Efficacy of Contrast-Enhanced Endoscopic Ultrasonography for the Differentiation of Non-Hodgkin’s Lymphoma: A Single-Center Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. EUS Procedure
2.3. Image Analysis
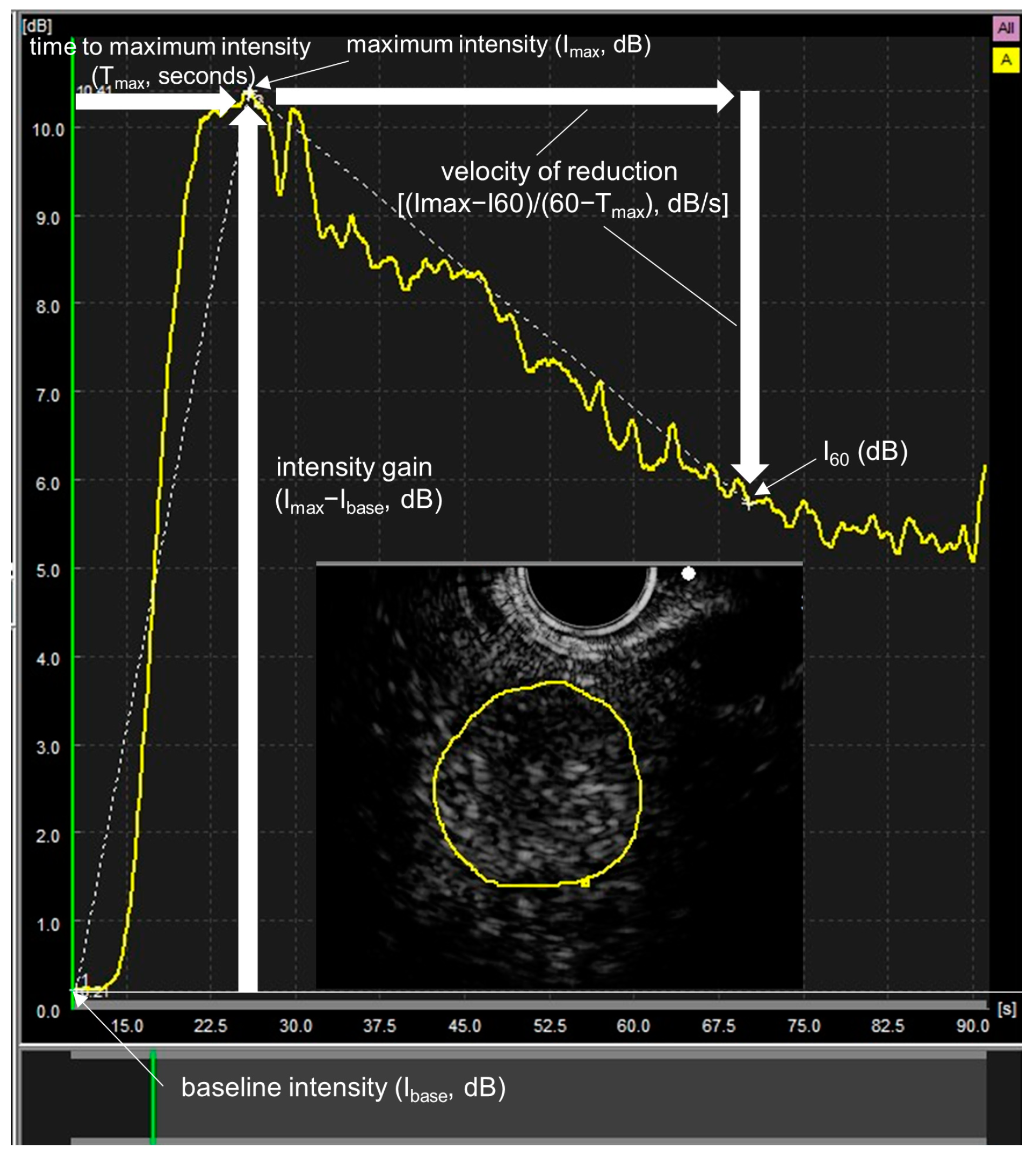
2.4. Time Intensity Curve (TIC) Analysis
2.5. Statistical Analysis
3. Results
3.1. Baseline Patient Characteristics
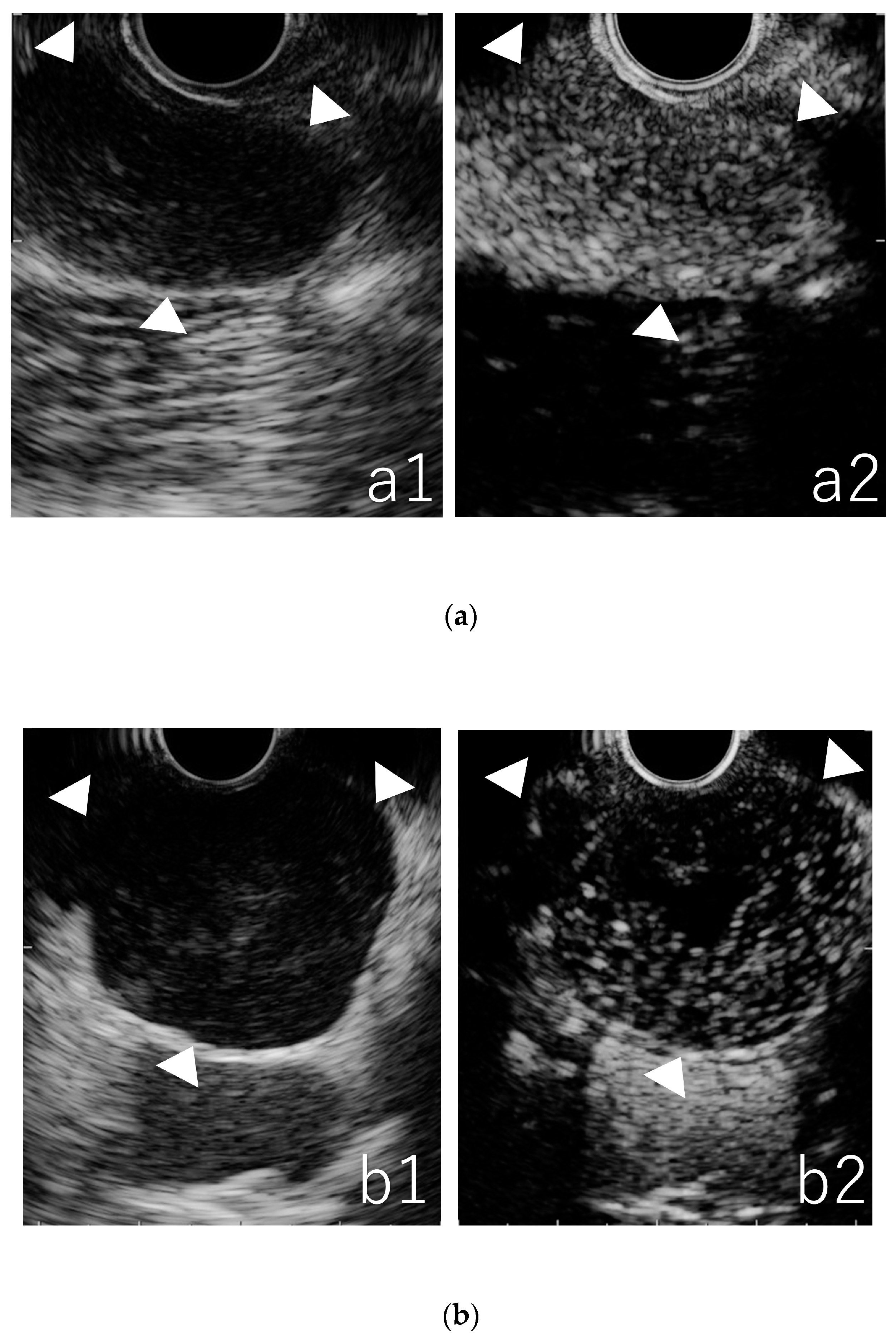
3.2. Qualitative Evaluation in B-Mode EUS
3.3. Qualitative Evaluation in CE-EUS
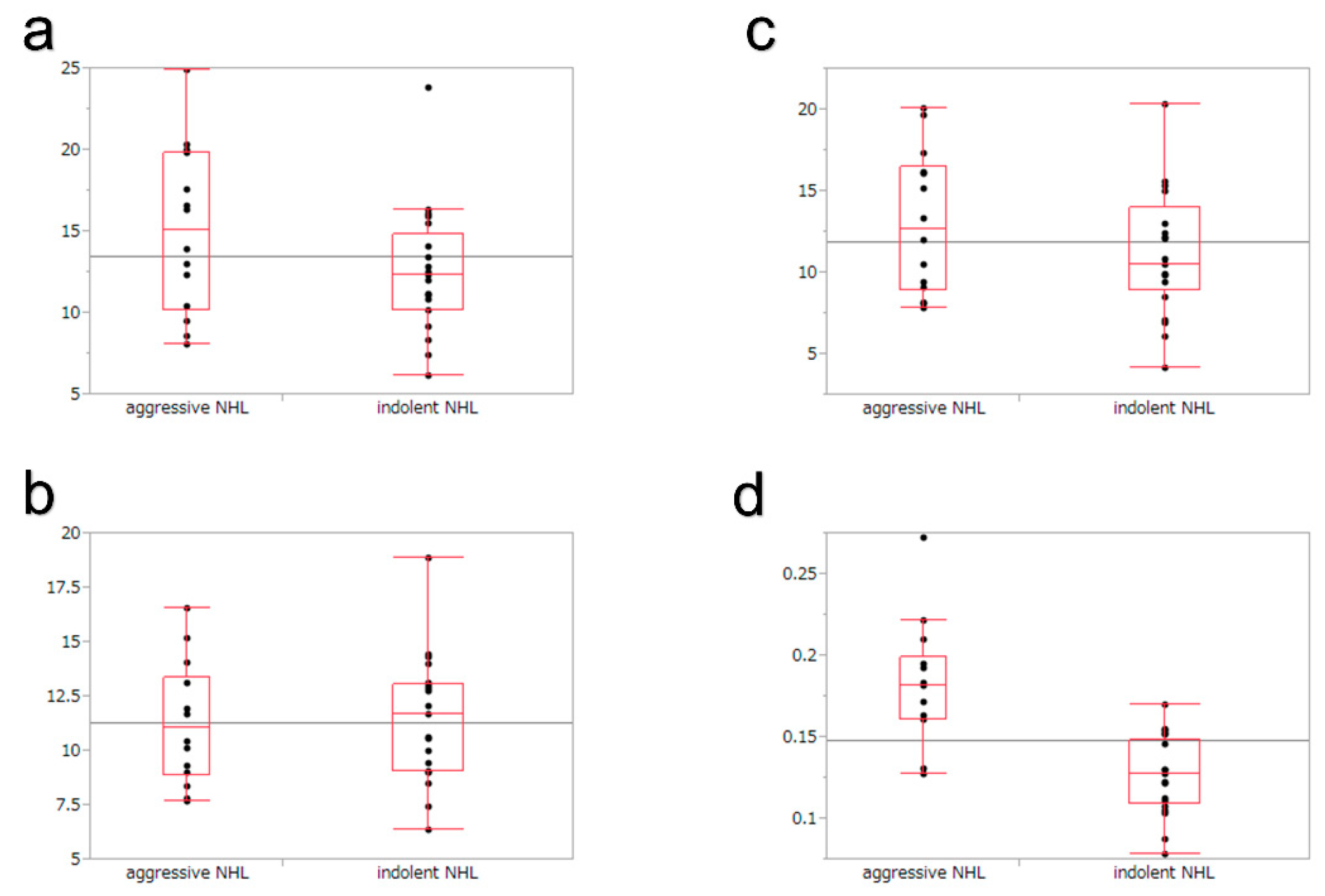
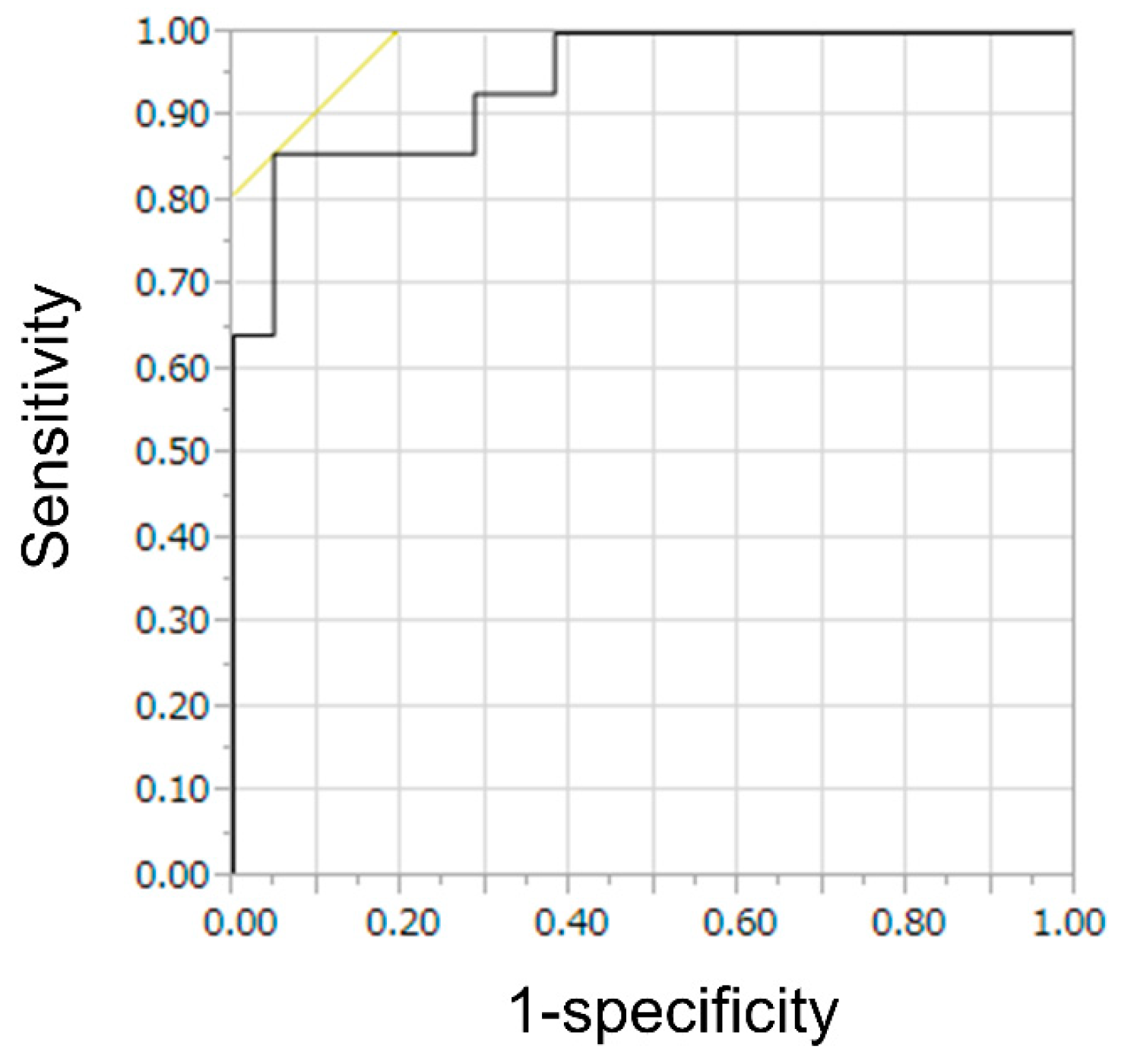
3.4. Quantitative Evaluation in TIC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Jakić-Razumović, J.; Aurer, I. The World Health Organization classification of lymphomas. Croat Med. J. 2002, 43, 527–534. [Google Scholar] [PubMed]
- Dumonceau, J.-M.; Deprez, P.H.; Jenssen, C.; Iglesias-Garcia, J.; Larghi, A.; Vanbiervliet, G.; Aithal, G.P.; Arcidiacono, P.G.; Bastos, P.; Carrara, S.; et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline—Updated January 2017. Endoscopy 2017, 49, 695–714. [Google Scholar] [CrossRef] [PubMed]
- Kanamori, A.; Hirooka, Y.; Itoh, A.; Hashimoto, S.; Kawashima, H.; Hara, K.; Uchida, H.; Goto, J.; Ohmiya, N.; Niwa, Y.; et al. Usefulness of contrast-enhanced endoscopic ultrasonography in the differentiation between malignant and benign lymphadenopathy. Am. J. Gastroenterol. 