Menopause Is Associated with Obstructive Sleep Apnea in a Population-Based Sample from Mecklenburg–Western Pomerania, Germany
Abstract
1. Introduction
1.1. Empirical Findings
1.2. Possible Mechanisms
1.3. The Apnea–Hypopnea Index
1.4. Aims and Scopes
2. Methods
2.1. Sample
2.2. Polysomnography
2.3. Data Analysis
2.4. Risk Indicators
2.5. Outcome
3. Results
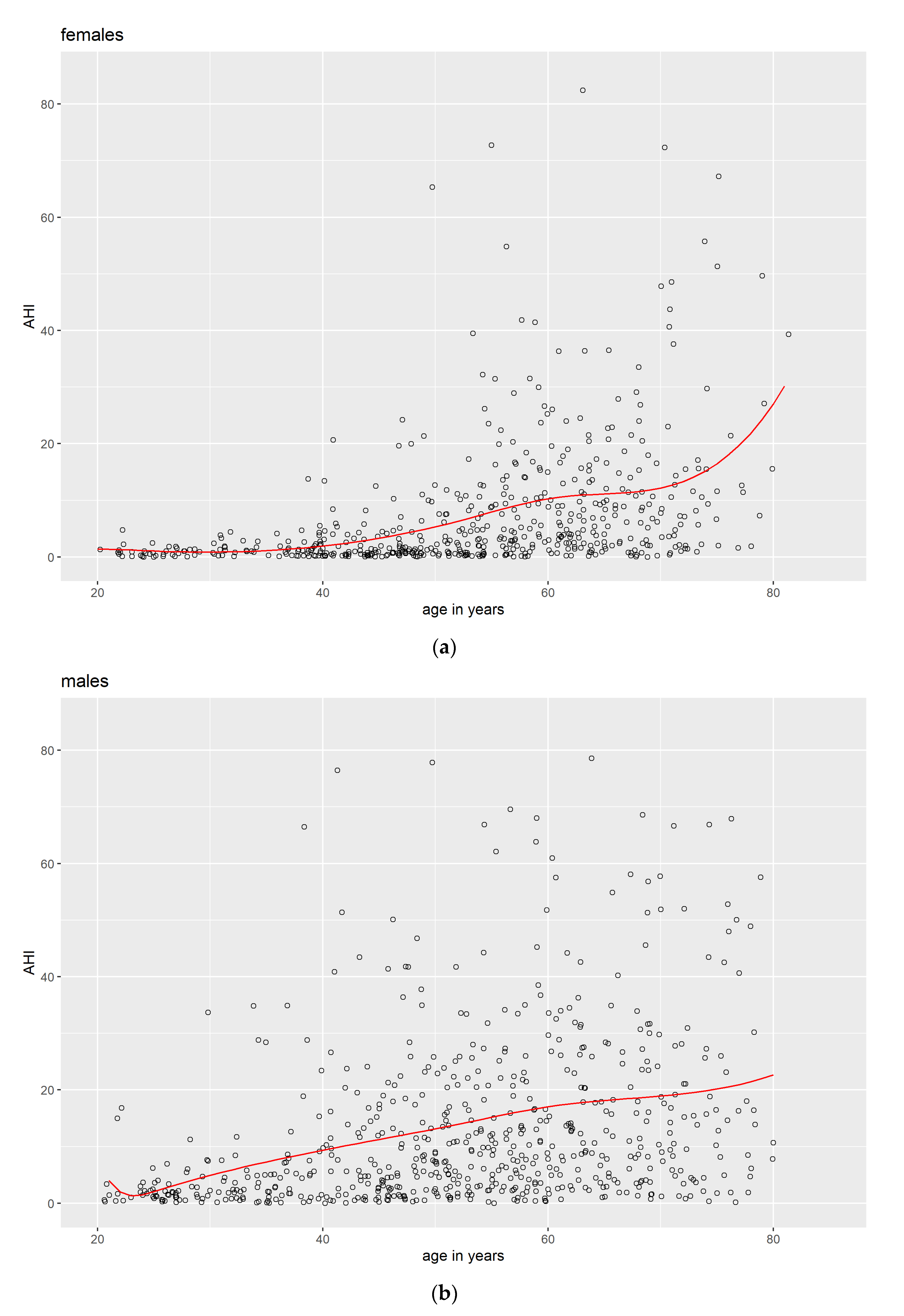
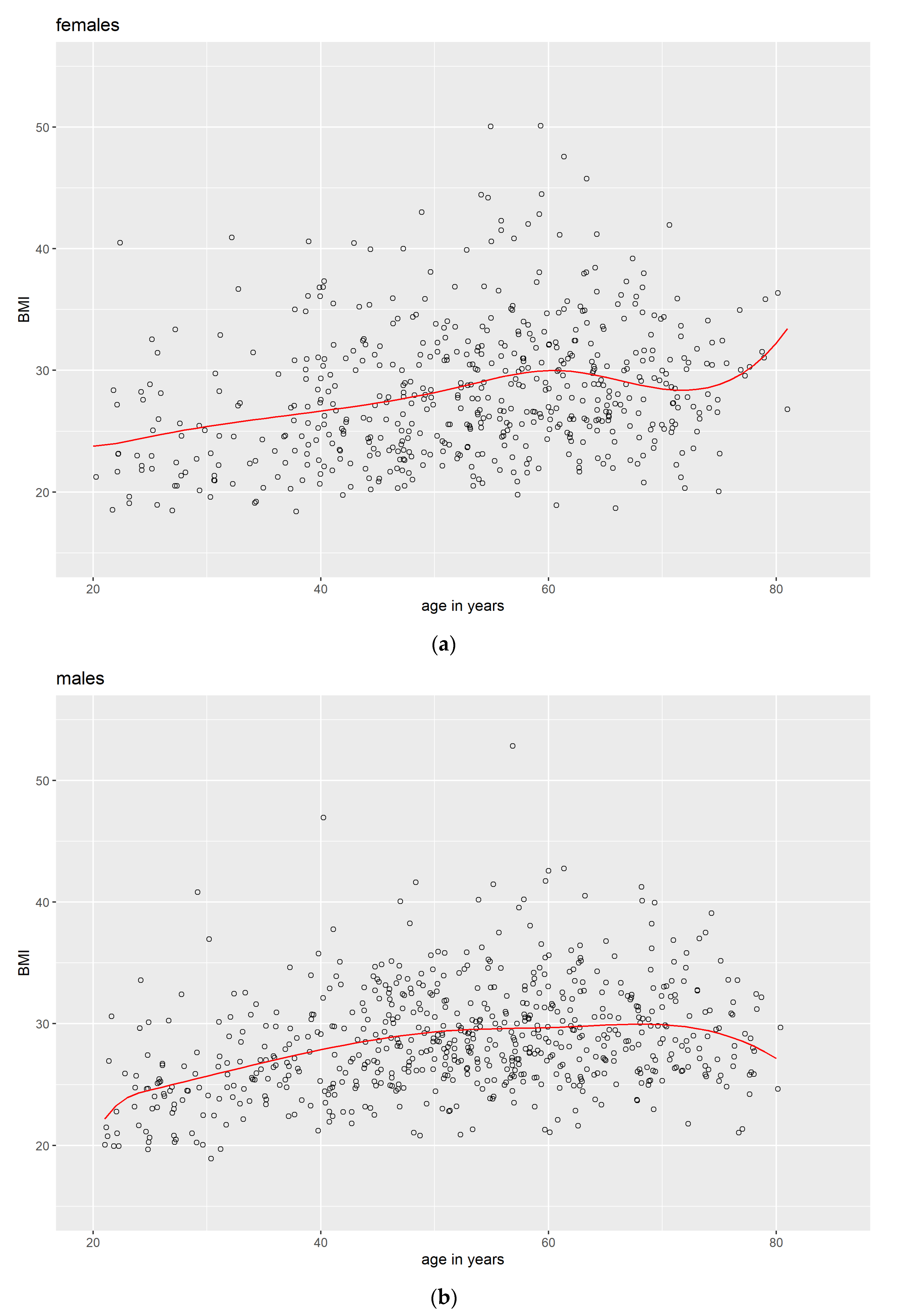
3.1. Descriptive Data
3.2. Ordinal Logistic Regression Analysis
4. Discussion
4.1. Strength and Limitations
4.2. Conclusions and Perspectives
Author Contributions
Funding
- This research was funded by the Deutsche Forschungsgemeinschaft (DFG), grant DA 1810/2-1 to Amro Daboul; the Bundesministerium für Bildung und Forschung, grants 01ZZ9603, 01ZZ0103, and 01ZZ0701; the Deutsche Stiftung für Herzforschung, grant F/34/10; and the Re-search Network of Community Medicine, University Medicine Greifswald.
