Prediction of the Postoperative Outcome in Liver Resection Using Perioperative Serum Lactate Levels
Abstract
:1. Introduction
2. Patients and Methods
2.1. Data Collection and Study Population
2.2. Outcome Measures
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
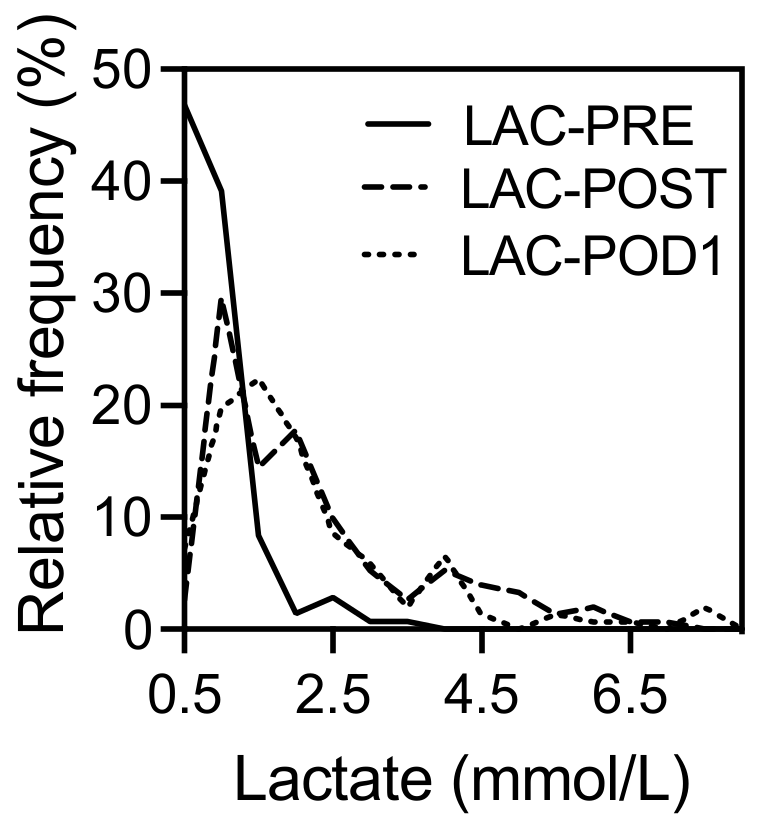
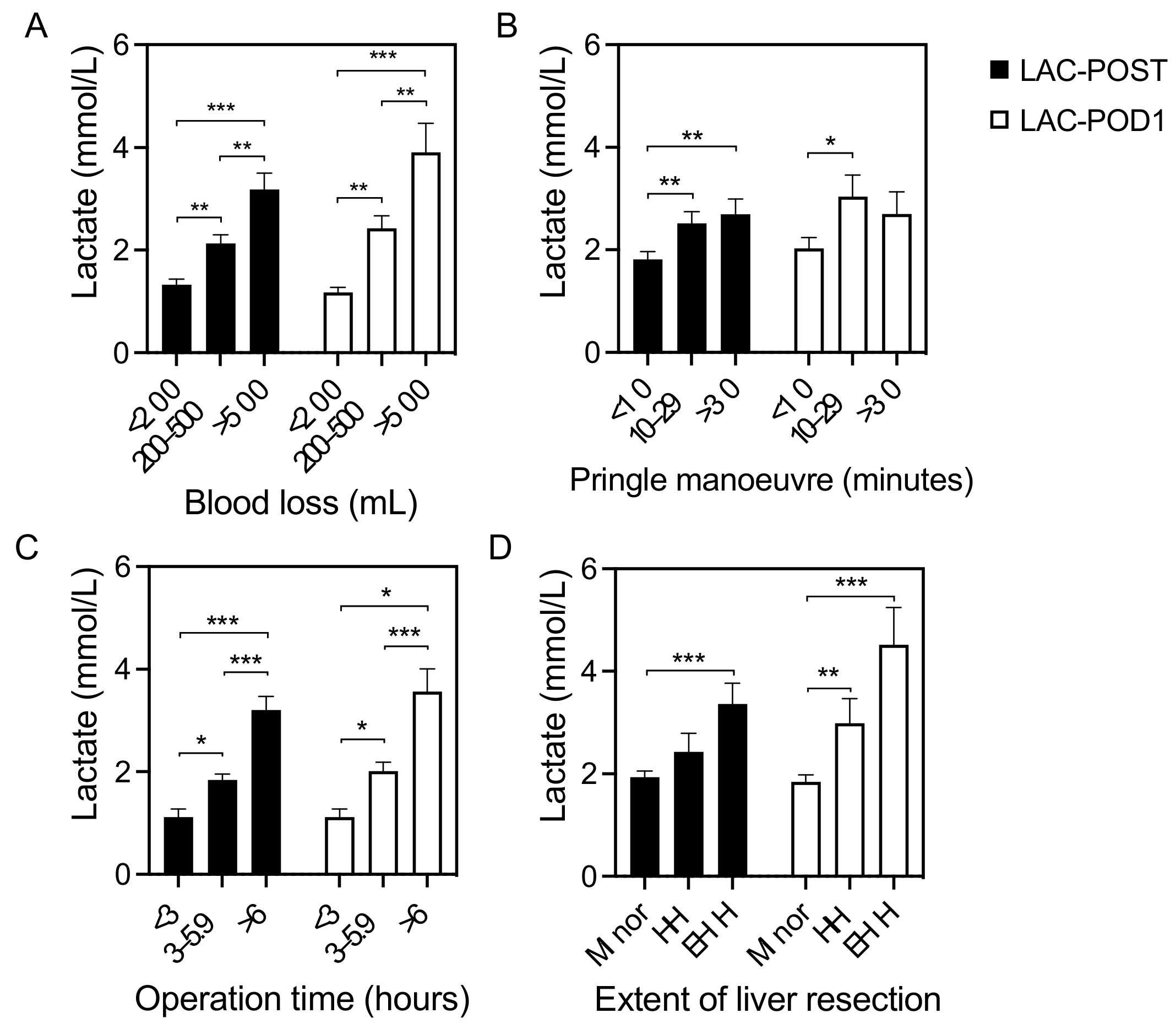
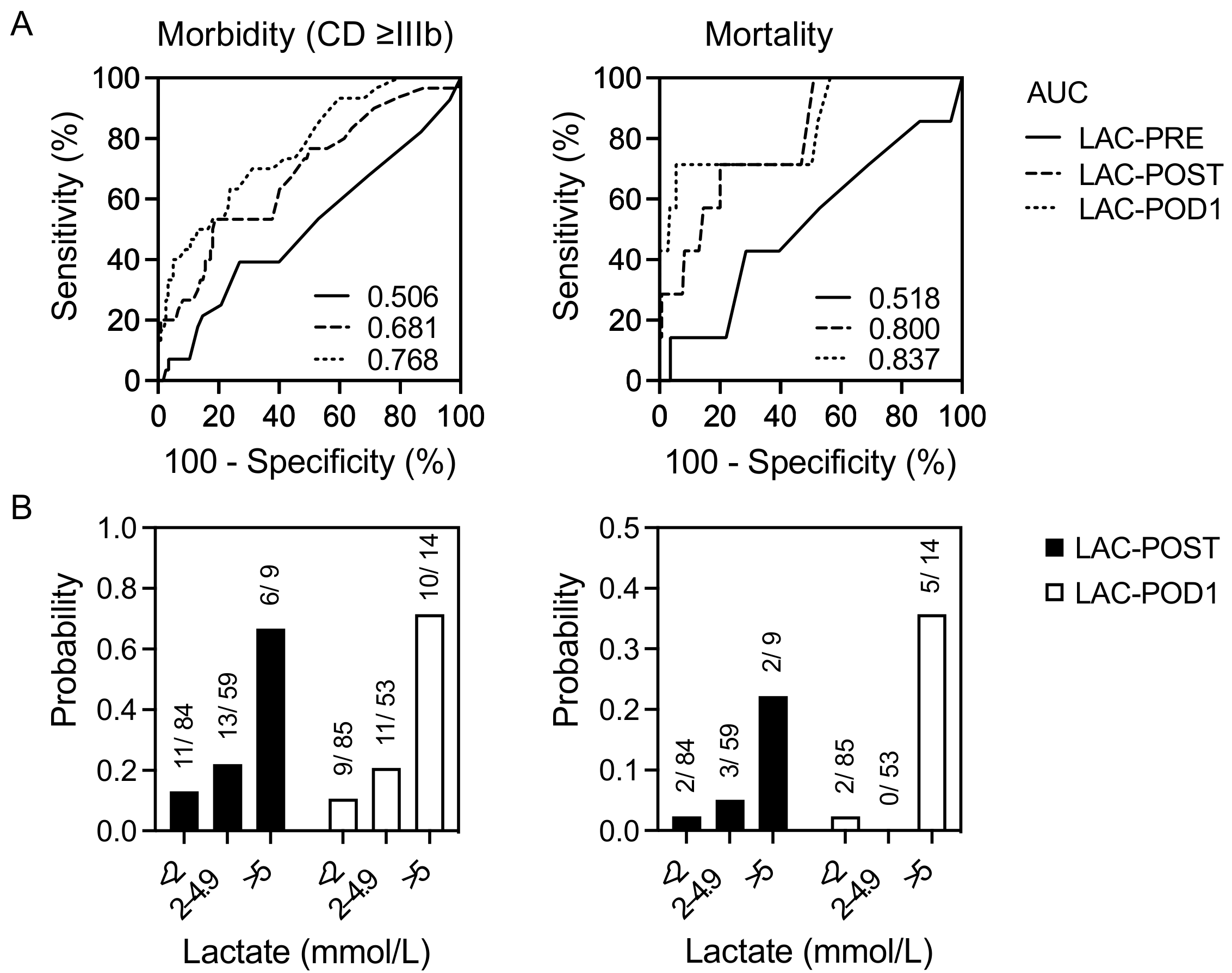
3.2. Correlation of Lactate Levels with Postoperative Outcomes
4. Discussion
4.1. Preoperative Lactate Level
4.2. Early Postoperative Lactate Level (Day 0)
4.3. Lactate Clearance (Day 1)
4.4. Postoperative Mortality
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASA | American Society of Anesthesiology |
| AUC | Area under the curve |
| BMI | Body mass index |
| CCA | Cholangiocarcinoma |
| CRLM | Colorectal liver metastases |
| FFP | Fresh frozen plasma |
| HALLR | Hand-assisted laparoscopic liver resection |
| HCC | Hepatocellular carcinoma |
| ICU | Intensive care unit |
| LAC-POD1 | Lactate on day one after liver resection |
| LAC-POST | Lactate after liver resection on day 0 |
| LAC-PRE | Lactate before liver resection |
| LLR | Laparoscopic liver resection |
| LR | Liver resection |
| MVA | Multivariate analysis |
| nCRLM | Non-colorectal liver metastases |
| OLR | Open liver resection |
| PM | Pringle maneuver |
| POD | Postoperative day |
| RBC | Red blood cells |
| SD | Standard deviation |
| UVA | Univariate analysis |
References
- Aloia, T.A.; Fahy, B.N.; Fischer, C.P.; Jones, S.L.; Duchini, A.; Galati, J.; Gaber, A.O.; Ghobrial, R.M.; Bass, B.L. Predicting poor outcome following hepatectomy: Analysis of 2313 hepatectomies in the NSQIP database. HPB 2009, 11, 510–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Virani, S.; Michaelson, J.S.; Hutter, M.M.; Lancaster, R.T.; Warshaw, A.L.; Henderson, W.G.; Khuri, S.F.; Tanabe, K.K. Morbidity and mortality after liver resection: Results of the patient safety in surgery study. J. Am. Coll. Surg. 2007, 204, 1284–1292. [Google Scholar] [CrossRef] [PubMed]
