Patient-Reported Outcome Measures of Psychosocial Quality of Life in Oropharyngeal Cancer Patients: A Scoping Review
Abstract
1. Introduction
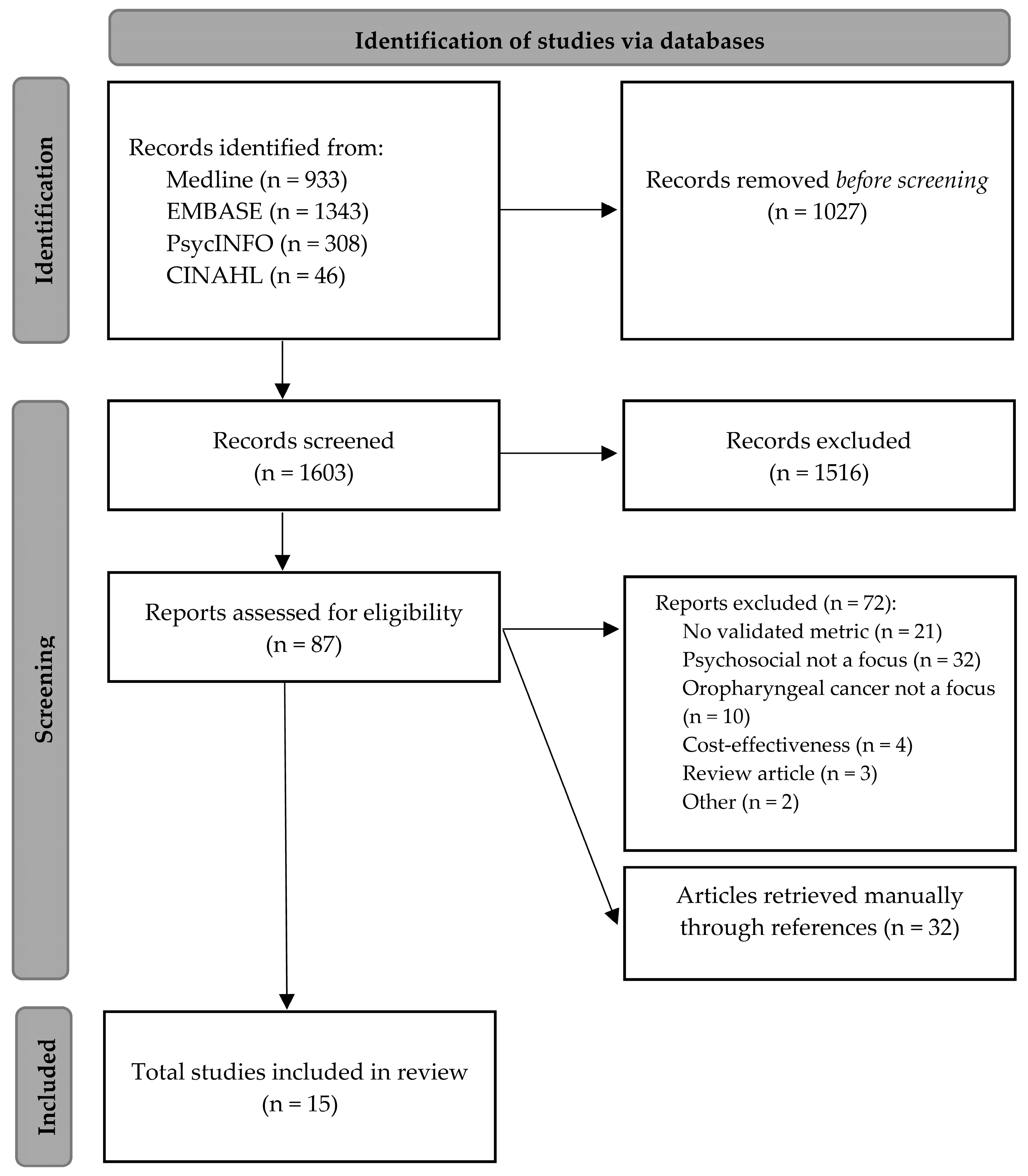
2. Methodology
2.1. Identifying the Research Question
2.2. Identifying Relevant Studies
2.3. Study Selection
2.4. Data Extraction
2.5. Collation, Summarizing and Reporting the Results
3. Results
3.1. Study Population and Demographics
3.2. Quality Assessment
3.3. QOL Metrics
3.4. Identification of Psychosocial QOL Themes and Thematic Analysis in Oropharyngeal Cancer Patients
3.4.1. Mental Health and Emotional Wellbeing
3.4.2. Social Wellbeing and Function
3.4.3. Stress
| Primary Author, Year | Study Design | Country | Participant Characteristics | Comparator | HPV/P16 Status of Participants | Cancer Stage | Treatment | Time Period | PROM | Summary of Results |
|---|---|---|---|---|---|---|---|---|---|---|
| Mental health and emotional wellbeing | ||||||||||
| Berg, 2021 [30] | Cross-sectional | Sweden | 190 patients with BOT cancer, aged 33–84 (median 63), 137 male, 53 female | Patients with tonsillar cancer, general population | Positive: 131 Negative: 20 Missing: 39 | Stage I-II: 27 Stage III-IV: 162 Missing: 1 (AJCC 7th edition) | RT: 56 CRT: 85 Surgery ± RT: 34 Surgery + CRT: 14 No adequate treatment: 1 | 15 months post-treatment | EORTC QLQ-C30, EORTC QLQ-H&N35 | Emotional function is higher in general population and in males, worse in HPV negative patients, same in tonsil cancer patients. |
| Casswell, 2021 [35] | Cross-sectional | Australia | 136 patients with HPV-associated oropharyngeal cancer, aged 42–87 (median 61), 114 male, 22 female | N/A | Positive: 136/136 | Stage I: 74 Stage II: 22 Stage III: 40 (AJCC 8th edition) | RT: 16 CRT: 120 Salvage surgery: 1 | Mean 2.8 years post-treatment (range 1–5.5 years) | EORTC QLQ-C30, MDASI-HN, PROMIS, Fear of Cancer Recurrence Inventory | Moderate levels of anxiety and depression were reported in 11% and 4% of patients, respectively. Severe levels of anxiety and depression were both reported in 1% of patients, respectively. PROMIS anxiety and depression scores were significantly associated with fear of cancer recurrence scores. |
| Janz, 2019 [28] | Prospective | USA | 21 patients with HPV-associated oropharyngeal cancer, aged 49–76 (mean 58.2), 19 male, 2 female | 17 patients with oral cavity cancer who smoke aged 32–76 (mean 55), 9 male, 8 female | Oropharynx cohort—Positive: 21/21 Oral cavity cohort—Positive 0/17 | Stage IV (oropharynx): 16 Stage IV (oral cavity): 11 (AJCC 7th edition) | Surgery: 13 RT: 16 Chemotx: 17 Combination therapies: Surgery + RT:2 CRT: 5 Surgery + CRT: 9 Other: 3 | 12 month follow-up | Cancer worry “Assessment of Survivor Concerns” instrument, CES-D, Cancer Behavior Inventory | At baseline: there was no difference in depression score between HPV positive OPSCC patients and smoking oral cavity patients (p = 0.041) At 12-months: depression decreased over time for the HPV positive cohort (p = 0.03) |
| Kaffenberger, 2021 [27] | Retrospective | USA | 44 patients with advanced oropharyngeal cancer treated with curative intent treated with primary CRT, with a mean age of 57.6, 37 male, 7 female | 29 patients with advanced oropharyngeal cancer treated with curative intent treated with surgery and adjuvant RT/CRT, with a mean age of 56.7, 25 male, 4 female | Positive: 66/73 Negative: 3/73 Unknown: 4/73 | Stage III: 10 Stage IVa: 62 Stage IVb: 1 (AJCC 7th edition) | CRT: 44 Surgery + RT: 9 Surgery + CRT: 20 | Median follow-up post treatment 29.7 months (range 6.1–133 months) | UW-QOL, PHQ-8, GAD-7, NDI, EAT-10 | On PHQ-8: no significant difference in depression scores between groups (p = 0.71) On GAD-7: no significant difference in anxiety scores between groups (p = 0.77), mean dose of RT delivered to the ipsilateral parotid correlated to more anxiety symptoms. |
| Korsten, 2021 [32] | Prospective | The Netherlands | 78 patients with HPV-associated oropharyngeal cancer, mean age 59.9, 59 male, 19 female | 120 patients with HPV-negative oropharyngeal cancer, mean age 59.9, 120 male, 72 female | Positive: 78/270 | Stage I: 37 Stage II: 57 Stage III: 59 Stave IV: 103 (AJCC 7th edition) | RT: 99 Surgery: 4 Combination: 89 | 24 months | EORTC QLQ-C30, EORTC QLQ-H&N35 | Emotional functioning mean scores were equal at baseline, 6 weeks and 3 months after treatment between HPV-positive and negative cohorts (p = 0.039). Scores improved more in HPV-positive patients at 6, 12, and 24 months compared HPV-negative patients. |
| Lee, 2022 [33] | Cross-sectional | USA | 25 patients with HPV-associated oropharyngeal cancer, aged 41–80 (median 58), 23 male, 2 female | N/A | Positive: 25/25 | Stage II: 1 Stage III: 2 Stage IVa: 21 Stage IVb: 1 (AJCC 7th edition | All received neoadjuvant chemotherapy and transoral robotic surgery | Mean 4.3 years (2.0–7.6 years) | UW-QOL | Patients treated with this protocol reported less anxiety compared to the normative cohort, demonstrating near-normal recovery in long-term outcomes (p = 0.005). There was no significant difference in mood scores of trial participants compared to controls (p = 0.288). |