- The Study of Health in Pomerania (SHIP) is part of the Community Medicine Research Network of the University Medicine Greifswald, which is supported by the German Federal State of Mecklenburg-West Pomerania, the Ministry of Cultural Affairs, and the Social Ministry of the Federal State of Mecklenburg—West Pomerania.
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AASM | American Academy of Sleep Medicine |
| AHI | Apnea–hypopnea index |
| ANOVA | Analysis of variance |
| BMI | Body mass index |
| CI | Confidence interval |
| DFG | Deutsche Forschungsgemeinschaft |
| EEG | Electroencephalogram |
| ECG | Electrocardiogram |
| EOG | Electrooculogram |
| OSA | Obstructive sleep apnea |
| PSQ | Polysomnography |
| SD | Standard deviation |
| SHIP | Study of Health in Pomerania |
Appendix A
Appendix B
| No OSA | Mild OSA | Moderate OSA | Severe OSA | Total | |
|---|---|---|---|---|---|
| Pre-menopausal | N = 180 | N = 14 | N = 3 | N = 0 | N = 197 |
| BMI = 26.0 (4.9) | BMI = 32.5 (3.9) | BMI = 37.7 (4.6) | BMI = 26.7 (5.3) | ||
| Post-menopausal | N = 170 | N = 110 | N = 57 | N = 25 | N = 362 |
| BMI = 27.0 (4.3) | BMI = 29.8 (5.2) | BMI = 32.7 (5.8) | BMI = 32.0 (6.0) | BMI = 29.1 (5.4) | |
| Total | N = 350 | N = 124 | N = 60 | N = 25 | |
| BMI = 26.5 (4.6) | BMI = 30.1 (5.1) | BMI = 33.0 (5.8) | BMI = 32.0 (6.0) |
References
- Clinical Guideline for the Evaluation, Management and Long-term Care of Obstructive Sleep Apnea in Adults. Available online: https://jcsm.aasm.org/doi/full/10.5664/jcsm.27497 (accessed on 11 August 2022).
