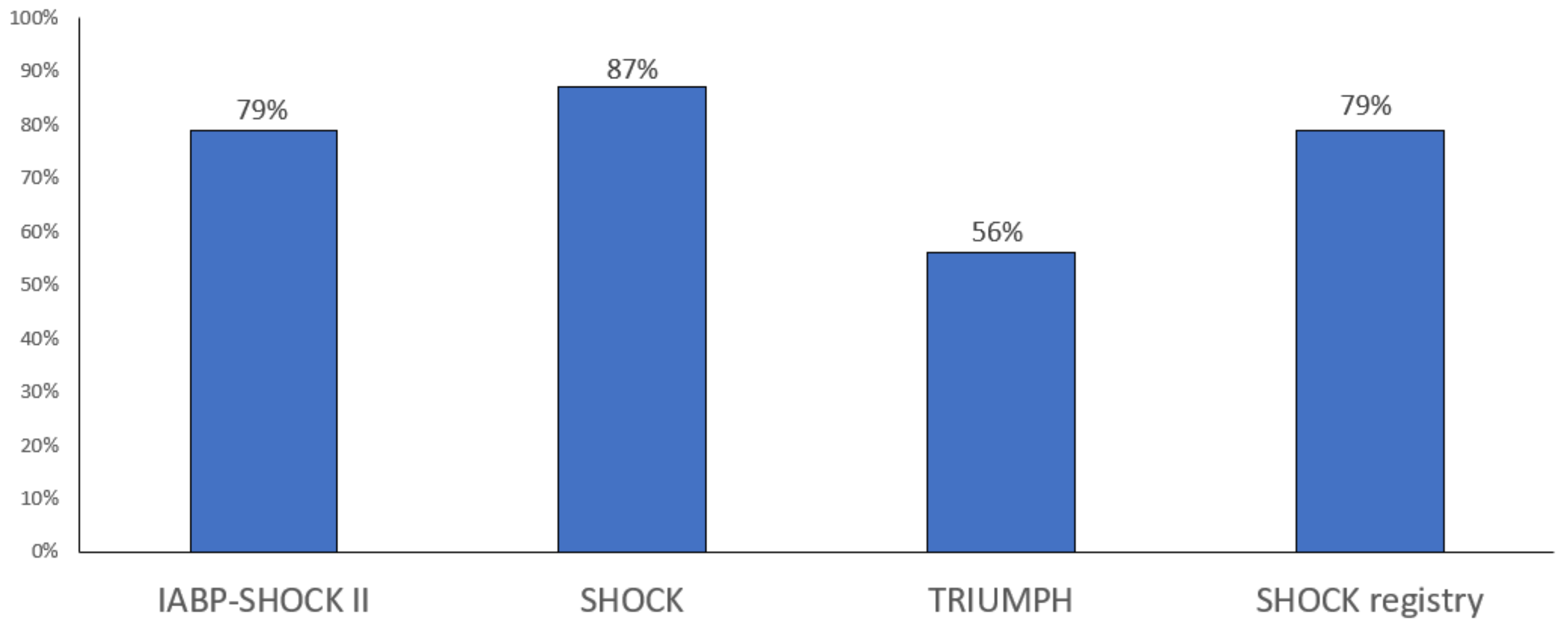
4. Classification and Prognosis
5. Management
5.1. Coronary Artery Revascularisation
5.2. Pharmacologic Therapies
5.3. Mechanical Circulatory Support Devices
5.4. Intra-Aortic Balloon Pump
5.5. Percutaneous Ventricular Assist Devices
5.6. Extracorporeal Life Support Systems
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Diepen, S.; Katz, J.N.; Albert, N.M.; Henry, T.D.; Jacobs, A.K.; Kapur, N.K.; Kilic, A.; Menon, V.; Ohman, E.M.; Sweitzer, N.K.; et al. Contemporary Management of Cardiogenic Shock: A Scientific Statement From the American Heart Association. Circulation 2017, 136, e232–e268. [Google Scholar] [CrossRef] [PubMed]
- De Luca, G.; Suryapranata, H.; Stone, G.W.; Antoniucci, D.; Tcheng, J.E.; Neumann, F.J.; Bonizzoni, E.; Topol, E.J.; Chiariello, M. Relationship between patient’s risk profile and benefits in mortality from ad-junctive abciximab to mechanical revascularization for ST-segment elevation myocardial infarction: A meta-regression analysis of randomized trials. J. Am. Coll. Cardiol. 2006, 47, 685–686. [Google Scholar] [CrossRef] [PubMed]
- De Luca, G.; Navarese, E.P.; Suryapranata, H. A meta-analytic overview of thrombectomy during primary angioplasty. Int. J. Cardiol. 2013, 166, 606–612. [Google Scholar] [CrossRef] [PubMed]
- De Luca, G.; Smits, P.; Hofma, S.H.; Di Lorenzo, E.; Vlachojannis, G.J.; Van’t Hof, A.W.; van Boven, A.J.; Kedhi, E.; Stone, G.W.; Suryapranata, H.; et al. Drug-Eluting Stent in Primary Angioplasty (DESERT 3) cooperation. Everolimus eluting stent vs. first generation drug-eluting stent in primary angioplasty: A pooled patient-level meta-analysis of randomized trials. Int. J. Cardiol. 2017, 244, 121–127. [Google Scholar] [CrossRef]
- Thiele, H.; Ohman, E.M.; de Waha-Thiele, S.; Zeymer, U.; Desch, S. Management of cardiogenic shock complicating myocardial infarction: An update 2019. Eur. Heart J. 2019, 40, 2671–2683. [Google Scholar] [CrossRef]
- De Luca, L.; Savonitto, S. Composite trends of cardiogenic shock complicating acute myocardialinfarction. Eur. J. Heart Fail. 2020, 22, 673–675. [Google Scholar] [CrossRef]
- Aissaoui, N.; Puymirat, E.; Tabone, X.; Charbonnier, B.; Schiele, F.; Lefevre, T.; Durand, E.; Blanchard, D.; Simon, T.; Cambou, J.-P.; et al. Improvedoutcome of cardiogenic shock at the acute stage of myocardial infarction: A report from the USIK 1995, USIC 2000, and FAST-MI French nationwideregistries. Eur. Heart J. 2012, 33, 2535–2543. [Google Scholar] [CrossRef]
- Zeymer, U.; Bueno, H.; Granger, C.B.; Hochman, J.; Huber, K.; Lettino, M.; Price, S.; Schiele, F.; Tubaro, M.; Vranckx, P.; et al. Acute Cardiovascular Care Association position statement for the diagnosis and treatment of patients with acute myocardialinfarctioncomplicated by cardiogenic shock: A document of the Acute Cardio-vascular Care Association of the European Society of Cardiology. Eur. Heart J. Acute Cardiovasc. Care. 2020, 9, 183–197. [Google Scholar]
- De Luca, G.; Suryapranata, H.; Thomas, K.; van’t Hof, A.W.; de Boer, M.J.; Hoorntje, J.C.; Zijlstra, F. Outcome in patients treated with primary angioplasty for acute myocardial infarction due to left main coronary artery occlusion. Am. J. Cardiol. 2003, 91, 235–238. [Google Scholar] [CrossRef]
- De Luca, G.; Savonitto, S.; Greco, C.; Parodi, G.; Ermolli NC, D.; Silva, C.; Lucci, D.; Gonzini, L.; Maggioni, A.P.; Cuccia, C.; et al. BLITZ Investigators.Cardiogenic shock developing in the coronary care unit in pa-tients with ST-elevation myocardial infarction. J. Cardiovasc. Med. 2008, 9, 1023–1029. [Google Scholar] [CrossRef]
- De Luca, G.; van’t Hof, A.W.; de Boer, M.J.; Hoorntje, J.C.; Gosselink, A.M.; Dambrink, J.H.E.; Ottervanger, J.P.; Zijlstra, F.; Suryapranata, H. Impaired myocardial perfusion is a major explanation of the poor outcome observed in patients undergoing primary angioplasty for ST-segment-elevation myocardial infarction and signs of heart failure. Circulation 2004, 109, 958–961. [Google Scholar] [CrossRef]
- De Luca, G.; Gibson, C.M.; Huber, K.; Zeymer, U.; Dudek, D.; Cutlip, D.; Bellandi, F.; Noc, M.; Emre, A.; Zorman, S.; et al. EGYPT Cooperation. Association between advanced Killip class at presentation and impaired myocardial perfusion among patients with ST-segment elevation myocardial infarction treated with primary angioplasty and adjunctive glycoprotein IIb-IIIa inhibitors. Am. Heart J. 2009, 158, 416–421. [Google Scholar] [CrossRef]