- Dokmak, S.; Ftériche, F.S.; Borscheid, R.; Cauchy, F.; Farges, O.; Belghiti, J. 2012 Liver resections in the 21st century: We are far from zero mortality. HPB 2013, 15, 908–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farges, O.; Goutte, N.; Bendersky, N.; Falissard, B.; ACHBT-French Hepatectomy Study Group. Incidence and risks of liver resection: An all-inclusive French nationwide study. Ann. Surg. 2012, 256, 697–704, discussion 704–705. [Google Scholar] [CrossRef]
- Filmann, N.; Walter, D.; Schadde, E.; Bruns, C.; Keck, T.; Lang, H.; Oldhafer, K.; Schlitt, H.J.; Schön, M.R.; Herrmann, E.; et al. Mortality after liver surgery in Germany. Br. J. Surg. 2019, 106, 1523–1529. [Google Scholar] [CrossRef]
- Tzeng, C.W.; Cooper, A.B.; Vauthey, J.N.; Curley, S.A.; Aloia, T.A. Predictors of morbidity and mortality after hepatectomy in elderly patients: Analysis of 7621 NSQIP patients. HPB 2014, 16, 459–468. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, K.; Hinz, U.; Stravodimos, C.; Knoblich, T.; Schön, M.R.; Büchler, M.W.; Mehrabi, A. Risk assessment for liver resection. Surgery 2018, 164, 998–1005. [Google Scholar] [CrossRef]
- Breitenstein, S.; DeOliveira, M.L.; Raptis, D.A.; Slankamenac, K.; Kambakamba, P.; Nerl, J.; Clavien, P.A. Novel and simple preoperative score predicting complications after liver resection in noncirrhotic patients. Ann. Surg. 2010, 252, 726–734. [Google Scholar] [CrossRef]
- McCormack, L.; Petrowsky, H.; Jochum, W.; Furrer, K.; Clavien, P.A. Hepatic Steatosis Is a Risk Factor for Postoperative Complications After Major Hepatectomy: A Matched Case-Control Study. Ann. Surg. 2007, 245, 923–930. [Google Scholar] [CrossRef]
- Sitzmann, J.V.; Greene, P.S. Perioperative predictors of morbidity following hepatic resection for neoplasm. A multivariate analysis of a single surgeon experience with 105 patients. Ann. Surg. 1994, 219, 13–17. [Google Scholar] [CrossRef]
- Strey, C.W.; Marquez-Pinilla, R.M.; Markiewski, M.M.; Siegmund, B.; Oppermann, E.; Lambris, J.D.; Bechstein, W.O. Early post-operative measurement of cytokine plasma levels combined with pre-operative bilirubin levels identify high-risk patients after liver resection. Int. J. Mol. Med. 2011, 27, 447–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casserly, B.; Phillips, G.S.; Schorr, C.; Dellinger, R.P.; Townsend, S.R.; Osborn, T.M.; Reinhart, K.; Selvakumar, N.; Levy, M.M. Lactate measurements in sepsis-induced tissue hypoperfusion: Results from the Surviving Sepsis Campaign database. Crit. Care Med. 2015, 43, 567–573. [Google Scholar] [CrossRef]