2006, 101, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Hocke, M.; Menges, M.; Topalidis, T.; Dietrich, C.F.; Stallmach, A. Contrast-enhanced endoscopic ultrasound in discrimination between benign and malignant mediastinal and abdominal lymph nodes. J. Cancer Res. Clin. Oncol. 2008, 134, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Kitano, M.; Kudo, M.; Imai, H.; Kamata, K.; Sakamoto, H.; Komaki, T. Characterization of intra-abdominal lesions of undetermined origin by contrast-enhanced harmonic EUS (with videos). Gastrointest Endosc. 2010, 72, 637–642. [Google Scholar] [CrossRef]
- Miyata, T.; Kitano, M.; Omoto, S.; Kadosaka, K.; Kamata, K.; Imai, H.; Sakamoto, H.; Nisida, N.; Harwani, Y.; Murakami, T.; et al. Contrast-enhanced harmonic endoscopic ultrasonography for assessment of lymph node metastases in pancreatobiliary carcinoma. World J. Gastroenterol. 2016, 22, 3381–3391. [Google Scholar] [CrossRef]
- Omoto, S.; Kitano, M.; Fukasawa, M.; Ashida, R.; Kato, H.; Shiomi, H.; Sugimori, K.; Kanno, A.; Chiba, Y.; Takano, S.; et al. Tissue harmonic versus contrast-enhanced harmonic endoscopic ultrasonography for the diagnosis of pancreatic tumors: Prospective multicenter study. Dig. Endosc. 2022, 34, 198–206. [Google Scholar] [CrossRef]
- Ishikawa, R.; Kamata, K.; Hara, A.; Tanaka, H.; Okamoto, A.; Yamazaki, T.; Nakai, A.; Omoto, S.; Minaga, K.; Yamao, K.; et al. Utility of contrast-enhanced harmonic endoscopic ultrasonography for predicting the prognosis of pancreatic neuroendocrine neoplasms. Dig. Endosc. 2021, 33, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Chen, K.; Cheng, M.-F.; Lin, C.; Wang, H.; Sung, C.; Chen, J.; Yen, R.