| McDowell, 2021 [36] | Cross-sectional | Australia | 136 patients with HPV-associated oropharyngeal cancer, aged 42-87 (median 61), 114 male, 22 female | N/A | Positive: 136/136 | Stage I: 74 Stage II: 22 Stage III: 40 (AJCC 8th edition) | RT: 16 CRT: 120 Salvage surgery: 1 | Mean 2.8 years post-treatment (range 1–5.5 years) | EORTC QLQ-C30, MDASI-HN, PROMIS, Fear of Cancer Recurrence Inventory | Anxiety (t-score 53.5 vs. 44.1, d = 0.80), and depression (t-score 42.8 vs. 51.3, d = 0.84) scores were significantly worse in the low functioning subgroup. PROMIS anxiety score: normal/low: 88.9%, moderate: 9.6%, severe: 1.5% PROMIS depression score: normal/low: 95.6%, moderate: 3.7%, severe: 0.7%. Increasing age is associated with worse anxiety scores (−0.2/year increase, p = 0.034) |
| Qualliotine, 2017 [31] | Retrospective | USA | 65 patients with oropharyngeal cancer between October 2011 and September 2014 who had completed the depression screening questionnaire prior to treatment, aged 44-88 (median 59.9), 55 male, 10 female | N/A | Positive: 50 Negative 15 | Stage I or II: 4 Stage III or IV: 61 (AJCC 7th edition) | N/A | N/A | CES-D | A lower proportion of HPV-associated OPSCC patients than HPV-negative patients reported using antidepressants (8% vs. 27%, p = 0.05). 44.9% of the patients screened positive for depression. No association of depression score and HPV status. |
| Rajeev-Kumar, 2019 [29] | Retrospective | USA | 69 patients treated with curative intent RT between 2013 and 2016 with up to 3 year follow-up, with a mean age of 58.3, 51 male, 18 female | N/A | Positive: 43 Negative: 26 | Stage I: 4 Stage II: 7 Stage III: 12 Stage IVa: 41 Stage IVb: 4 (AJCC 7th edition) | Pre-RT surgery: 37 RT: 69 Induction chemotx: 16 Concurrent CRT 38 | 12 months post-RT | UW-QOL | Of the 51 patients with active alcohol use, 11.8% had a severe mood score and 33.3% had a severe anxiety score before starting RT. After 12 months, 88% of those patients returned to baseline or better mood (only 52% response). At consultation, anxiety was worse than mood score. At 12 months, anxiety remained mildly worse than mood but both were better than pre-treatment. Multivariate regression: no association between worse emotional status and patient/disease characteristics at 12 months, PEG placement, surgery versus CRT, HPV infection. Longer duration of treatment is more likely to be associated with worse mood (>50 days of treatment). Physical symptom worsening is associated with worse anxiety (taste scores, saliva scores) and with worse mood (swallow scores). |
| Shinn, 2016 [34] | Prospective | USA | 130 patients diagnosed with new diagnosis of oropharyngeal cancer between March 2005 and June 2007 treated with RT, aged 28.4–78.5 (mean 56.8), 94 male and 108 male, 22 female | N/A | Positive: 15/22 Negative 7/22 (Only 22 patients tested) | Stage I or II: 10 Stage III or IV: 119 Missing: 1 (AJCC 7th edition) | RT: 130 Neoadjuvant chemotx: 47 Concurrent CRT: 51 | Median of 4.9 years (range of 0.1–6 years) | PHQ-9, CES-D | 19 patients (15%) screened positive for depression at baseline. In the univariate analysis of the PHQ-9, depression’s association with survival was borderline (p = 0.061) but significant in the multivariate analysis (p = 0.022). Dichotomized, PHQ-9 positive depression was associated with overall survival (p = 0.022). As a multivariate model, for every increased unit of the PHQ-9, the risk for reduced survival increased by a factor of 10%. Depression was associated with disease recurrence in univariate (p = 0.028) and multivariate analysis (p = 0.025). For every increased unit of the PHQ-9, the risk for recurrence increased by a factor of 10%. No association of HPV status and depression |
| Social wellbeing and function | ||||||||||
| Berg, 2021 [30] | Cross-sectional | Sweden | 190 patients with BOT cancer, aged 33–84 (median 63), 137 male, 53 female | Patients with tonsillar cancer, general population | Positive: 131 Negative: 20 Missing: 39 | Stage I–II: 27 Stage III–IV: 162 Missing: 1 (AJCC 7th edition) | RT: 56 CRT: 85 Surgery ± RT: 34 Surgery + CRT: 14 No adequate treatment: 1 | 15 months post-treatment | EORTC QLQ-C30, EORTC QLQ-H&N35 | Compared to the general population, BOT patients have worse social function (p < 0.001), social eating (p < 0.001), social contact (p < 0.001). No difference in social domains in BOT patients who are stage I-II versus III-IV, males versus females, HPV+ versus HPV-, different treatment modalities or adjuvant treatment regimens. Patients with BOT cancer had worse social eating scores than patients with tonsil cancer (p = 0.001). |