- Gyawali, B.; Ramakrishna, K.; Dhamoon, A.S. Sepsis: The evolution in definition, pathophysiology, and management. SAGE Open Med. 2019, 7, 2050312119835043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.M.; An, W.S. New clinical criteria for septic shock: Serum lactate level as new emerging vital sign. J. Thorac. Dis. 2016, 8, 1388–1390. [Google Scholar] [CrossRef] [PubMed]
- Vitin, A.A.; Azamfirei, L.; Tomescu, D.; Lang, J.D. Perioperative Management of Lactic Acidosis in End-Stage Liver Disease Patient. J. Crit. Care Med. (Targu Mures) 2017, 3, 55–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giustiniano, E.; Procopio, F.; Costa, G.; Rocchi, L.; Ruggieri, N.; Cantoni, S.; Zito, P.C.; Gollo, Y.; Torzilli, G.; Raimondi, F. Serum lactate in liver resection with intermittent Pringle maneuver: The “square-root- shape. J. Hepatobiliary Pancreat. Sci. 2017, 24, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Lemke, M.; Karanicolas, P.J.; Habashi, R.; Behman, R.; Coburn, N.G.; Hanna, S.S.; Law, C.H.L.; Hallet, J. Elevated Lactate is Independently Associated with Adverse Outcomes Following Hepatectomy. World J. Surg. 2017, 41, 3180–3188. [Google Scholar] [CrossRef]
- Meguro, M.; Mizuguchi, T.; Kawamoto, M.; Nishidate, T.; Ishii, M.; Tatsumi, H.; Kimura, Y.; Furuhata, T.; Hirata, K. Highest intraoperative lactate level could predict postoperative infectious complications after hepatectomy, reflecting the Pringle maneuver especially in chronic liver disease. J. Hepatobiliary Pancreat. Sci. 2014, 21, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Vibert, E.; Boleslawski, E.; Cosse, C.; Adam, R.; Castaing, D.; Cherqui, D.; Naili, S.; Régimbeau, J.M.; Cunha, A.S.; Truant, S.; et al. Arterial Lactate Concentration at the End of an Elective Hepatectomy Is an Early Predictor of the Postoperative Course and a Potential Surrogate of Intraoperative Events. Ann. Surg. 2015, 262, 787–792, discussion 792–793. [Google Scholar] [CrossRef]