-F.; Hsu, C.; Shih, S. Endoscopic ultrasound ablation in a patient with multiple metastatic pancreatic tumors from adrenocorticotropic hormone-producing thymic neuroendocrine neoplasm. Dig. Endosc. 2021, 33, 458–463. [Google Scholar] [CrossRef]
- Iwashita, T.; Uemura, S.; Mita, N.; Iwasa, Y.; Ichikawa, H.; Senjyu, A.; Yasuda, I.; Shimizu, M. Utility of endoscopic ultrasound and endoscopic ultrasound-guided fine-needle aspiration for the diagnosis and management of pancreatic cystic lesions: Differences between the guidelines. Dig. Endosc. 2020, 32, 251–262. [Google Scholar] [CrossRef]
- Yoshida, K.; Iwashita, T.; Uemura, S.; Mita, N.; Iwata, K.; Mukai, T.; Yasuda, I.; Shimizu, M. Efficacy of contrast-enhanced EUS for lymphadenopathy: A prospective multicenter pilot study (with videos). Gastrointest Endosc. 2019, 90, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.K. The new World Health Organization classification of lymphomas: The past, the present and the future. Hematol. Oncol. 2001, 19, 129–150. [Google Scholar] [CrossRef]
- Gu, L.-S.; Cui, N.-Y.; Wang, Y.; Che, S.-N.; Zou, S.-M.; He, W.; Liu, J.-Y.; Gong, X.-T. Comparison of sonographic characteristics of primary thyroid lymphoma and anaplastic thyroid carcinoma. J. Thorac. Dis. 2017, 9, 4774–4784. [Google Scholar] [CrossRef] [PubMed]