| Dziegielewski, 2013 [38] | Prospective | USA | 81 patients with oropharyngeal cancer treated with transoral robotic surgery | N/A | HPV positive: 51 HPV negative: 20 p16 positive: 60 p16 negative: 11 Missing: 10 | Stage I: 7 Stage III: 9 Stage IV: 63 Missing: 2 (AJCC 7th edition | Surgery: 81 Adjuvant RT: 69 Adjuvant CRT: 49 | 12 month post-operatively | HNCI | All health-related quality of life scores declined at 3 weeks post = operatively, including social scores, which continued to drop but reached the nadir at 3 months. Social scores recovered and were indifferent from baseline (p > 0.05) at 12 months. No difference of social function (p = 0.81) or social attitude (p = 0.57) when in HPV+ or HPV-patients. |
| Kaffenberger, 2021 [27] | Retrospective | USA | 44 patients with advanced oropharyngeal cancer treated with curative intent treated with primary CRT, with a mean age of 57.6, 37 male, 7 female | 29 patients with advanced oropharyngeal cancer treated with curative intent treated with surgery and adjuvant RT/CRT, with a mean age of 56.7, 25 male, 4 female | Positive: 66/73 Negative: 3/73 Unknown: 4/73 | Stage III: 10 Stage IVa: 62 Stage IVb: 1 (AJCC 7th edition) | CRT: 44 Surgery + RT: 9 Surgery + CRT: 20 | Median follow-up post treatment 29.7 months (range 6.1–133 months) | UW-QOL, PHQ-8, GAD-7, NDI, EAT-10 | The mean dose delivered to the ipsilateral parotid gland was correlated with worse scores on the social aspects of the UWQOL No difference in social score based on treatment modality. |
| Korsten, 2021 [32] | Prospective | Canada | 78 patients with HPV-associated oropharyngeal cancer, mean age 59.9, 59 male, 19 female | 120 patients with HPV-negative oropharyngeal cancer, mean age 59.9, 120 male, 72 female | 78/270 | Stage I: 37 Stage II: 57 Stage III: 59 Stave IV: 103 (AJCC 7th edition) | RT: 99 Surgery: 4 Combination: 89 | 24 months | EORTC QLQ-C30, EORTC QLQ-H&N35 | For HPV-associated patients, social functioning was better before treatment, worsened during treatment, and recovered better and faster at follow-up compared to patients with an HPV-negative cancer (p = 0.033). On mixed model analysis, social contact and social eating did not demonstrate a significant difference between HPV-positive and negative patients. |
| Stress | ||||||||||
| Casswell, 2021 [35] | Cross-sectional | Australia | 136 patients with HPV-associated oropharyngeal cancer, aged 42-87 (median 61), 114 male, 22 female | N/A | Positive: 136/136 | Stage I: 74 Stage II: 22 Stage III: 40 (AJCC 8th edition) | RT: 16 CRT: 120 Salvage surgery: 1 | Mean 2.8 years post-treatment (range 1–5.5 years) | EORTC QLQ-C30, MDASI-HN, PROMIS, Fear of Cancer Recurrence Inventory | Clinically significant fear of cancer recurrence was reported in 53% of patients (72/135). Younger patients were more likely to report high fear of cancer recurrence (−0.9/5 years; p = 0.031). Those with higher fear of cancer recurrence also had lower global QOL (−0.8/10 unit increase; p = 0.012), had higher symptom interference with daily activities (0.8/unit increase; p = 0.17) (MDASI-HN), and greater anxiety (0.4/unit; p < 0.001) and depression scores (0.3/unit; p < 0.001) (PROMIS). |
| Dziegielewski, 2013 [38] | Prospective | USA | 81 patients with oropharyngeal cancer treated with transoral robotic surgery | N/A | HPV positive: 51 HPV negative: 20 p16 positive: 60 p16 negative: 11 Missing: 10 | Stage I: 7 Stage III: 9 Stage IV: 63 Missing: 2 (AJCC 7th edition | Surgery: 81 Adjuvant RT: 69 Adjuvant CRT: 49 | 12 month post-operatively | HNCI | There was a significant change of overall attitude from baseline, but small clinically important difference and a good recovery at 12 months. No difference of overall attitude in HPV+ or HPV− patients (p = 0.56). Significant differences in overall attitude in patients who received adjuvant RT (p = 0.003) and those receiving adjuvant CRT (p = 0.04). |
| Goepfert, 2017 [40] | Cross-sectional | USA | 935 patients diagnosed with oropharyngeal cancer between January 2000 and December 2014, aged 32–84 (median 56), 791 male, 144 female | N/A | Positive: 456 Negative: 59 Unknown: 420 | RT alone: 276 CRT: 628 Surgery alone: 8 Surgery + CRT: 17 RT + salvage surgery: 6 | 1.5–15.6 years (median 6) | Decision regret scale, MDASI-HN | Patients reported a low level of decisional regret: mean score of 12.7/100 = “mild” 38.6% had no regret, 45.8% had “mild” regret, 15.5% of cohort had ”mod-strong” regret Regret significantly associated with higher T classification, combination treatment (surgery + RT/CRT), smoking at diagnosis, high MDASI-HN symptom score (associated with dysphagia symptom). | |
| Janz, 2019 [28] | Prospective | USA | 21 patients with HPV-associated oropharyngeal cancer, aged 49–76 (mean 58.2), 19 male, 2 female | 17 patients with oral cavity cancer who smoke aged 32–76 (mean 55), 9 male, 8 female | Oropharynx cohort—Positive: 21/21 Oral cavity cohort—Positive 0/17 | Stage IV (oropharynx): 16 Stage IV (oral cavity): 11 (AJCC 7th edition) | Surgery: 13 RT: 16 Chemotx: 17 Combination therapiess: Surgery + RT:2 CRT: 5 Surgery + CRT: 9 Other: 3 | 12 month follow-up | Assessment of Survivor Concerns instrument, CES-D, Cancer Behavior Inventory | At baseline, the HPV+ OPSCC patients had a mean cancer worry score of 2.8 and the oral cavity cohort had a score of 3.25 (p = 0.1) At baseline, the HPV+ OPSCC patients had a self-efficacy score of 97.8 and the oral cavity cohort had a scope of 96.3 (p = 0.79) Cancer worry decreased over time but was not statistically significant (2.8 to 2.4, p = 0.11) |