- Wiggans, M.G.; Starkie, T.; Shahtahmassebi, G.; Woolley, T.; Birt, D.; Erasmus, P.; Anderson, I.; Bowles, M.J.; Aroori, S.; Stell, D.A. Serum arterial lactate concentration predicts mortality and organ dysfunction following liver resection. Perioper Med. 2013, 2, 21. [Google Scholar] [CrossRef] [Green Version]
- Connolly, C.; Stättner, S.; Niederwieser, T.; Primavesi, F. Systematic review on peri-operative lactate measurements to predict outcomes in patients undergoing liver resection. J. Hepatobiliary Pancreat. Sci. 2020, 27, 359–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niederwieser, T.; Braunwarth, E.; Dasari, B.V.M.; Pufal, K.; Szatmary, P.; Hackl, H.; Haselmann, C.; Connolly, C.E.; Cardini, B.; Öfner, D.; et al. Early postoperative arterial lactate concentrations to stratify risk of post-hepatectomy liver failure. Br. J. Surg. 2021, 108, 1360–1370. [Google Scholar] [CrossRef] [PubMed]
- Pagano, D.; Tropea, A.; Cintorino, D.; Biondi, A.; Spada, M.; Gruttadauria, S. The unreliability of continuous postoperative lactate monitoring after extended hepatectomies: Single center experience. Updates Surg. 2015, 67, 33–37. [Google Scholar] [CrossRef]
- Popescu, M.; Dima, S.; Brasoveanu, V.; Tudor, A.; Simionescu, M.; Tomescu, D. High perioperative lactate levels and decreased lactate clearance are associated with increased incidence of posthepatectomy liver failure. Hepatobiliary Pancreat. Dis. Int. 2021, 20, 592–594. [Google Scholar] [CrossRef] [PubMed]
- Riediger, C.; Mueller, M.W.; Hapfelmeier, A.; Bachmann, J.; Friess, H.; Kleeff, J. Preoperative Serum Bilirubin and Lactate Levels Predict Postoperative Morbidity and Mortality in Liver Surgery: A Single-Center Evaluation. Scand. J. Surg. 2015, 104, 176–184. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, I.; Mayumi, T.; Arishima, T.; Takahashi, H.; Shikano, T.; Nakao, A.; Nagino, M.; Nimura, Y.; Takezawa, J. Hyperlactemia can predict the prognosis of liver resection. Shock 2007, 28, 35–38. [Google Scholar] [CrossRef]
