The Effect of Two-Generation Exposure to a High-Fat Diet on Craniofacial Morphology in Rats
Abstract
1. Introduction
2. Materials and Methods
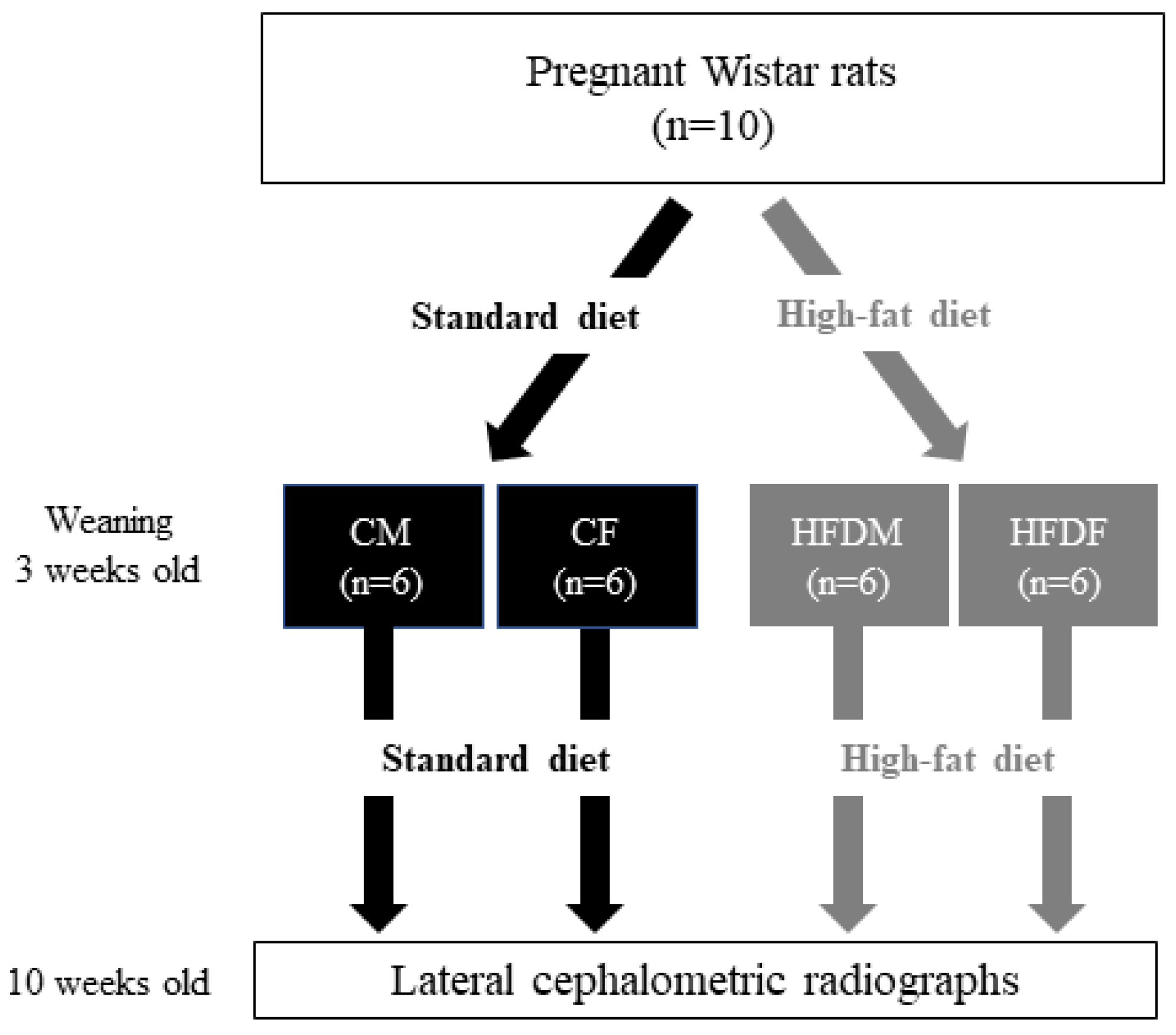
2.1. Animals and Experimental Design