- Castroagudín, J.F.; Molina, E.; Abdulkader, I.; Forteza, J.; Delgado, M.B.; Dominguez-Munoz, J.E. Sonographic Features of Liver Involvement by Lymphoma. J. Ultrasound Med. 2007, 26, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Xue, H.; Wang, Q.; Zhang, X.; Wang, Z.; Zhao, C. Value of contrast-enhanced ultrasound and PET/CT in assessment of extramedullary lymphoma. Eur. J. Radiol. 2018, 99, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Ling, W.; Xia, F.; Zhang, Y.; Zhu, C.; He, J. Application of Contrast-Enhanced Ultrasound (CEUS) in Lymphomatous Lymph Nodes: A Comparison between PET/CT and Contrast-Enhanced CT. Contrast Media Mol. Imaging. 2019, 2019, 5709698. [Google Scholar] [CrossRef]
- Alobthani, G.; Romanov, V.; Isohashi, K.; Matsunaga, K.; Watabe, T.; Kato, H.; Tatsumi, M.; Shimosegawa, E.; Hatazawa, J. Value of 18F-FDG PET/CT in discrimination between indolent and aggressive non-Hodgkin’s lymphoma: A study of 328 patients. Hell J. Nucl. Med. 2018, 21, 7–14. [Google Scholar]
- Schöder, H.; Noy, A.; Gönen, M.; Weng, L.; Green, D.; Erdi, Y.E.; Larson, S.M.; Yeung, H.W. Intensity of 18fluorodeoxyglucose uptake in positron emission tomography distinguishes between indolent and aggressive non-Hodgkin’s lymphoma. J. Clin. Oncol. 2005, 23, 4643–4651. [Google Scholar] [CrossRef]
- Ngeow, J.Y.Y.; Quek, R.H.H.; Ng, D.C.E.; Hee, S.W.; Tao, M.; Lim, L.C.; Tan, Y.H.; Lim, S.T. High SUV uptake on FDG-PET/CT predicts for an aggressive B-cell lymphoma in a prospective study of primary FDG-PET/CT staging in lymphoma. Ann. Oncol. 2009, 20, 1543–1547. [Google Scholar] [CrossRef]
- Ribeiro, A.; Pereira, D.; Escalón, M.P.; Goodman, M.; Byrne, G.E. EUS-guided biopsy for the diagnosis and classification of lymphoma. Gastrointest Endosc. 2010, 71, 851–855. [Google Scholar] [CrossRef] [PubMed]