- Fietze, I.; Penzel, T.; Alonderis, A.; Barbe, F.; Bonsignore, M.R.; Calverly, P.; Mallin, W. Management of obstructive sleep apnea in Europe. Sleep Med. 2011, 12, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Fietze, I.; Laharnar, N.; Bargiotas, P.; Basoglu, O.K.; Dogas, Z.; Drummond, M.; Penzel, T. Management of obstructive sleep apnea in Europe–A 10-year follow-up. Sleep Med. 2022, 97, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Young, T.; Skatrud, J.; Peppard, P.E. Risk Factors for Obstructive Sleep Apnea in Adults. JAMA 2004, 291, 2013–2016. [Google Scholar] [CrossRef]
- Kapsimalis, F.; Kryger, M.H. Gender and Obstructive Sleep Apnea Syndrome, Part 1: Clinical Features. Sleep 2002, 25, 409–416. [Google Scholar] [CrossRef]
- Shaver, J.L.F.; Zenk, S.N. Review: Sleep Disturbance in Menopause. J. Women’s Health Gend. Based Med. 2000, 9, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Perger, E.; Mattaliano, P.; Lombardi, C. Menopause and Sleep Apnea. Maturitas 2019, 124, 35–38. [Google Scholar] [CrossRef]
- Hachul, H.; Frange, C.; Bezerra, A.G.; Hirotsu, C.; Pires, G.N.; Andersen, M.L.; Tufik, S. The effect of menopause on objective sleep parameters: Data from an epidemiologic study in São Paulo, Brazil. Maturitas 2015, 80, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Young, T.; Finn, L.; Austin, D.; Peterson, A. Menopausal Status and Sleep-disordered Breathing in the Wisconsin Sleep Cohort Study. Am. J. Respir. Crit. Care Med. 2003, 167, 1181–1185. [Google Scholar] [CrossRef]
- Mirer, A.G.; Young, T.; Palta, M.; Benca, R.M.; Rasmuson, A.; Peppard, P.E. Sleep-Disordered Breathing and the Menopausal Transition among Participants in the Sleep in Midlife Women Study. Menopause 2017, 24, 157–162. [Google Scholar] [CrossRef]
- Zolfaghari, S.; Yao, C.; Thompson, C.; Gosselin, N.; Desautels, A.; Dang-Vu, T.T.; Carrier, J. Effects of menopause on sleep quality and sleep disorders: Canadian Longitudinal Study on Aging. Menopause 2020, 27, 295–304. [Google Scholar] [CrossRef]
- Netzer, N.C.; Eliasson, A.H.; Strohl, K.P. Women with Sleep Apnea Have Lower Levels of Sex Hormones. Sleep Breath 2003, 7, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Toth, M.J.; Tchernof, A.; Sites, C.K.; Poehlman, E.T. Menopause-Related Changes in Body Fat Distribution. Ann. N. Y. Acad. Sci. 2000, 904, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Resta, O.; Bonfitto, P.; Sabato, R.; De Pergola, G.; Barbaro, M.P.F. Prevalence of obstructive sleep apnoea in a sample of obese women: Effect of menopause. Diabetes Nutr Metab. 2004, 17, 296–303. [Google Scholar] [PubMed]
- Qiu, Q.; Mateika, J.H. Pathophysiology of Obstructive Sleep Apnea in Aging Women. Curr. Sleep Med. Rep. 2021, 7, 177–185. [Google Scholar] [CrossRef]
- Huang, T.; Lin, B.M.; Redline, S.; Curhan, G.C.; Hu, F.B.; Tworoger, S.S. Type of Menopause, Age at Menopause, and Risk of Developing Obstructive Sleep Apnea in Postmenopausal Women. Am. J. Epidemiol. 2018, 187, 1370–1379. [Google Scholar] [CrossRef] [PubMed]