| Shaverdian, 2019 [39] | Retrospective | USA | 24 consecutive patients enrolled in the CCRO-22 phase II clinical trial for locally advanced HPV-positive oropharyngeal cancer between March 2014 to March 2015, aged 49–83 (median 62), 21 male, 3 female. | N/A | Positive: 24 | Stage III/IV: 24 (AJCC 7th edition) | Induction chemotherapy: 24 CRT: 24 (15 = 54 Gy, 10 = 60 Gy) | 24 months (range of 16–30 months) | Decision Regret Scale, Chicago Priorities Scale | 83% were “totally satisfied” with their treatment and its result. 17% said that they were “somewhat satisfied”. None had any level of dissatisfaction with the treatment. 92% “strongly agree” that their decision to proceed with de-escalated therapy was the “right decision”, 8% “agree”. 92% strongly disagreeing to the statement “I regret the choice I made”, none “agree” or “strongly agree”. 75% “strongly agree” with the statement “I would go for the same choice if I had to do it again”, 21% “agree” and the remaining 1 patient selected “neither agree nor disagree”. 92% “strongly agree” that their decision to receive de-escalated therapy was a “wise one”, with the remaining 8% patients selecting “agree”. The fear of disease recurrence was greater than expected in 42%, as expected in 33% and less than originally expected in 25%. |
| Relationship and sexual behavior | ||||||||||
| Berg, 2021 [30] | Cross-sectional | Sweden | 190 patients with BOT cancer, aged 33–84 (median 63), 137 male, 53 female | Patients with tonsillar cancer, general population | Positive: 131 Negative: 20 Missing: 39 | Stage I–II: 27 Stage III–IV: 162 Missing: 1 (AJCC 7th edition) | RT: 56 CRT: 85 Surgery ± RT: 34 Surgery + CRT: 14 No adequate treatment: 1 | 15 months post-treatment | EORTC QLQ-C30, EORTC QLQ-H & N35 | BOT cancer patients treated with radiotherapy alone reported worse sexuality scores than those treated with surgery and adjuvant CRT (40 versus 28). BOT cancer patients have worse (but not statistically significant) sexuality than the general population (p = 0.002), from tonsillar cancer patients (p = 0.16), comparing genders (p = 0.27), nor tumor stage (p = 0.44). HPV-negative patients report worse sexuality than HPV-positive patients (p = 0.05) |
| Casswell, 2021 [37] | Cross-sectional | Australia | 136 patients with HPV-associated oropharyngeal cancer, aged 42–87 (median 61), 114 male, 22 female | N/A | Positive: 136/136 | Stage I: 74 Stage II: 22 Stage III: 40 (AJCC 8th edition) | RT: 16 CRT: 120 Salvage surgery: 1 | Mean 2.8 years post-treatment (range 1–5.5 years) | EORTC QLQ-C30, EORTC QLQ-SH22, MDASI-HN, PROMIS, Fear of Cancer Recurrence Inventory | An active sex life was considered important to the majority of survivors (60%) Only 20% of patients reported “quite a bit”/”very much” sexual activity in the 4 weeks prior Among those that reported high importance of an active sex life, 72% reported “little to no sexual activity” No difference in importance of sexual activity or recent sexual activity in patients who reported knowing if their cancer was caused by HPV Patients aware of the HPV association did not report negative changes more frequently in their general relationship (20% versus 7%), nor in their sexual relationship (39% versus 39%). |
| Taberna, 2017 [41] | Prospective | USA | 172 patients with oropharyngeal cancer who self-reported that they were in a partnered relationship, aged 18–89, 125 male, 17 female (HPV+ cohort demographics) | 90 patients with oral cavity cancer 81 partners of patients with oropharyngeal cancer | Positive: 142 Negative: 30 | HPV+ cohort: Stage I: 5 Stage II: 7 Stage III: 43 Stage IV: 78 (AJCC 7th edition) | HPV+ cohort; Surgery: 45 CRT: 89 RT: 7 Chemo 1 Unknown 1 | 6-month follow up | Dyadic Adjustment Scale | Few patients or partners reported distressed relationships at baseline or at 6-months, with no significant difference when analyzed by HPV-status. Patients reported high relationship satisfaction; confided in their partner almost always (>85%), rarely/never regretted the relationship (~95%), and had high confidence in the latter (>75%). Strong majorities also described their relationships as happy/very happy (>90%). Demonstrations of affection: >65% agreed with their partner about sexual relations. The majority reported no issues in the relationship with regards to being too tired for sex (>65%) or not showing love (>80%). Very few patients reported relationship distress (T-score ≤ 40) in any subscale. 38% of HPV-positive patients reported that their relationship with their partner had not changed. When a change was perceived, it was generally positive, namely feeling supported by their partner (92%) and that their relationship had become stronger (69%). Approximately 25% of patients either blamed themselves for their cancer diagnosis (26%) or felt guilty about exposing their partner to HPV (28%). |