- Samsky, M.D.; Morrow, D.A.; Proudfoot, A.G.; Hochman, J.S.; Thiele, H.; Rao, S.V. Cardiogenic Shock After Acute Myocardial Infarction. JAMA 2021, 326, 1840–1850. [Google Scholar] [CrossRef]
- Vincent, J.-L.; e Silva, A.Q.; Couto, L.; Taccone, F.S. The value of blood lactate kinetics in critically ill patients: A systematic review. Crit. Care 2016, 20, 257. [Google Scholar] [CrossRef]
- Lampert, B.C. Right Heart Catheterization. In Encyclopedia of Cardiovascular Researchand Medicine; Vasan, R.S., Sawyer, D.B., Eds.; Elsevier: Oxford, UK, 2018; pp. 298–306. [Google Scholar]
- Nalluri, N.; Patel, N.J.; Atti, V.; Kumar, V.; Basir, M.B.; O’Neill, W.W. Temporal Trends in Utilization of Right-Sided Heart Catheterization Among Percutaneous Ventricular Assist Device Recipients in Acute Myocardial Infarction Complicated by Cardiogenic Shock. Am. J. Cardiol. 2018, 122, 2014–2017. [Google Scholar] [CrossRef]
- Basir, M.B.; Schreiber, T.; Dixon, S.; Alaswad, K.; Patel, K.; Almany, S.; Khandelwal, A.; Hanson, I.; George, A.; Ashbrook, M.; et al. Feasibility of early mechanical circulatory support in acute myocardial infarction complicated by cardiogenic shock: The Detroit cardiogenic shock initiative. Catheter. Cardiovasc. Interv. 2017, 91, 454–461. [Google Scholar] [CrossRef]
- Binanay, C.; Califf, R.M.; Hasselblad, V.; O’Connor, C.M.; Shah, M.R.; Sopko, G.; Stevenson, L.W.; Francis, G.S.; Leier, C.V.; Miller, L.W.; et al. Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness. JAMA 2005, 294, 1625–1633. [Google Scholar] [CrossRef]
- Baran, D.A.; Grines, C.L.; Bailey, S.; Burkhoff, D.; Hall, S.A.; Henry, T.D.; Hollenberg, S.M.; Kapur, N.K.; O’Neill, W.; Ornato, J.P.; et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: This document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter. Cardiovasc. Interv. 2019, 94, 29–37. [Google Scholar] [CrossRef]
- Jentzer, J.C.; van Diepen, S.; Barsness, G.W.; Henry, T.D.; Menon, V.; Rihal, C.S.; Naidu, S.S.; Baran, D.A. Cardiogenic Shock Classification to Predict Mortality in the Cardiac Intensive Care Unit. J. Am. Coll. Cardiol. 2019, 74, 2117–2128. [Google Scholar] [CrossRef]
- Naidu, S.S.; Baran, D.A.; Jentzer, J.C.; Hollenberg, S.M.; van Diepen, S.; Basir, M.B.; Grines, C.L.; Diercks, D.B.; Hall, S.; Kapur, N.K.; et al. SCAI SHOCK Stage Classification Expert Consensus Update: A Review and Incorporation of Validation Studies: This statement was endorsed by the American College of Cardiology (ACC), American College of Emergency Physicians (ACEP), American Heart Association (AHA), European Society of Cardiology (ESC) As-sociation for Acute Cardiovascular Care (ACVC), International Society for Heart and Lung Transplantation (ISHLT), Society of Critical Care Medicine (SCCM), and Society of Thoracic Surgeons (STS) in December 2021. J. Am. Coll. Cardiol. 2022, 79, 933–946. [Google Scholar]
- Pöss, J.; Köster, J.; Fuernau, G.; Eitel, I.; de Waha, S.; Ouarrak, T.; Lassus, J.; Harjola, V.-P.; Zeymer, U.; Thiele, H.; et al. Risk Stratification for Patients in Cardiogenic Shock After Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2017, 69, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Thiele, H.; Zeymer, U.; Neumann, F.-J.; Ferenc, M.; Olbrich, H.-G.; Hausleiter, J.; Richardt, G.; Hennersdorf, M.; Empen, K.; Fuernau, G.; et al. Intraaortic Balloon Support for Myocardial Infarction with Cardiogenic Shock. N. Engl. J. Med. 2012, 367, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Ceglarek, U.; Schellong, P.; Rosolowski, M.; Scholz, M.; Willenberg, A.; Kratzsch, J.; Zeymer, U.; Fuernau, G.; de Waha-Thiele, S.; Büttner, P.; et al. The novel cystatin C, lactate, interleukin-6, and N-terminal pro-B-type natriuretic peptide (CLIP)-based mortality risk score in cardiogenic shock after acute myocardial infarction. Eur. Heart J. 2021, 42, 2344–2352. [Google Scholar] [CrossRef] [PubMed]
- Thiele, H.; Akin, I.; Sandri, M.; Fuernau, G.; De Waha, S.; Meyer-Saraei, R.; Nordbeck, P.; Geisler, T.; Landmesser, U.; Skurk, C.; et al. PCI Strategies in Patients with Acute Myocardial Infarction and Cardiogenic Shock. N. Engl. J. Med. 2017, 377, 2419–2432. [Google Scholar] [CrossRef]