- Stockmann, M.; Lock, J.F.; Malinowski, M.; Niehues, S.M.; Seehofer, D.; Neuhaus, P. The LiMAx test: A new liver function test for predicting postoperative outcome in liver surgery. HPB 2010, 12, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Strasberg, S.M. Nomenclature of hepatic anatomy and resections: A review of the Brisbane 2000 system. J. Hepatobiliary Pancreat. Surg. 2005, 12, 351–355. [Google Scholar] [CrossRef]
- Rahbari, N.N.; Garden, O.J.; Padbury, R.; Brooke-Smith, M.; Crawford, M.; Adam, R.; Koch, M.; Makuuchi, M.; Dematteo, R.P.; Christophi, C.; et al. Posthepatectomy liver failure: A definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 2011, 149, 713–724. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Habibzadeh, F.; Habibzadeh, P.; Yadollahie, M. On determining the most appropriate test cut-off value: The case of tests with continuous results. Biochem. Med. 2016, 26, 297–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adeva-Andany, M.; López-Ojén, M.; Funcasta-Calderón, R.; Ameneiros-Rodríguez, E.; Donapetry-García, C.; Vila-Altesor, M.; Rodríguez-Seijas, J. Comprehensive review on lactate metabolism in human health. Mitochondrion 2014, 17, 76–100. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, G.; Bellomo, R.; Bakker, J. The ten pitfalls of lactate clearance in sepsis. Intensive Care Med. 2019, 45, 82–85. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Xu, X. Lactate clearance is a useful biomarker for the prediction of all-cause mortality in critically ill patients: A systematic review and meta-analysis. Crit. Care Med. 2014, 42, 2118–2125. [Google Scholar] [CrossRef] [PubMed]
- Seehofer, D.; Kamphues, C.; Neuhaus, P. Resektion von Klatskin-Tumoren [Resection of Klatskin tumors]. Chirurg 2012, 83, 221–228. [Google Scholar] [CrossRef] [PubMed]
| Variables | N = 152 |
|---|---|
| Patient Characteristics | |
| Mean age, years | 60.5 ± 15.4 |
| Gender, male/female, n (%) | 86 (56.6)/66 (43.4) |
| BMI, kg/m2 | 26.1 ± 4.7 |
| ASA score, I/II/III, n (%) | 6 (3.9)/74 (48.7)/72 (47.4) |
| Parenchymal liver disease, n (%) | |
| Steatosis hepatis/fibrosis/cirrhosis | 21 (13.8)/6 (3.9)/20 (13.2) |
| Diagnosis, n (%) | |
| Benign/malign | 36 (23.7)/116 (76.3) |