3.4.4. Relationship and Sexual Behavior
3.5. Association of Psychosocial QOL and Treatment Modality
3.6. Association of Psychosocial QOL and HPV Status
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaturvedi, A.K.; Engels, E.A.; Pfeiffer, R.M.; Hernandez, B.Y.; Xiao, W.; Kim, E.; Jiang, B.; Goodman, M.T.; Sibug-Saber, M.; Cozen, W.; et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J. Clin. Oncol. 2011, 29, 4294–4301. [Google Scholar] [CrossRef]
- Gillison, M.L.; Chaturvedi, A.K.; Anderson, W.F.; Fakhry, C. Epidemiology of Human Papillomavirus-Positive Head and Neck Squamous Cell Carcinoma. J. Clin. Oncol. 2015, 33, 3235–3242. [Google Scholar] [CrossRef]
- Blitzer, G.C.; Smith, M.A.; Harris, S.L.; Kimple, R.J. Review of the clinical and biologic aspects of human papillomavirus-positive squamous cell carcinomas of the head and neck. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 761–770. [Google Scholar] [CrossRef]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef]
- Fakhry, C.; Westra, W.H.; Li, S.; Cmelak, A.; Ridge, J.A.; Pinto, H.; Forastiere, A.; Gillison, M.L. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J. Natl. Cancer Inst. 2008, 100, 261–269. [Google Scholar] [CrossRef]
- Fakhry, C.; Andersen, K.K.; Eisele, D.W.; Gillison, M.L. Oropharyngeal cancer survivorship in Denmark, 1977–2012. Oral Oncol. 2015, 51, 982–984. [Google Scholar] [CrossRef]
- Ledeboer, Q.C.; Velden, L.A.; Boer, M.F.; Feenstra, L.; Pruyn, J.F. Physical and psychosocial correlates of head and neck cancer: An update of the literature and challenges for the future (1996–2003). Clin. Otolaryngol. 2005, 30, 303–319. [Google Scholar] [CrossRef]
- Hammerlid, E.; Bjordal, K.; Ahlner-Elmqvist, M.; Boysen, M.; Evensen, J.F.; Biörklund, A.; Jannert, M.; Kaasa, S.; Sullivan, M.; Westin, T. A prospective study of quality of life in head and neck cancer patients. Part I: At diagnosis. Laryngoscope 2001, 111, 669–680. [Google Scholar] [CrossRef]
- De Boer, M.F.; McCormick, L.K.; Pruyn, J.F.; Ryckman, R.M.; van den Borne, B.W. Physical and psychosocial correlates of head and neck cancer: A review of the literature. Otolaryngol. Head Neck Surg. 1999, 120, 427–436. [Google Scholar] [CrossRef]
- Infante-Cossio, P.; Torres-Carranza, E.; Cayuela, A.; Hens-Aumente, E.; Pastor-Gaitan, P.; Gutierrez-Perez, J.L. Impact of treatment on quality of life for oral and oropharyngeal carcinoma. Int. J. Oral Maxillofac. Surg. 2009, 38, 1052–1058. [Google Scholar] [CrossRef]
- Bahig, H.; Lambert, L.; Filion, E.; Soulières, D.; Guertin, L.; Ayad, T.; Christopoulos, A.; Bissada, E.; Alizadeh, M.; Bélair, M.; et al. Phase II study of de-intensified intensity-modulated radiotherapy and concurrent carboplatin/5-fluorouracil in lateralized p16-associated oropharyngeal carcinoma. Head Neck 2020, 42, 3479–3489. [Google Scholar] [CrossRef]
- Silver, J.A.; Turkdogan, S.; Roy, C.F.; Subramaniam, T.; Henry, M.; Sadeghi, N. De-Escalation Strategies for Human Papillomavirus-Associated Oropharyngeal Squamous Cell Carcinoma-Where Are We Now? Curr. Oncol. 2022, 29, 3668–3697. [Google Scholar] [CrossRef]
- Price, K.A.R.; Nichols, A.C.; Shen, C.J.; Rammal, A.; Lang, P.; Palma, D.A.; Rosenberg, A.J.; Chera, B.S.; Agrawal, N. Novel Strategies to Effectively De-escalate Curative-Intent Therapy for Patients With HPV-Associated Oropharyngeal Cancer: Current and Future Directions. Am. Soc. Clin. Oncol. Educ. Book 2020, 40, 257–269. [Google Scholar] [CrossRef]
- Murphy, B.A.; Ridner, S.; Wells, N.; Dietrich, M. Quality of life research in head and neck cancer: A review of the current state of the science. Crit. Rev. Oncol. Hematol. 2007, 62, 251–267. [Google Scholar] [CrossRef]
- WHO Quality of Life Assessment Group. What Quality of Life? World Health Forum: Geneva, Switzerland, 1996. [Google Scholar]
- Sehlen, S.; Lenk, M.; Herschbach, P.; Aydemir, U.; Dellian, M.; Schymura, B.; Hollenhorst, H.; Dühmke, E. Depressive symptoms during and after radiotherapy for head and neck cancer. Head Neck 2003, 25, 1004–1018. [Google Scholar] [CrossRef]
- Kam, D.; Salib, A.; Gorgy, G.; Patel, T.D.; Carniol, E.T.; Eloy, J.A.; Baredes, S.; Park, R.C. Incidence of Suicide in Patients With Head and Neck Cancer. JAMA Otolaryngol. Head Neck Surg. 2015, 141, 1075–1081. [Google Scholar] [CrossRef]
- Osazuwa-Peters, N.; Simpson, M.C.; Zhao, L.; Boakye, E.A.; Olomukoro, S.I.; Deshields, T.; Loux, T.M.; Varvares, M.A.; Schootman, M. Suicide risk among cancer survivors: Head and neck versus other cancers. Cancer 2018, 124, 4072–4079. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Levac, D.; Colquhoun, H.; O’Brien, K.K. Scoping studies: Advancing the methodology. Implement. Sci. 2010, 5, 69. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Peters, M.D.; Godfrey, C.; McInerney, P.; Munn, Z.; Tricco, A.C.; Khalil, H. Scoping reviews. Joanna Briggs Inst. Rev. Man. 2017, 2015, 1–24. [Google Scholar]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- NVivo (Released in March 2020); QSR International Pty Ltd.: Burlington, MA, USA, 2020.