- Auffret, V.; Cottin, Y.; Leurent, G.; Gilard, M.; Beer, J.-C.; Zabalawi, A.; Chagué, F.; Filippi, E.; Brunet, D.; Hacot, J.-P.; et al. Predicting the development of in-hospital cardiogenic shock in patients with ST-segment elevation myocardial infarction treated by primary percutaneous coronary intervention: The ORBI risk score. Eur. Heart J. 2018, 39, 2090–2102. [Google Scholar] [CrossRef]
- van Nunen, L.X.; Veer, M.V.; Schampaert, S.; Rutten, M.C.; van de Vosse, F.N.; Patel, M.R.; Schampaert, S.; Pijls, N.H. Intra-aortic balloon counterpulsation reduces mortality in large anterior myocardial infarction complicated by persistent ischaemia: A CRISP-AMI substudy. Eurointervention 2015, 11, 286–292. [Google Scholar] [CrossRef]
- Combes, A.; Leprince, P.; Luyt, C.-E.; Bonnet, N.; Trouillet, J.-L.; Léger, P.; Pavie, A.; Chastre, J. Outcomes and long-term quality-of-life of patients supported by extracorporeal membrane oxygenation for refractory cardiogenic shock. Crit. Care Med. 2008, 36, 1404–1411. [Google Scholar] [CrossRef]
- Schmidt, M.; Burrell, A.; Roberts, L.; Bailey, M.; Sheldrake, J.; Rycus, P.T.; Hodgson, C.; Scheinkestel, C.; Cooper, D.J.; Thiagarajan, R.R.; et al. Predicting survival after ECMO for refractory cardiogenic shock: The survival after veno-arterial-ECMO (SAVE)-score. Eur. Heart J. 2015, 36, 2246–2256. [Google Scholar] [CrossRef]
- Goldberg, R.J.; Spencer, F.A.; Gore, J.M.; Lessard, D.; Yarzebski, J. Thirty-year trends (1975 to 2005) in the magnitude of, manage-ment of, and hospital death rates associated with cardiogenic shock in patients with acute myocardial infarction: A population-based perspective. Circulation 2009, 119, 1211–1219. [Google Scholar] [CrossRef]
- Nguyen, H.L.; Yarzebski, J.; Lessard, D.; Gore, J.M.; McManus, D.D.; Goldberg, R.J. Ten-Year (2001–2011) Trends in the Incidence Rates and Short-Term Outcomes of Early Versus Late Onset Cardiogenic Shock After Hospitalization for Acute Myocardial Infarction. J. Am. Heart Assoc. 2017, 6, e005566. [Google Scholar] [CrossRef]
- Shah, R.U.; de Lemos, J.A.; Wang, T.Y.; Chen, A.Y.; Thomas, L.; Sutton, N.R.; Fang, J.C.; Scirica, B.M.; Henry, T.D.; Granger, C.B. Post-Hospital Outcomes of Patients With Acute Myocardial Infarction With Cardiogenic Shock. J. Am. Coll. Cardiol. 2016, 67, 739–747. [Google Scholar] [CrossRef]
- Henry, T.D.; Tomey, M.I.; Tamis-Holland, J.E.; Thiele, H.; Rao, S.V.; Menon, V.; Klein, D.G.; Naka, Y.; Piña, I.L.; Kapur, N.K.; et al. Invasive Management of Acute Myocardial Infarction Complicated by Cardiogenic Shock: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e815–e829. [Google Scholar] [CrossRef]
- Hochman, J.S.; Sleeper, L.A.; Webb, J.G.; Sanborn, T.A.; White, H.D.; Talley, J.D.; Christopher, E.B.; Jacobs, A.K.; Slater, J.N.; Col, J.; et al. Early Revascularization in Acute Myocardial Infarction Complicated by Cardiogenic Shock. N. Engl. J. Med. 1999, 341, 625–634. [Google Scholar] [CrossRef]
- Hochman, J.S.; Sleeper, L.A.; White, H.D.; Dzavik, V.; Wong, S.C.; Menon, V.; Webb, J.G.; Steingart, R.; Picard, M.; Menegus, M.A.; et al. One-Year Survival Following Early Revascularization for Cardiogenic Shock. JAMA 2001, 285, 190–192. [Google Scholar] [CrossRef]
- Hochman, J.S.; Sleeper, L.A.; Webb, J.G.; Dzavik, V.; Buller, C.E.; Aylward, P.; Col, J.; White, H.D.; for the SHOCK Investigators. Early Revascularization and Long-term Survival in Cardiogenic Shock Complicating Acute Myocardial Infarction. JAMA 2006, 295, 2511–2515. [Google Scholar] [CrossRef]
- Goldberg, R.J.; Gore, J.M.; Alpert, J.S.; Osganian, V.; de Groot, J.; Bade, J.; Chen, Z.; Frid, D.; Dalen, J.E. Cardiogenic Shock after Acute Myocardial Infarction. Incidence and mortality from a community-wide perspective, 1975 to 1988. N. Engl. J. Med. 1991, 325, 1117–1122. [Google Scholar] [CrossRef]
- Aissaoui, N.; Puymirat, E.; Delmas, C.; Ortuno, S.; Durand, E.; Bataille, V.; Drouet, E.; Bonello, L.; Bonnefoy-Cudraz, E.; Lesmeles, G.; et al. Trends in cardiogenic shock complicating acute myocardial infarction. Eur. J. Heart Fail. 2020, 22, 664–672. [Google Scholar] [CrossRef]
- De Luca, L.; Olivari, Z.; Farina, A.; Gonzini, L.; Lucci, D.; Di Chiara, A.; Casella, G.; Chiarella, F.; Boccanelli, A.; Di Pasquale, G.; et al. Temporal trends in the epidemiology, management, and outcome of patients with cardiogenic shock complicating acute coronary syndromes. Eur. J. Heart Fail. 2015, 17, 1124–1132. [Google Scholar] [CrossRef]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef]