| Aggressive NHL | Indolent NHL | p Value | ||
|---|---|---|---|---|
| Age, year old, median (Range) | 69 (31–86) | 68 (54–83) | 0.9077 | |
| Sex, n | Male | 20 | 16 | 0.6653 |
| Female | 13 | 13 | ||
| Location, n | Mediastinum | 3 | 1 | 0.3545 |
| Abdominal | 30 | 28 | ||
| Aggressive NHL, n | Diffuse large B-cell lymphoma | 27 | ||
| Peripheral T-cell lymphoma | 2 | |||
| Extranodal NK/T-cell lymphoma | 1 | |||
| Plasmablastic lymphoma | 1 | |||
| T-cell lymphoma/histiocyte rich B-cell lymphoma | 1 | |||
| Adult T-cell leukemia/lymphoma | 1 | |||
| Total | 33 | |||
| Indolent NHL, n | Follicular lymphoma | 27 | ||
| Mantle cell lymphoma | 1 | |||
| MALT lymphoma | 1 | |||
| Total | 29 |
| Aggressive NHL (N = 33) | Indolent NHL (N = 29) | p Value | ||
|---|---|---|---|---|
| Size of region, mm, median (range) | short axis | 21 (7–75) | 17 (4–85) | 0.3057 |
| long axis | 29 (10–80) | 23 (10–98) | 0.4629 | |
| Shape, n | round | 17 | 17 | 0.5745 |
| oval | 16 | 12 | ||
| Border, n | sharp | 15 | 18 | 0.1895 |
| fuzzy | 18 | 11 | ||
| Echogenecity, n | hyperechoic | 2 | 1 | 0.6324 |
| hypoechoic | 31 | 28 | ||
| Echotexture, n | heterogeneous | 21 | 14 | 0.2236 |
| homogeneous | 12 | 15 |
| Hypervascular/Homogeneous | Hypervascular/Heterogeneous | Hypovascular/Heterogenous | p Value | |
|---|---|---|---|---|
| Aggressive NHL, n | 13 | 5 | 15 | |
| Diffuse large B-cell lymphoma, n | 10 | 5 | 12 | 0.5601 |