- NIH. Study Quality Assessment Tools National Heart, Lung, and Blood Institute (NHLBI). 2021. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 2 December 2022).
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Kaffenberger, T.M.; Patel, A.K.; Lyu, L.; Li, J.; Wasserman-Wincko, T.; Zandberg, D.P.; Clump, D.A.; Johnson, J.T.; Nilsen, M.L. Quality of life after radiation and transoral robotic surgery in advanced oropharyngeal cancer. Laryngoscope Investig. Otolaryngol. 2021, 6, 983–990. [Google Scholar] [CrossRef]
- Janz, T.A.; Momin, S.R.; Sterba, K.R.; Kato, M.G.; Armeson, K.E.; Day, T.A. Comparison of psychosocial factors over time among HPV+ oropharyngeal cancer and tobacco-related oral cavity cancer patients. Am. J. Otolaryngol. 2019, 40, 40–45. [Google Scholar] [CrossRef]
- Rajeev-Kumar, G.; Moreno, J.; Kelley, A.; Sharma, S.; Gupta, V.; Bakst, R. Emotional Quality of Life After Radiation Therapy for Oropharyngeal Carcinoma. Adv. Radiat. Oncol. 2019, 4, 674–682. [Google Scholar] [CrossRef]
- Berg, M.; Adnan, A.; Hogmo, A.; Sjodin, H.; Gebre-Medhin, M.; Laurell, G.; Reizenstein, J.; Farnebo, L.; Norberg, L.S.; Notstam, I.; et al. A national study of health-related quality of life in patients with cancer of the base of the tongue compared to the general population and to patients with tonsillar carcinoma. Head Neck 2021, 43, 3843–3856. [Google Scholar] [CrossRef]
- Qualliotine, J.R.; Califano, J.A.; Li, R.J.; Gold, D.; Messing, B.; Lee, G.; Ha, P.; Fakhry, C. Human papillomavirus tumour status is not associated with a positive depression screen for patients with oropharyngeal cancer. J. Laryngol. Otol. 2017, 131, 760–767. [Google Scholar] [CrossRef]
- Korsten, L.H.A.; Jansen, F.; Lissenberg-Witte, B.I.; Vergeer, M.; Brakenhoff, R.H.; Leemans, C.R.; Verdonck-de Leeuw, I.M. The course of health-related quality of life from diagnosis to two years follow-up in patients with oropharyngeal cancer: Does HPV status matter? Support. Care Cancer 2021, 29, 4473–4483. [Google Scholar] [CrossRef]
- Lee, E.; Crowder, H.R.; Gorelik, D.; Badger, C.; Schottler, J.; Li, N.W.; Siegel, R.; Sadeghi, N.; Thakkar, P.G.; Joshi, A.S.; et al. Comparison of quality of life outcomes in a de-intensification treatment regimen for p16 + oropharyngeal cancer. Eur. Arch. Otorhinolaryngol. 2022, 279, 4533–4540. [Google Scholar] [CrossRef]
- Shinn, E.H.; Valentine, A.; Jethanandani, A.; Basen-Engquist, K.; Fellman, B.; Urbauer, D.; Atkinson, E.; Yusuf, S.W.; Lenihan, D.; Woods, M.L.; et al. Depression and Oropharynx Cancer Outcome. Psychosom. Med. 2016, 78, 38–48. [Google Scholar] [CrossRef]
- Casswell, G.; Gough, K.; Drosdowsky, A.; Bressel, M.; Coleman, A.; Shrestha, S.; D’Costa, I.; Fua, T.; Tiong, A.; Liu, C.; et al. Fear of Cancer Recurrence in Survivors of Human Papillomavirus-Associated Oropharyngeal Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2021, 13, 13. [Google Scholar] [CrossRef]
- McDowell, L.; Casswell, G.; Bressel, M.; Drosdowsky, A.; Rischin, D.; Coleman, A.; Shrestha, S.; D’Costa, I.; Fua, T.; Tiong, A.; et al. Symptom burden, quality of life, functioning and emotional distress in survivors of human papillomavirus associated oropharyngeal cancer: An Australian cohort. Oral Oncol. 2021, 122, 105560. [Google Scholar] [CrossRef]
- Casswell, G.; Gough, K.; Drosdowsky, A.; Bressel, M.; Coleman, A.; Shrestha, S.; D’Costa, I.; Fua, T.; Tiong, A.; Liu, C.; et al. Sexual Health and Interpersonal Relationships After Chemoradiation Therapy for Human Papillomavirus-Associated Oropharyngeal Cancer: A Cross-sectional Study. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 382–393. [Google Scholar] [CrossRef]
- Dziegielewski, P.T.; Teknos, T.N.; Durmus, K.; Old, M.; Agrawal, A.; Kakarala, K.; Marcinow, A.; Ozer, E. Transoral robotic surgery for oropharyngeal cancer: Long-term quality of life and functional outcomes. JAMA Otolaryngol. Head Neck Surg. 2013, 139, 1099–1108. [Google Scholar] [CrossRef]
- Shaverdian, N.; Hegde, J.V.; Felix, C.; Hsu, S.; Basehart, V.; Steinberg, M.L.; Chen, A.M. Patient perspectives and treatment regret after de-escalated chemoradiation for human papillomavirus-positive oropharyngeal cancer: Findings from a phase II trial. Head Neck 2019, 41, 2768–2776. [Google Scholar] [CrossRef]
- Goepfert, R.P.; Fuller, C.D.; Gunn, G.B.; Hanna, E.Y.; Lewin, J.S.; Zaveri, J.S.; Hubbard, R.M.; Barrow, M.P.; Hutcheson, K.A. Symptom burden as a driver of decisional regret in long-term oropharyngeal carcinoma survivors. Head Neck 2017, 39, 2151–2158. [Google Scholar] [CrossRef]
- Taberna, M.; Inglehart, R.C.; Pickard, R.K.; Fakhry, C.; Agrawal, A.; Katz, M.L.; Gillison, M.L. Significant changes in sexual behavior after a diagnosis of human papillomavirus-positive and human papillomavirus-negative oral cancer. Cancer 2017, 123, 1156–1165. [Google Scholar] [CrossRef]