- Kochar, A.; Al-Khalidi, H.R.; Hansen, S.M.; Shavadia, J.S.; Roettig, M.L.; Fordyce, C.B.; Doerfler, S.; Gersh, B.J.; Henry, T.D.; Berger, P.B.; et al. Delays in Primary Percutaneous Coronary Intervention in ST-Segment Elevation Myocardial Infarction Patients Presenting With Cardiogenic Shock. JACC: Cardiovasc. Interv. 2018, 11, 1824–1833. [Google Scholar] [CrossRef] [PubMed]
- Sanborn, T.A.; Sleeper, L.A.; Webb, J.G.; French, J.K.; Bergman, G.; Parikh, M.; Wong, S.; Boland, J.; Pfisterer, M.; Slater, J.N.; et al. Correlates of one-year survival inpatients with cardiogenic shock complicating acute myocardial infarction: Angiographic findings from the SHOCK trial. J. Am. Coll. Cardiol. 2003, 42, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- TRIUMPH Investigators; Alexander, J.H.; Reynolds, H.R.; Stebbins, A.L.; Dzavik, V.; Harrington, R.A.; Van de Werf, F.; Hochman, J.S. Effect of tilarginine acetate in patients with acute myocardial infarction and cardiogenic shock: The TRIUMPH randomized controlled trial. JAMA 2007, 297, 1657–1666. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.H.; Lopes, R.D.; Ballotta, A.; Frigiola, A.; Sketch, M.H.; Bossone, E.; Bates, E.R. Percutaneous coronary intervention or coronary artery bypass surgery for cardiogenic shock and multivessel coronary artery disease? Am. Heart J. 2010, 159, 141–147. [Google Scholar] [CrossRef]
- Thiele, H.; Akin, I.; Sandri, M.; De Waha-Thiele, S.; Meyer-Saraei, R.; Fuernau, G.; Eitel, I.; Nordbeck, P.; Geisler, T.; Landmesser, U.; et al. One-Year Outcomes after PCI Strategies in Cardiogenic Shock. N. Engl. J. Med. 2018, 379, 1699–1710. [Google Scholar] [CrossRef]
- Lee, J.M.; Rhee, T.; Kim, H.K.; Hwang, D.; Lee, S.H.; Choi, K.H.; Kim, J.; Park, T.K.; Yang, J.H.; Bin Song, Y.; et al. Comparison of Long-Term Clinical Outcome Between Multivessel Percutaneous Coronary Intervention Versus Infarct-Related Artery–Only Revascularization for Patients With ST-Segment–Elevation Myocardial Infarction With Cardiogenic Shock. J. Am. Heart Assoc. 2019, 8, e013870. [Google Scholar] [CrossRef]
- De Luca, L.; Tomai, F.; Verdoia, M.; De Luca, G. Evaluation and management of special subgroups after primary percutaneous coronary intervention. Am. Heart J. 2010, 160 (Suppl. S6), S22–S27. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart-Fail. 2016, 18, 891–975. [Google Scholar]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure [published correction appears in EurHeart J. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Unverzagt, S.; Wachsmuth, L.; Hirsch, K.; Thiele, H.; Buerke, M.; Haerting, J.; Werdan, K.; Prondzinsky, R. Inotropic agents and vasodilator strategies for acute myocardial infarction complicated by cardiogenic shock or low cardiac output syndrome. Cochrane Database Syst. Rev. 2014, CD009669. [Google Scholar] [CrossRef]
- Maack, C.; Eschenhagen, T.; Hamdani, N.; Heinzel, F.R.; Lyon, A.R.; Manstein, D.J.; Metzger, J.; Papp, Z.; Tocchetti, C.G.; Yilmaz, M.B.; et al. Treatments targeting inotropy. Eur. Heart J. 2018, 40, 3626–3644. [Google Scholar] [CrossRef]
- De Backer, D.; Biston, P.; Devriendt, J.; Madl, C.; Chochrad, D.; Aldecoa, C.; Brasseur, A.; Defrance, P.; Gottignies, P.; Vincent, J.-L.; et al. Comparison of dopamine and norepinephrine in the treatment of shock. N. Engl. J. Med. 2010, 362, 779–789. [Google Scholar] [CrossRef]
- Levy, B.; Clere-Jehl, R.; Legras, A.; Morichau-Beauchant, T.; Leone, M.; Frederique, G.; Quenot, J.-P.; Kimmoun, A.; Cariou, A.; Lassus, J.; et al. Epinephrine Versus Norepinephrine for Cardiogenic Shock After Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2018, 72, 173–182. [Google Scholar] [CrossRef]
- De Luca, L.; Colucci, W.S.; Nieminen, M.S.; Massie, B.M.; Gheorghiade, M. Evidence-based use of levosimendan in different clinical settings. Eur. Heart J. 2006, 27, 1908–1920. [Google Scholar] [CrossRef]
- Tarvasmäki, T.; Lassus, J.; Varpula, M.; Sionis, A.; Sund, R.; Køber, L.; Spinar, J.; Parissis, J.; Banaszewski, M.; Cardoso, J.S.; et al. Current real-life use of vasopressors and inotropes in cardiogenic shock—Adrenaline use is associated with excess organ injury and mortality. Crit. Care. 2016, 20, 208. [Google Scholar] [CrossRef]