| Peripheral T-cell lymphoma, n | 1 | 0 | 1 | 0.754 |
| Extranodal NK/T-cell lymphoma, n | 0 | 0 | 1 | 0.3121 |
| Plasmablastic lymphoma, n | 1 | 0 | 0 | 0.1668 |
| T-cell lymphoma/histiocyte rich B-cell lymphoma, n | 0 | 0 | 1 | 0.3121 |
| Adult T-cell leukemia/lymphoma, n | 1 | 0 | 0 | 0.1668 |
| Indolent NHL, n | 21 | 0 | 8 | |
| Follicular lymphoma, n | 20 | 0 | 7 | 0.4855 |
| Mantle cell lymphoma, n | 0 | 0 | 1 | 0.1022 |
| MALT lymphoma, n | 1 | 0 | 0 | 0.4169 |
| Homogeneous | Heterogeneous | |||||
|---|---|---|---|---|---|---|
| Indolent NHL | Aggressive NHL | p Value | Indolent NHL | Aggressive NHL | p Value | |
| Peak intensity, dB | 12.3 | 13.9 | 0.178 | 4.9 | 5 | 0.3597 |
| Time to peak, seconds | 11.7 | 10.5 | 0.6577 | 11.7 | 11.1 | 0.8988 |
| Intensity gain, dB | 10.5 | 12 | 0.4565 | 4.5 | 4.7 | 0.3467 |
| Velocity of reduction, dB/s | 0.127 | 0.182 | <0.0001 | 0.057 | 0.066 | 0.4014 |
| Diagnosis of CE-EUS | ||
|---|---|---|
| Indolent NHL | Aggressive NHL | |
| Indolent NHL, n | 20 | 9 |
| Follicular lymphoma, n | 19 | 8 |
| Mantle cell lymphoma, n | 0 | 1 |
| MALT lymphoma, n | 1 | 0 |
| Aggressive NHL, n | 2 | 31 |
| Diffuse large B-cell lymphoma, n | 1 | 26 |
| Peripheral T-cell lymphoma, n | 0 | 2 |
| Extranodal NK/T-cell lymphoma, n | 0 | 1 |
| Plasmablastic lymphoma, n | 0 | 1 |
| T-cell lymphoma/histiocyte rich B-cell lymphoma, n | 0 | 1 |
| Adult T-cell leukemia/lymphoma, n | 1 | 0 |
| Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | Accuracy (95% CI) | |
|---|---|---|---|---|---|