- Ringash, J.; Fisher, R.; Peters, L.; Trotti, A.; O’Sullivan, B.; Corry, J.; Kenny, L.; Nuyts, S.; Wratten, C.; Rischin, D. Effect of p16 Status on the Quality-of-Life Experience During Chemoradiation for Locally Advanced Oropharyngeal Cancer: A Substudy of Randomized Trial Trans-Tasman Radiation Oncology Group (TROG) 02.02 (HeadSTART). Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 678–686. [Google Scholar] [CrossRef]
- Xiao, C.; Beitler, J.J.; Higgins, K.A.; Glazer, T.; Huynh, L.K.; Paul, S.; Felger, J.C.; Wommack, E.C.; Saba, N.F.; Shin, D.M.; et al. Associations among human papillomavirus, inflammation, and fatigue in patients with head and neck cancer. Cancer 2018, 124, 3163–3170. [Google Scholar] [CrossRef]
- Gascon, B.; Panjwani, A.A.; Mazzurco, O.; Li, M. Screening for Distress and Health Outcomes in Head and Neck Cancer. Curr. Oncol. 2022, 29, 3793–3806. [Google Scholar] [CrossRef]
- Krebber, A.M.; Buffart, L.M.; Kleijn, G.; Riepma, I.C.; de Bree, R.; Leemans, C.R.; Becker, A.; Brug, J.; van Straten, A.; Cuijpers, P.; et al. Prevalence of depression in cancer patients: A meta-analysis of diagnostic interviews and self-report instruments. Psychooncology 2014, 23, 121–130. [Google Scholar] [CrossRef]
- Schmidt, T.; Valuck, T.; Perkins, B.; Riposo, J.; Patel, P.; Westrich, K.; Basch, E.; McClellan, M. Improving patient-reported measures in oncology: A payer call to action. J. Manag Care Spec. Pharm 2021, 27, 118–126. [Google Scholar] [CrossRef]
- Tyner, T.E.; Freysteinson, W.M. A concept analysis of decision regret in women with breast cancer. Nurs. Forum 2022, 57, 112–120. [Google Scholar] [CrossRef]
- Windon, M.J.; Le, D.; D’Souza, G.; Bigelow, E.; Pitman, K.; Boss, E.; Eisele, D.W.; Fakhry, C. Treatment decision-making among patients with oropharyngeal squamous cell cancer: A qualitative study. Oral Oncol. 2021, 112, 105044. [Google Scholar] [CrossRef]
- Langendijk, J.A.; Doornaert, P.; Verdonck-de Leeuw, I.M.; Leemans, C.R.; Aaronson, N.K.; Slotman, B.J. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J. Clin. Oncol. 2008, 26, 3770–3776. [Google Scholar] [CrossRef]
- Nguyen, N.P.; Sallah, S.; Karlsson, U.; Antoine, J.E. Combined chemotherapy and radiation therapy for head and neck malignancies: Quality of life issues. Cancer 2002, 94, 1131–1141. [Google Scholar] [CrossRef]
- Jensen, K.; Bonde Jensen, A.; Grau, C. The relationship between observer-based toxicity scoring and patient assessed symptom severity after treatment for head and neck cancer. A correlative cross sectional study of the DAHANCA toxicity scoring system and the EORTC quality of life questionnaires. Radiother. Oncol. 2006, 78, 298–305. [Google Scholar] [CrossRef]
- Jellema, A.P.; Doornaert, P.; Slotman, B.J.; Leemans, C.R.; Langendijk, J.A. Does radiation dose to the salivary glands and oral cavity predict patient-rated xerostomia and sticky saliva in head and neck cancer patients treated with curative radiotherapy? Radiother. Oncol. 2005, 77, 164–171. [Google Scholar] [CrossRef]
- Ferris, R.L.; Flamand, Y.; Weinstein, G.S.; Li, S.; Quon, H.; Mehra, R.; Garcia, J.J.; Chung, C.H.; Gillison, M.L.; Duvvuri, U.; et al. Phase II Randomized Trial of Transoral Surgery and Low-Dose Intensity Modulated Radiation Therapy in Resectable p16+ Locally Advanced Oropharynx Cancer: An ECOG-ACRIN Cancer Research Group Trial (E3311). J. Clin. Oncol. 2022, 40, 138–149. [Google Scholar] [CrossRef]
- Swisher-McClure, S.; Lukens, J.N.; Aggarwal, C.; Ahn, P.; Basu, D.; Bauml, J.M.; Brody, R.; Chalian, A.; Cohen, R.B.; Fotouhi-Ghiam, A.; et al. A Phase 2 Trial of Alternative Volumes of Oropharyngeal Irradiation for De-intensification (AVOID): Omission of the Resected Primary Tumor Bed After Transoral Robotic Surgery for Human Papilloma Virus-Related Squamous Cell Carcinoma of the Oropharynx. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.J.; Price, K.A.; Moore, E.J.; Patel, S.H.; Hinni, M.L.; Garcia, J.J.; Graner, D.E.; Foster, N.R.; Ginos, B.; Neben-Wittich, M.; et al. Phase II Evaluation of Aggressive Dose De-Escalation for Adjuvant Chemoradiotherapy in Human Papillomavirus-Associated Oropharynx Squamous Cell Carcinoma. J. Clin. Oncol. 2019, 37, 1909–1918. [Google Scholar] [CrossRef] [PubMed]
| Abstract Criteria | ||
|---|---|---|
| Study Characteristics | Inclusion Criteria | Exclusion Criteria |
| Participants (population) |
|
|
| Study design (concept) |
|
|
| Outcome measures (context) |
|
|
| Other (publication) |
|
|
| Full-text criteria (additional criteria) | ||
| Study design |
| |
| Outcome measures |
|
|
| Study | HPV-Related Results |
|---|---|
| Mental Health and Emotional Wellbeing | |
| Berg, 2021 [30] | HPV-positive BOT cancer patients had better emotional functioning (p = 0.004) than the HPV-negative cohort on EORTCQLQ-C30 (Primary text, Table 5) |