- Delle Karth, G.; Buberl, A.; Geppert, A.; Neunteufl, T.; Huelsmann, M.; Kopp, C.; Nikfardjam, M.; Berger, R.; Heinz, G. Hemodynamic effects of a continuous infusion of levosimendan in critically ill patients with cardiogenic shock requiring catecholamines. Acta Anaesthesiol. Scand. 2003, 47, 1251–1256. [Google Scholar] [CrossRef]
- Felker, G.M.; Benza, R.L.; Chandler, A.B.; Leimberger, J.D.; Cuffe, M.S.; Califf, R.M.; Gheorghiade, M.; O’Connor, C.; OPTIME-CHF Investigators. Heart failure etiology and response to milrinone in decompensated heart failure: Results from the OPTIME-CHF study. J. Am. Coll. Cardiol. 2003, 41, 997–1003. [Google Scholar] [CrossRef]
- Mullens, W.; Abrahams, Z.; Francis, G.S.; Skouri, H.N.; Starling, R.C.; Young, J.B.; Taylor, D.O.; Tang, W.W. Sodium Nitroprusside for Advanced Low-Output Heart Failure. J. Am. Coll. Cardiol. 2008, 52, 200–207. [Google Scholar] [CrossRef]
- Fuernau, G.; Beck, J.; Desch, S.; Eitel, I.; Jung, C.; Erbs, S.; Mangner, N.; Lurz, P.; Fengler, K.; Jobs, A.; et al. Mild Hypothermia in Cardiogenic Shock Complicating Myocardial Infarction. Circulation 2019, 139, 448–457. [Google Scholar] [CrossRef]
- Levy, B.; Girerd, N.; Amour, J.; Besnier, E.; Nesseler, N.; Helms, J.; Delmas, C.; Sonneville, R.; Guidon, C.; Rozec, B.; et al. HYPO-ECMO Trial Group and the International ECMO Network (ECMONet).Effect of Moderate Hypothermia vs. Nor-mothermia on 30-Day Mortality in Patients With Cardiogenic Shock Receiving Venoarterial Extracorporeal Membrane Oxygenation: A Randomized Clinical Trial. JAMA 2022, 327, 442–453. [Google Scholar] [CrossRef]
- Stretch, R.; Sauer, C.M.; Yuh, D.D.; Bonde, P. National Trends in the Utilization of Short-Term Mechanical Circulatory Support. J. Am. Coll. Cardiol. 2014, 64, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Shapero, K.; Ahn, S.S.; Goldsweig, A.M.; Desai, N.; Altin, S.E. Outcomes of mechanical circulatory support for acute myo-cardial infarction complicated by cardiogenic shock. Catheter. Cardiovasc. Interv. 2022, 99, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Prondzinsky, R.; Unverzagt, S.; Russ, M.; Lemm, H.; Swyter, M.; Wegener, N.; Buerke, U.; Raaz, U.; Ebelt, H.; Schlitt, A.; et al. Hemodynamic effects of intra-aortic balloon counterpulsation in patients with acute my-ocardial infarction complicated by cardiogenic shock: The prospective, randomized IABP shock trial. Shock 2012, 37, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Sanborn, T.A.; Sleeper, L.A.; Bates, E.R.; Jacobs, A.; Boland, J.; French, J.K.; Dens, J.; Dzavik, V.; Palmeri, S.T.; Webb, J.G.; et al. Impact of thrombolysis, intra-aortic balloon pump counterpulsation, and their combination in cardiogenic shock complicating acute myocardial infarction: A report from the SHOCK Trial Registry. Should we emergently revascularize Occluded Coronaries for cardiogenicshock? J. Am. Coll. Cardiol. 2000, 36, 1123–1129. [Google Scholar] [CrossRef]
- Barron, H.V.; Every, N.R.; Parsons, L.S.; Angeja, B.; Goldberg, R.J.; Gore, J.M.; Chou, T.M. The use of intra-aortic balloon counterpulsation in patients with cardiogenic shock complicating acute myocardial infarction: Data from the National Registry of Myocardial Infarction 2. Am. Heart J. 2001, 141, 933–939. [Google Scholar] [CrossRef]
- Thiele, H.; Zeymer, U.; Neumann, F.-J.; Ferenc, M.; Olbrich, H.-G.; Hausleiter, J.; de Waha, A.; Richardt, G.; Hennersdorf, M.; Empen, K.; et al. Intra-aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP-SHOCK II): Final 12 month results of a randomised, open-label trial. Lancet 2013, 382, 1638–1645. [Google Scholar] [CrossRef]
- Thiele, H.; Zeymer, U.; Thelemann, N.; Neumann, F.J.; Hausleiter, J.; Abdel-Wahab, M.; Meyer-Saraei, R.; Fuernau, G.; Eitel, I.; Hambrecht, R.; et al. Intraaortic Balloon Pump in Cardiogenic Shock Complicating Acute Myocardial Infarction: Long-Term 6-Year Outcome of the Randomized IABP-SHOCK II Trial. Circulation 2019, 139, 395–403. [Google Scholar] [CrossRef]
- Seyfarth, M.; Sibbing, D.; Bauer, I.; Fröhlich, G.; Bott-Flügel, L.; Byrne, R.; Dirschinger, J.; Kastrati, A.; Schömig, A. A randomizedclinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus in-tra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J. Am. Coll. Cardiol. 2008, 52, 1584–1588. [Google Scholar] [CrossRef]