| Primary malign hepatic tumors, HCC/CCA/others | 30 (19.7)/22 (14.5)/6 (3.9) |
| Hepatic metastases, CRLM/nCRLM | 44 (28.9)/14 (9.2) |
| Benign lesions, benign tumors/others | 27 (17.8)/9 (5.9) |
| Preoperative laboratory parameters | |
| Base excess, mmol/L | −1.0 ± 2.9 |
| Lactate, mmol/L | 0.8 ± 0.5 |
| Bilirubine, µmol/L | 7.7 ± 15.4 |
| Creatinine, µmol/L | 73 ± 27.3 |
| Portal vein embolization/in-situ split | 10 (6.6)/1 (0.7) |
| Surgery | |
| OLR/HALLR/LLR, n (%) | 107 (70.4)/9 (5.9)/36 (23.7) |
| Minor/major resection, n (%) | 102 (67.1)/50 (32.9) |
| Type of resection, n (%) | |
| Atypical | 20 (13.2) |
| Segmentectomy, −/+ atypical resection | 41 (27.0)/27 (17.8) |
| Bisegmentectomy −/+ atypical resection | 6 (3.9)/8 (5.3) |
| Hemihepatectomy left/right | 10 (6.6)/9 (5.9) |
| Extended hemihepatectomy left/right | 9 (5.9)/22 (14.5) |
| Complex resection, n (%) | 28 (18.4) |
| Biliodigestive anastomosis, n (%) | 14 (9.2) |
| Lymphadenectomy, n (%) | 56 (36.8) |
| T-tube, n (%) | 46 (30.3) |
| Pringle manoeuvre (PM), n (%) | 93 (61.2) |
| PM duration, minutes | 17 ± 16.3 |
| Intraoperative blood loss, mL | 335 ± 445 |
| Transfusion, n (%) | |
| RBC/FFP/albumin | 6 (3.9)/8 (5.3)/10 (6.6) |
| Operation time, minutes | 312 ± 118 |
| Variables | N = 152 |
|---|---|
| ICU | |
| ICU stay, days | 1.0 ± 2.7 |
| Mechanical ventilation, n (%) | 20 (13.2) |
| Transfusion, n (%) | |
| RBC/FFP/albumin | 12 (7.9)/3 (2.0)/5 (3.3) |
| Morbidity, n (%) | |
| Overall complications | 59 (38.8) |
| Total events | 116 |
| Systematic sepsis | 5 (3.3) |
| Liver/biliary | |
| Liver failure/bile leakage/ascites/liver abscess/cholangitis | 7 (4.6)/7 (4.6)/4 (2.6)/3 (2.0)/3 (2.0) |
| Pulmonary | |
| Pleural effusion/pneumonia/unplanned intubation/ventilator requirement > 48 h/pulmonary embolism/pneumothorax | 12 (7.9)/2 (1.3)/4 (2.6)/2 (1.3)/4 (2.6)/3 (2.0) |
| Genitourinary | |
| Renal failure | 8 (5.3) |
| Wounds | |
| Delayed wound healing/fascial dehiscence/wound infection | 26 (17.1)/7 (4.6)/5 (3.3) |
| Vascular | |
| Bleeding/hematoma/embolism liver arteria/deep venous thrombosis | 5 (3.3)/2 (1.3)/1 (0.7)/1 (0.7) |
| Other | |