- Ruehland, W.R.; Rochford, P.D.; O’Donoghue, F.J.; Pierce, R.J.; Singh, P.; Thornton, A.T. The New Aasm Criteria for Scoring Hypopneas: Impact on the Apnea Hypopnea Index. Sleep 2009, 32, 150–157. [Google Scholar] [CrossRef]
- Pevernagie, D.A.; Gnidovec-Strazisar, B.; Grote, L.; Heinzer, R.; McNicholas, W.T.; Penzel, T.; Arnardottir, E.S. On the rise and fall of the apnea−hypopnea index: A historical review and critical appraisal. J. Sleep Res. 2020, 29, e13066. [Google Scholar] [CrossRef]
- Völzke, H.; Alte, D.; Schmidt, C.O.; Radke, D.; Lorbeer, R.; Friedrich, N.; Hoffmann, W. Cohort Profile: The Study of Health in Pomerania. Int. J. Epidemiol. 2011, 40, 294–307. [Google Scholar] [CrossRef]
- Völzke, H.; Schössow, J.; Schmidt, C.O.; Jürgens, C.; Richter, A.; Werner, A.; Kocher, T. Cohort Profile Update: The Study of Health in Pomerania (SHIP). Int. J. Epidemiol. 2022, 51, e372–e383. [Google Scholar] [CrossRef]
- Stubbe, B.; Penzel, T.; Fietze, I.; Obst, A.; Garcia, C.; Zimmermann, S.; Diecker, B.; Glos, M.; Schmidt, C.O.; Lau, K.; et al. Polysomnography in a Large Population Based Study-the Study of Health in Pomerania Protocol. J. Sleep Disord Manag. 2016, 2, 1–5. [Google Scholar] [CrossRef]
- Sateia, M.J. International Classification of Sleep Disorders—Third Edition. Chest 2014, 146, 1387–1394. [Google Scholar] [CrossRef]
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.; Morrell, M.J.; Malhotra, A. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Fietze, I.; Laharnar, N.; Obst, A.; Ewert, R.; Felix, S.B.; Garcia, C.; Penzel, T. Prevalence and association analysis of obstructive sleep apnea with gender and age differences–Results of SHIP-Trend. J. Sleep Res. 2019, 28, e12770. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria. 2018. Available online: https://www.R-project.org/ (accessed on 26 July 2022).
- Wickham, H.; Miller, E.; Smith, D. Haven: Import and Export “SPSS”, “Stata” and “SAS” Files. 2022. Available online: https://CRAN.R-project.org/package=haven (accessed on 26 July 2022).
- Wickham, H.; François, R.; Henry, L.; Müller, K. Dplyr: A Grammar of Data Manipulation. 2022. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 26 July 2022).
- Wickham, H.; Girlich, M.; RStudio. Tidyr: Tidy Messy Data. 2022. Available online: https://CRAN.R-project.org/package=tidyr (accessed on 9 February 2022).
- Ripley, B.; Venables, B.; Bates, D.M.; Hornik, K.; Gebhardt, A.; Firth, D. MASS: Support Functions and Datasets for Venables and Ripley’s MASS. 2022. Available online: https://CRAN.R-project.org/package=MASS (accessed on 26 July 2022).
- Kassambara, A. Rstatix: Pipe-Friendly Framework for Basic Statistical Tests. 2021. Available online: https://CRAN.R-project.org/package=rstatix (accessed on 26 July 2022).
- Wickham, H.; Chang, W.; Henry, L.; Pedersen, T.L.; Takahashi, K.; Wilke, C.; Woo, K.; Yutani, H.; Dunnington, D. Ggplot2: Create Elegant Data Visualisations Using the Grammar of Graphics. 2022. Available online: https://CRAN.R-project.org/package=ggplot2 (accessed on 26 July 2022).