- Ouweneel, D.M.; Eriksen, E.; Sjauw, K.D.; van Dongen, I.M.; Hirsch, A.; Packer, E.J.; Vis, M.M.; Wykrzykowska, J.J.; Koch, K.T.; Baan, J.; et al. Percutaneous Mechanical Circulatory Support Versus Intra-Aortic Balloon Pump in Cardiogenic Shock After Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2017, 69, 278–287. [Google Scholar] [CrossRef]
- Thiele, H.; Jobs, A.; Ouweneel, D.M.; Henriques, J.P.S.; Seyfarth, M.; Desch, S.; Eitel, I.; Pöss, J.; Fuernau, G.; De Waha, S. Percutaneous short-term active mechanical support devices in cardiogenic shock: A systematic review and collaborative meta-analysis of randomized trials. Eur. Heart J. 2017, 38, 3523–3531. [Google Scholar] [CrossRef]
- Schrage, B.; Ibrahim, K.; Loehn, T.; Werner, N.; Sinning, J.M.; Pappalardo, F.; Pieri, M.; Skurk, C.; Lauten, A.; Landmesser, U.; et al. ImpellaSupport for Acute Myocardial Infarction Complicated by Cardiogenic Shock. Circulation 2019, 139, 1249–1258. [Google Scholar] [CrossRef]
- Amin, A.P.; Spertus, J.A.; Curtis, J.P.; Desai, N.; Masoudi, F.A.; Bach, R.G.; McNeely, C.; Al-Badarin, F.; House, J.A.; Kulkarni, H.; et al. The Evolving Landscape of Impella Use in the United States Among Patients Undergo-ing Percutaneous Coronary Intervention With Mechanical Circulatory Support. Circulation 2020, 141, 273–284. [Google Scholar] [CrossRef]
- Miller, P.E.; Bromfield, S.G.; Ma, Q.; Crawford, G.; Whitney, J.; DeVries, A.; Desai, N.R. Clinical Outcomes and Cost Associated With an Intravascular Microaxial Left Ventricular Assist Device vs. Intra-aortic Balloon Pump in Patients Presenting With Acute Myocardial Infarction Complicated by Cardiogenic Shock. JAMA Intern. Med. 2022, 182, 926. [Google Scholar] [CrossRef]
- Dhruva, S.S.; Ross, J.S.; Mortazavi, B.J.; Hurley, N.C.; Krumholz, H.M.; Curtis, J.P.; Berkowitz, A.P.; Masoudi, F.A.; Messenger, J.C.; Parzynski, C.S.; et al. Use of Mechanical Circulatory Support Devices Among Patients With Acute Myocardial Infarction Complicated by Cardiogenic Shock. JAMA Netw. Open 2021, 4, e2037748. [Google Scholar] [CrossRef]
- Møller, J.E.; Gerke, O.; DanGer Shock Investigators. Danish-German cardiogenic shock trial-DanGer shock: Trial design update. Am. Heart J. 2023, 255, 90–93. [Google Scholar] [CrossRef]
- Anderson, M.B.; Goldstein, J.; Milano, C.; Morris, L.D.; Kormos, R.L.; Bhama, J.; Kapur, N.K.; Bansal, A.; Garcia, J.; Baker, J.N.; et al. Benefits of a novelpercutaneousventricular assist device for right heart failure: The prospective RECOVER RIGHT study of the Impella RP device. J. Heart Lung Transplant. 2015, 34, 1549–1560. [Google Scholar] [CrossRef]
- Briceno, N.; Annamalai, S.K.; Reyelt, L.; Crowley, P.; Qiao, X.; Swain, L.; Pedicini, R.; Foroutanjazi, S.; Jorde, L.; Yesodharan, G.; et al. Left Ventricular Unloading Increases the Coronary Collateral Flow Index Before Reperfusion and Reduces Infarct Size in a Swine Model of Acute Myocardial Infarction. J. Am. Heart Assoc. 2019, 8, e013586. [Google Scholar] [CrossRef]
- Kapur, N.K.; Alkhouli, M.; DeMartini, T.J.; Faraz, H.; George, Z.H.; Goodwin, M.J.; Hernandez-Montfort, J.A.; Iyer, V.S.; Josephy, N.; Kalra, S.; et al. Unloading the Left Ventricle Before Reperfusion in Patients With Anterior ST-Segment–Elevation Myocardial Infarction. Circulation 2019, 139, 337–346. [Google Scholar] [CrossRef]
- Kapur, N.K.; Kim, R.J.; Moses, J.W.; Stone, G.W.; Udelson, J.E.; Ben-Yehuda, O.; Redfors, B.; Issever, M.O.; Josephy, N.; Polak, S.J.; et al. Primary left ventricular unloading with delayed reperfusion in patients with anterior ST-elevation myocardial infarc-tion: Rationale and design of the STEMI-DTU randomized pivotal trial. Am. Heart J. 2022, 254, 122–132. [Google Scholar] [CrossRef]
- Becher, P.M.; Schrage, B.; Sinning, C.R.; Schmack, B.; Fluschnik, N.; Schwarzl, M.; Waldeyer, C.; Lindner, D.; Seiffert, M.; Neumann, J.; et al. Venoarterial Extracorporeal Membrane Oxygenation for Cardiopulmonary Support. Circulation 2018, 138, 2298–2300. [Google Scholar] [CrossRef]
- Guglin, M.; Zucker, M.J.; Bazan, V.M.; Bozkurt, B.; El Banayosy, A.; Estep, J.D.; Gurley, J.; Nelson, K.; Malyala, R.; Panjrath, G.S.; et al. Venoarterial ECMO for Adults. J. Am. Coll. Cardiol. 2019, 73, 698–716. [Google Scholar] [CrossRef] [PubMed]