| Cardiac arrest/cerebral vascular accident/intestinal ischemia/lymphoceles | 1 (0.7)/1 (0.7)/1 (0.7)/2 (1.3) |
| Mortality, n (%) | 7 (4.6) |
| Clavien–Dindo I/II/IIIa/IIIb/IV/V | 18 (11.8)/4 (2.6)/7 (4.6)/14 (9.2)/9 (5.9)/7 (4.6) |
| Hospital stay, days | 9.0 ± 12.2 |
| Variable | Morbidity (CD ≥ IIIb) | p-Value | Mortality | p-Value | ||
|---|---|---|---|---|---|---|
| No | Yes | No | Yes | |||
| Lactate, mmol/L | ||||||
| LAC-PRE | 0.8 ± 0.5 | 0.8 ± 0.5 | 0.809 | 0.8 ± 0.5 | 0.8 ± 0.7 | 0.638 |
| LAC-POST | 1.6 ± 1.2 | 2.9 ± 2.4 | <0.001 | 1.8 ± 1.4 | 4.0 ± 3.4 | <0.001 |
| LAC-POD1 | 1.6 ± 1.5 | 3.0 ± 4.1 | <0.001 | 1.7 ± 1.8 | 7.6 ± 5.9 | <0.001 |
| Variables | Morbidity (Clavien-Dindo ≥ IIIb) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| ROC | UVA | Multivariate Analysis | |||||||
| AUC | Cut-Off Value | Sen (%) | Spe (%) | LRa | p-Value | OR | 95% CI | p-Value | |
| Patient characteristic | |||||||||
| Age | 0.676 | 64.9 years | 73.3 | 67.2 | 2.24 | <0.001 | 7.604 | 2.474–23.370 | <0.001 |
| ASA score I–II vs. III | 0.007 | NS | NS | NS | |||||
| Laboratory tests | |||||||||
| Bilirubin-PRE | 0.632 | 9.25 µmol/L | 60.0 | 64.8 | 1.70 | 0.015 | NS | NS | NS |
| Lactate | |||||||||
| LAC-POST | 0.681 | 2.8 mmol/L | 53.3 | 81.2 | 2.83 | <0.001 | 9.283 | 2.883–29.898 | <0.001 |
| LAC-POD1 | 0.768 | 2.4 mmol/L | 63.3 | 76.2 | 2.66 | 0.001 | NS | NS | NS |
| or: | |||||||||
| LAC-DIFF | 0.710 | −0.7 mmol/L | 53.3 | 86.1 | 3.83 | <0.001 | 5.518 | 1.953–15.590 | 0.001 |
| Surgery | |||||||||
| OLR vs. LLR | 0.015 | NS | NS | NS | |||||
| Extent of LR | |||||||||
| Minor vs. major LR | 0.001 | NS | NS | NS | |||||
| or: | |||||||||
| EHH | 0.002 | NS | NS | NS | |||||
| Complex resection | <0.001 | 4.144 | 1.310–13.106 | 0.016 | |||||
| Lymphadenectomy | 0.014 | NS | NS | NS | |||||
| Duration PM | 0.592 | 20.5 min | 80.0 | 45.9 | 1.48 | 0.013 | 3.957 | 1.184–13.225 | 0.025 |
| Blood loss | 0.634 | 175 mL | 96.4 | 24.5 | 1.28 | 0.037 | NS | NS | NS |
| RBC substitution | 0.013 | NS | NS | NS | |||||
| Operation time | 0.688 | 312 min | 76.7 | 56.6 | 1.77 | 0.005 | NS | NS | NS |
| Variables | Mortality | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| ROC | UVA | Multivariate Analysis | |||||||