- Krüger, M.; Obst, A.; Bernhardt, O.; Ewert, R.; Penzel, T.; Stubbe, B.; Daboul, A. Socioeconomic factors do not predict sleep apnea in a population sample from Mecklenburg-Western Pomerania, Germany. Sleep Breath 2022, 1–9. [Google Scholar] [CrossRef]
- Vogler, K.; Daboul, A.; Obst, A.; Fietze, I.; Ewert, R.; Biffar, R.; Krüger, M. Quality of Life in patients with Obstructive Sleep Apnea: Results from the Study of Health in Pomerania. J. Sleep Res. 2023, 32, e13702. [Google Scholar] [CrossRef]
- Perperoglou, A.; Sauerbrei, W.; Abrahamowicz, M.; Schmid, M. A review of spline function procedures in R. BMC Med. Res. Methodol. 2019, 19, 46. [Google Scholar] [CrossRef]
- Davis, S.R.; Castelo-Branco, C.; Chedraui, P.; Lumsden, M.A.; Nappi, R.E.; Shah, D. Understanding weight gain at menopause. Climacteric 2012, 15, 419–429. [Google Scholar] [CrossRef]
- Eichling, P.S.; Sahni, J. Menopause Related Sleep Disorders. J. Clin. Sleep Med. 2005, 1, 291–300. [Google Scholar] [CrossRef]
- Kozakowski, J.; Gietka-Czernel, M.; Leszczyńska, D.; Majos, A. Obesity in menopause–our negligence or an unfortunate inevitability? Menopause Rev. 2017, 16, 61–65. [Google Scholar] [CrossRef]
- Szentkirályi, A.; Stefani, A.; Hackner, H.; Czira, M.; Teismann, I.K.; Völzke, H.; Berger, K. Prevalence and associated risk factors of periodic limb movement in sleep in two German population-based studies. Sleep 2019, 42, zsy237. [Google Scholar] [CrossRef] [PubMed]
- Vivian-Taylor, J.; Hickey, M. Menopause and depression: Is there a link? Maturitas 2014, 79, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Young, T.; Rabago, D.; Zgierska, A.; Austin, D.; Finn, L. Objective and Subjective Sleep Quality in Premenopausal, Perimenopausal, and Postmenopausal Women in the Wisconsin Sleep Cohort Study. Sleep 2003, 26, 667–672. [Google Scholar] [CrossRef]
- Fox, H.; Arzt, M.; Bergmann, M.W.; Bitter, T.; Linz, D.; Oldenburg, O.; Skobel, C.E. Positionspapier „Schlafmedizin in der Kardiologie“, Update 2021. Kardiologe 2021, 15, 429–461. [Google Scholar] [CrossRef]
- Galea, S.; Tracy, M. Participation Rates in Epidemiologic Studies. Ann. Epidemiol. 2007, 17, 643–653. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krüger, M.; Obst, A.; Ittermann, T.; Bernhardt, O.; Ivanovska, T.; Zygmunt, M.; Ewert, R.; Fietze, I.; Penzel, T.; Biffar, R.; et al. Menopause Is Associated with Obstructive Sleep Apnea in a Population-Based Sample from Mecklenburg–Western Pomerania, Germany. J. Clin. Med. 2023, 12, 2101. https://doi.org/10.3390/jcm12062101
Krüger M, Obst A, Ittermann T, Bernhardt O, Ivanovska T, Zygmunt M, Ewert R, Fietze I, Penzel T, Biffar R, et al. Menopause Is Associated with Obstructive Sleep Apnea in a Population-Based Sample from Mecklenburg–Western Pomerania, Germany. Journal of Clinical Medicine. 2023; 12(6):2101. https://doi.org/10.3390/jcm12062101
Chicago/Turabian StyleKrüger, Markus, Anne Obst, Till Ittermann, Olaf Bernhardt, Tatyana Ivanovska, Marek Zygmunt, Ralf Ewert, Ingo Fietze, Thomas Penzel, Reiner Biffar, and et al. 2023. "Menopause Is Associated with Obstructive Sleep Apnea in a Population-Based Sample from Mecklenburg–Western Pomerania, Germany" Journal of Clinical Medicine 12, no. 6: 2101. https://doi.org/10.3390/jcm12062101
APA StyleKrüger, M., Obst, A., Ittermann, T., Bernhardt, O., Ivanovska, T., Zygmunt, M., Ewert, R., Fietze, I., Penzel, T., Biffar, R., & Daboul, A. (2023). Menopause Is Associated with Obstructive Sleep Apnea in a Population-Based Sample from Mecklenburg–Western Pomerania, Germany. Journal of Clinical Medicine, 12(6), 2101. https://doi.org/10.3390/jcm12062101