- Meani, P.; Gelsomino, S.; Natour, E.; Johnson, D.M.; La Rocca, H.-P.B.; Pappalardo, F.; Bidar, E.; Makhoul, M.; Raffa, G.; Heuts, S.; et al. Modalities and Effects of Left Ventricle Unloading on Extracorporeal Life support: A Review of the Current Literature. Eur. J. Heart Fail. 2017, 19, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Russo, J.J.; Aleksova, N.; Pitcher, I.; Couture, E.; Parlow, S.; Faraz, M.; Visintini, S.; Simard, T.; Di Santo, P.; Mathew, R.; et al. Left Ventricular Unloading During Extracorporeal Membrane Oxygenation in Patients With Cardiogenic Shock. J. Am. Coll. Cardiol. 2019, 73, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-S.; Lin, J.-W.; Yu, H.-Y.; Ko, W.-J.; Jerng, J.-S.; Chang, W.-T.; Chen, W.-J.; Huang, S.-C.; Chi, N.-H.; Wang, C.-H.; et al. Cardiopulmonary resuscitation with assisted extracorporeal life-support versus conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest: An observational study and propensity analysis. Lancet 2008, 372, 554–561. [Google Scholar] [CrossRef]
- Yannopoulos, D.; Bartos, J.; Raveendran, G.; Walser, E.; Connett, J.; A Murray, T.; Collins, G.; Zhang, L.; Kalra, R.; Kosmopoulos, M.; et al. Advanced reperfusion strategies for patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation (ARREST): A phase 2, single centre, open-label, randomised controlled trial. Lancet 2020, 396, 1807–1816. [Google Scholar] [CrossRef]
- Belohlavek, J.; Smalcova, J.; Rob, D.; Franek, O.; Smid, O.; Pokorna, M.; Horák, J.; Mrazek, V.; Kovarnik, T.; Zemanek, D.; et al. Effect of Intra-arrest Transport, Extracorporeal Cardiopulmonary Resuscitation, and Immediate Invasive Assessment and Treatment on Functional Neurologic Outcome in Refractory Out-of-Hospital Cardiac Arrest: A Randomized Clinical Trial. JAMA 2022, 327, 737–747. [Google Scholar] [CrossRef]
- Suleiman, T.; Scott, A.; Tong, D.; Khanna, V.; Kunadian, V. Contemporary device management of cardiogenic shock following acute myocardial infarction. Heart Fail. Rev. 2021, 27, 915–925. [Google Scholar] [CrossRef]
- Ostadal, P.; Rokyta, R.; Karasek, J.; Kruger, A.; Vondrakova, D.; Janotka, M.; Naar, J.; Smalcova, J.; Hubatova, M.; Hromadka, M.; et al. Extracorporeal Membrane Oxygenation in the Therapy of Cardiogenic Shock: Results of the ECMO-CS Randomized Clinical Trial. Circulation 2023, 147, 454–464. [Google Scholar] [CrossRef]
- Brunner, S.; Guenther, S.P.; Lackermair, K.; Peterss, S.; Orban, M.; Boulesteix, A.-L.; Michel, S.; Hausleiter, J.; Massberg, S.; Hagl, C. Extracorporeal Life Support in Cardiogenic Shock Complicating Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2019, 73, 2355–2357. [Google Scholar] [CrossRef]
- Ouweneel, D.M.; Schotborgh, J.V.; Limpens, J.; Sjauw, K.D.; Engström, A.E.; Lagrand, W.K.; Cherpanath, T.G.V.; Driessen, A.H.G.; De Mol, B.A.J.M.; Henriques, J.P.S. Extracorporeal life support during cardiac arrest and cardiogenic shock: A systematic review and meta-analysis. Intensiv. Care Med. 2016, 42, 1922–1934. [Google Scholar] [CrossRef]
- Pavasini, R.; Cirillo, C.; Campo, G.; Menezes, M.N.; Biscaglia, S.; Tonet, E.; Ferrari, R.; Patel, B.V.; Price, S. Extracorporeal Circulatory Support in Acute Coronary Syndromes. Crit. Care Med. 2017, 45, e1173–e1183. [Google Scholar] [CrossRef]
- Cheng, R.; Hachamovitch, R.; Kittleson, M.; Patel, J.; Arabia, F.; Moriguchi, J.; Esmailian, F.; Azarbal, B. Complications of Extracorporeal Membrane Oxygenation for Treatment of Cardiogenic Shock and Cardiac Arrest: A Meta-Analysis of 1866 Adult Patients. Ann. Thorac. Surg. 2014, 97, 610–616. [Google Scholar] [CrossRef]
- Banning, A.S.; Adriaenssens, T.; Berry, C.; Bogaerts, K.; Erglis, A.; Distelmaier, K.; Guagliumi, G.; Haine, S.; Kastrati, A.; Massberg, S.; et al. Veno-arterial extracorporeal membrane oxygenation (ECMO) in patients with cardiogenic shock: Rationale and design of the randomised, multicentre, open-label EURO SHOCK trial. Eurointervention 2021, 16, e1227–e1236. [Google Scholar] [CrossRef]
- Zeymer, U.; Thiele, H. What to expect from upcoming MCS randomized trials? Eur. Heart J. Acute. Cardiovasc. Care 2022, 11, 841–843. [Google Scholar] [CrossRef]
- Assessment of ECMO in Acute Myocardial Infarction Cardiogenic Shock (ANCHOR). Available online: https://clinicaltrials.gov/ct2/show/NCT04184635 (accessed on 5 February 2023).