| AUC | Cut-Off Value | Sen (%) | Spe (%) | LRa | p-Value | OR | 95% CI | p-Value | |
| Laboratory tests | |||||||||
| Bilirubin-PRE | 0.827 | 13.85 µmol/L | 71.4 | 76.6 | 4.32 | 0.003 | NS | NS | NS |
| Lactate | |||||||||
| LAC-POST | 0.800 | 3.1 mmol/L | 71.4 | 80.0 | 3.57 | 0.009 | 11.685 | 1.757–77.730 | 0.011 |
| LAC-POD1 | 0.838 | 5.4 mmol/L | 71.4 | 94.5 | 12.95 | 0.021 | NS | NS | NS |
| or: | |||||||||
| LAC-DIFF | 0.822 | −2.4 mmol/L | 71.4 | 95.9 | 17.26 | <0.001 | 20.724 | 2.722–157.812 | 0.003 |
| Surgery | |||||||||
| Extent of LR | |||||||||
| Minor vs. major LR | 0.017 | NS | NS | NS | |||||
| or: | |||||||||
| EHH | 0.004 | NS | NS | NS | |||||
| Complex resection | 0.001 | 39.925 | 4.032–375.805 | 0.002 | |||||
| Operation time | 0.858 | 426 min | 85.7 | 82.8 | 4.97 | 0.004 | NS | NS | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Recknagel, S.; Rademacher, S.; Höhne, C.; Lederer, A.A.; Lange, U.G.; Herta, T.; Seehofer, D.; Sucher, R.; Scheuermann, U. Prediction of the Postoperative Outcome in Liver Resection Using Perioperative Serum Lactate Levels. J. Clin. Med. 2023, 12, 2100. https://doi.org/10.3390/jcm12062100
Recknagel S, Rademacher S, Höhne C, Lederer AA, Lange UG, Herta T, Seehofer D, Sucher R, Scheuermann U. Prediction of the Postoperative Outcome in Liver Resection Using Perioperative Serum Lactate Levels. Journal of Clinical Medicine. 2023; 12(6):2100. https://doi.org/10.3390/jcm12062100
Chicago/Turabian StyleRecknagel, Sebastian, Sebastian Rademacher, Claudia Höhne, Andri A. Lederer, Undine G. Lange, Toni Herta, Daniel Seehofer, Robert Sucher, and Uwe Scheuermann. 2023. "Prediction of the Postoperative Outcome in Liver Resection Using Perioperative Serum Lactate Levels" Journal of Clinical Medicine 12, no. 6: 2100. https://doi.org/10.3390/jcm12062100
APA StyleRecknagel, S., Rademacher, S., Höhne, C., Lederer, A. A., Lange, U. G., Herta, T., Seehofer, D., Sucher, R., & Scheuermann, U. (2023). Prediction of the Postoperative Outcome in Liver Resection Using Perioperative Serum Lactate Levels. Journal of Clinical Medicine, 12(6), 2100. https://doi.org/10.3390/jcm12062100