| CE-EUS qualitative evaluation (%) | 61 (44–75) | 72 (54–85) | 71 (53–85) | 62 (45–76) | 66 (54–77) |
| CE-EUS combined evaluation (%) | 94 (80–98) | 69 (51–83) | 78 (62–88) | 91 (72–97) | 82 (71–90) † |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, K.; Iwashita, T.; Mita, N.; Iwasa, Y.; Uemura, S.; Shimizu, M. Efficacy of Contrast-Enhanced Endoscopic Ultrasonography for the Differentiation of Non-Hodgkin’s Lymphoma: A Single-Center Retrospective Cohort Study. J. Clin. Med. 2023, 12, 2054. https://doi.org/10.3390/jcm12052054
Yoshida K, Iwashita T, Mita N, Iwasa Y, Uemura S, Shimizu M. Efficacy of Contrast-Enhanced Endoscopic Ultrasonography for the Differentiation of Non-Hodgkin’s Lymphoma: A Single-Center Retrospective Cohort Study. Journal of Clinical Medicine. 2023; 12(5):2054. https://doi.org/10.3390/jcm12052054
Chicago/Turabian StyleYoshida, Kensaku, Takuji Iwashita, Naoki Mita, Yuhei Iwasa, Shinya Uemura, and Masahito Shimizu. 2023. "Efficacy of Contrast-Enhanced Endoscopic Ultrasonography for the Differentiation of Non-Hodgkin’s Lymphoma: A Single-Center Retrospective Cohort Study" Journal of Clinical Medicine 12, no. 5: 2054. https://doi.org/10.3390/jcm12052054
APA StyleYoshida, K., Iwashita, T., Mita, N., Iwasa, Y., Uemura, S., & Shimizu, M. (2023). Efficacy of Contrast-Enhanced Endoscopic Ultrasonography for the Differentiation of Non-Hodgkin’s Lymphoma: A Single-Center Retrospective Cohort Study. Journal of Clinical Medicine, 12(5), 2054. https://doi.org/10.3390/jcm12052054