- Thiele, H.; Freund, A.; Gimenez, M.R.; de Waha-Thiele, S.; Akin, I.; Pöss, J.; Feistritzer, H.-J.; Fuernau, G.; Graf, T.; Nef, H.; et al. Extracorporeal life support in patients with acute myocardial infarction complicated by cardiogenic shock—Design and rationale of the ECLS-SHOCK trial. Am. Heart J. 2021, 234, 1–11. [Google Scholar] [CrossRef]

| Shock | Iabp Shock II | Culprit-Shock | Esc Heart Failure Guidelines | Orbi Risk Score |
|---|---|---|---|---|
|
|
| SBP < 90 mmHg with adequate volume and clinical or laboratory signs of hypoperfusion Clinical hypoperfusion: Cold extremities, oliguria, mental confusion, dizziness, and narrow pulse pressure. Laboratory hypoperfusion: Metabolic acidosis Elevated lactate Elevated creatinine | SBP ≤ 90 mmHg for >30 min following exclusion of hypovolaemia, with clinical evidence of hypoperfusion, inotrope dependence, or mechanical left ventricular support to correct this situation |
| Iabp | Impella | Tandemheart | Ecmo | |
|---|---|---|---|---|
| Structure | Pneumatic pump | Axial pump | Centrifugal pump | Centrifugal pump |
| Cannula size | 7–8 Fr | 14 Fr (Impella CP) 21 Fr (Impella 5.0) | 21 Fr venous (inflow) 12–19 Fr arterial (outflow) | 17–21 Fr venous (inflow) 14–19 Fr artery (outflow) |
| Insertion/Placement | Femoral artery | Femoral artery (CP) Transaortic/Transubclavian (for Impella 5.5) | Femoral artery Femoral vein for left atrial access | Femoral artery Femoral vein |
| Cardiac output | 0.5–0.8 L/min | 3.7–5 L/min | 4.5 L/min | >4.5 L/min |
| Max. no. of implantation days | weeks | 5–7 day (up to 30 days for Impella 5.5) | 14 day | weeks |
| Complexity of insertion | low | Medium (High for Impella 5.0) | high | Medium |
| Risk of hemolysis | low | Moderate | Moderate | Moderate |
| Risk of complications: (1) Ischemic (2) Hemorrhagic (3) Infectious | Low Low Low | Moderate (High for Impella 5.0) Moderate Low (moderate for Impella 5.0) | High High Moderate | High High Moderate |
| MCS | Trial | Study Population | Active Treatment and Comparator | Primary Study Endpoint |
|---|---|---|---|---|
| Impella CP® | DanGer Shock (77) NCT01633502 | 330 STEMI patients complicated by CS patients undergoing PCI | Impella CP vs. conventional circulatory support | death from all causes through 180 days |
| Impella CP® | STEMI-DTU (81) NCT03947619 | 668 patients with anterior STEMI | Impella CP placement prior to reperfusion with primary PCI vs. primary PCI alone | Infarct size (evaluated by cardiac magnetic resonance at 3–5 days after PCI) |
| VA ECMO | ANCHOR (97) NCT04184635 | 400 patients with STEMI complicated by CS | ECMO and IABP vs. optimal medical therapy | Treatment failure at Day 30 |
| VA ECMO | ECLS-SHOCK (98) NCT03637205 | 420 patients with STEMI complicated by CS | ECMO vs. optimal medical therapy (in addition to early revascularization) | 30-day mortality |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Luca, L.; Mistrulli, R.; Scirpa, R.; Thiele, H.; De Luca, G. Contemporary Management of Cardiogenic Shock Complicating Acute Myocardial Infarction. J. Clin. Med. 2023, 12, 2184. https://doi.org/10.3390/jcm12062184
De Luca L, Mistrulli R, Scirpa R, Thiele H, De Luca G. Contemporary Management of Cardiogenic Shock Complicating Acute Myocardial Infarction. Journal of Clinical Medicine. 2023; 12(6):2184. https://doi.org/10.3390/jcm12062184
Chicago/Turabian StyleDe Luca, Leonardo, Raffaella Mistrulli, Riccardo Scirpa, Holger Thiele, and Giuseppe De Luca. 2023. "Contemporary Management of Cardiogenic Shock Complicating Acute Myocardial Infarction" Journal of Clinical Medicine 12, no. 6: 2184. https://doi.org/10.3390/jcm12062184
APA StyleDe Luca, L., Mistrulli, R., Scirpa, R., Thiele, H., & De Luca, G. (2023). Contemporary Management of Cardiogenic Shock Complicating Acute Myocardial Infarction. Journal of Clinical Medicine, 12(6), 2184. https://doi.org/10.3390/jcm12062184