| Janz, 2019 [28] | At baseline, HPV-positive OPSCC cohort had a non-significant difference in mean depression score compared to smoking oral cavity patients (12 versus 14, p = 0.41). |
| Depression decreased significantly over time for the HPV-positive OPSCC patients (12 to 9.9, p = 0.03) and non-significantly in the oral cavity patients (14 to 9.73, p = 0.1) from baseline to 12 months. | |
| Korsten, 2021 [32] | Emotional functioning was significantly different between HPV-positive and negative patients: average scores were equal at baseline and in close follow-up (6 weeks and 3 months), but scores improved more in HPV-positive patients (p = 0.039). |
| Qualliotine, 2017 [31] | There was no significant association noted between depression and HPV status (p > 0.1) (Primary text: Figure 1). |
| Rajeev-Kumar, 2019 [29] | There is no statistically significant relationship between anxiety or mood and human papillomavirus infection status (p = 0.089 for anxiety; p = 0.731 for mood). |
| Shinn, 2016 [34] | There was no significant difference in depression scores between HPV-positive and HPV-negative patients. |
| Social wellbeing and function | |
| Berg, 2021 [30] | HPV-positive BOT cancer patients had better social functioning (p = 0.01) than the HPV-negative cohort on EORTCQLQ-C30 (Primary text, Table 5) |
| Dziegielewski, 2013 [38] | HPV status did not correlate with any quality of life domain (i.e., social function, social attitude, overall attitude) in the HNCI (p > 0.5 for all domains, Primary text: Table 5) |
| Korsten, 2021 [32] | Social functioning recovered faster and to a better degree in HPV-positive patients (p = 0.033) (Primary text: Figure 2). |
| Stress | |
| Goepfert, 2017 [40] | There was no significant difference in MDASI-HN symptom scores (p = 0.27) or proportional decisional regret (p = 0.37) based on HPV status (Primary text: Table 3) |
| Janz, 2019 [28] | At baseline, HPV-positive OPSCC cohort had a non-significant difference in mean cancer worry compared to smoking oral cavity patients (2.8 versus 3.25, p = 0.1). |
| Cancer worry decreased non-significantly over time in both the HPV-positive OPSCC patients (2.8 to 2.4, p = 0.11) and the oral cavity patients (3.2 to 2.7, p = 0.07). (Primary text: Table 2) | |
| Relationship and sexual behavior | |
| Taberna, 2017 [41] | At baseline, there was no statistically significant differences in levels of relationship distress between HPV-positive and HPV-negative patients. At 6 months follow up, a non-significant trend was noted of higher distress in the affection expression subscale of the DAS for HPV-positive patients compared to HPV-negative. 38% of HPV-positive patients reported that their relationship with their partner had stayed the same, and those who reported a change felt it was positive. 70% of partners reported favorable changes in their relationship since diagnosis. A higher proportion of partners reported more stress in their relationship since the cancer diagnosis than the patients (39% versus 14%, p < 0.01). Approximately a quarter of patients blamed themselves for their cancer diagnosis or felt guilty about exposing their partner to HPV. 14% of partners felt guilty for possibly exposing their partner to HPV or were concerned that the HPV infection may have been a result of an extramarital relationship (their or their partner’s). There was a significant decline in sexual behavior frequency in both HPV-positive and HPV-negative cohorts (Primary text: Figure 2, p < 0.01). |
| No comparison | |
| Casswell, 2021 [35] | N/A |
| Casswell, 2021 [37] | N/A |
| Kaffenberger, 2021 [27] | N/A |
| Lee, 2022 [33] | N/A |
| McDowell, 2021 [36] | N/A |
| Shaverdian, 2019 [39] | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silver, J.A.; Schwartz, R.; Roy, C.F.; Sadeghi, N.; Henry, M. Patient-Reported Outcome Measures of Psychosocial Quality of Life in Oropharyngeal Cancer Patients: A Scoping Review. J. Clin. Med. 2023, 12, 2122. https://doi.org/10.3390/jcm12062122
Silver JA, Schwartz R, Roy CF, Sadeghi N, Henry M. Patient-Reported Outcome Measures of Psychosocial Quality of Life in Oropharyngeal Cancer Patients: A Scoping Review. Journal of Clinical Medicine. 2023; 12(6):2122. https://doi.org/10.3390/jcm12062122
Chicago/Turabian StyleSilver, Jennifer A., Russell Schwartz, Catherine F. Roy, Nader Sadeghi, and Melissa Henry. 2023. "Patient-Reported Outcome Measures of Psychosocial Quality of Life in Oropharyngeal Cancer Patients: A Scoping Review" Journal of Clinical Medicine 12, no. 6: 2122. https://doi.org/10.3390/jcm12062122
APA StyleSilver, J. A., Schwartz, R., Roy, C. F., Sadeghi, N., & Henry, M. (2023). Patient-Reported Outcome Measures of Psychosocial Quality of Life in Oropharyngeal Cancer Patients: A Scoping Review. Journal of Clinical Medicine, 12(6), 2122. https://doi.org/10.3390/jcm12062122