2.2. Body Weight and Fasting Blood Glucose Measurement
2.3. Cephalometric Analyses
2.4. Statistical Analysis
3. Results
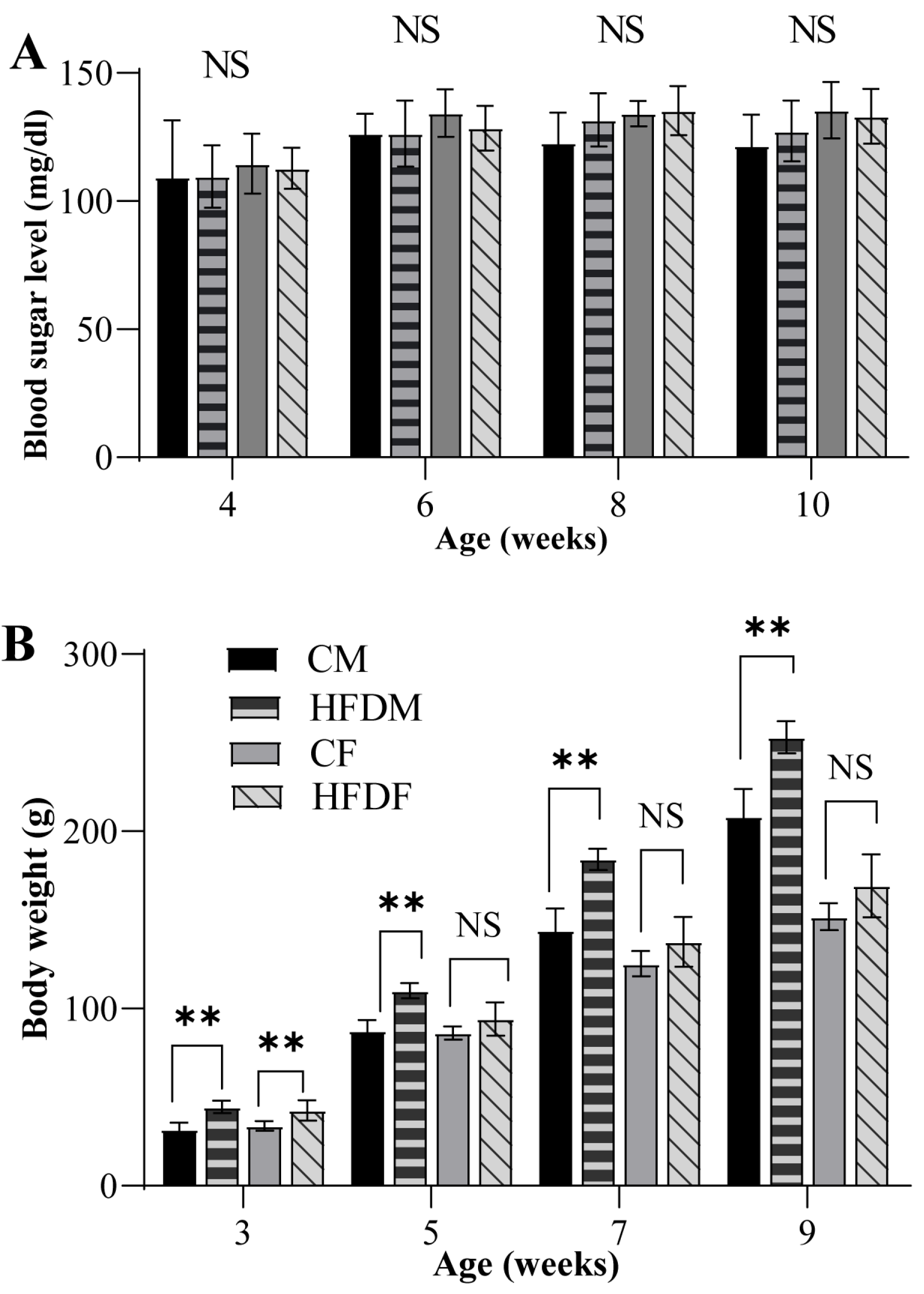
3.1. Changes in Body Weight but Not FBS in Offspring
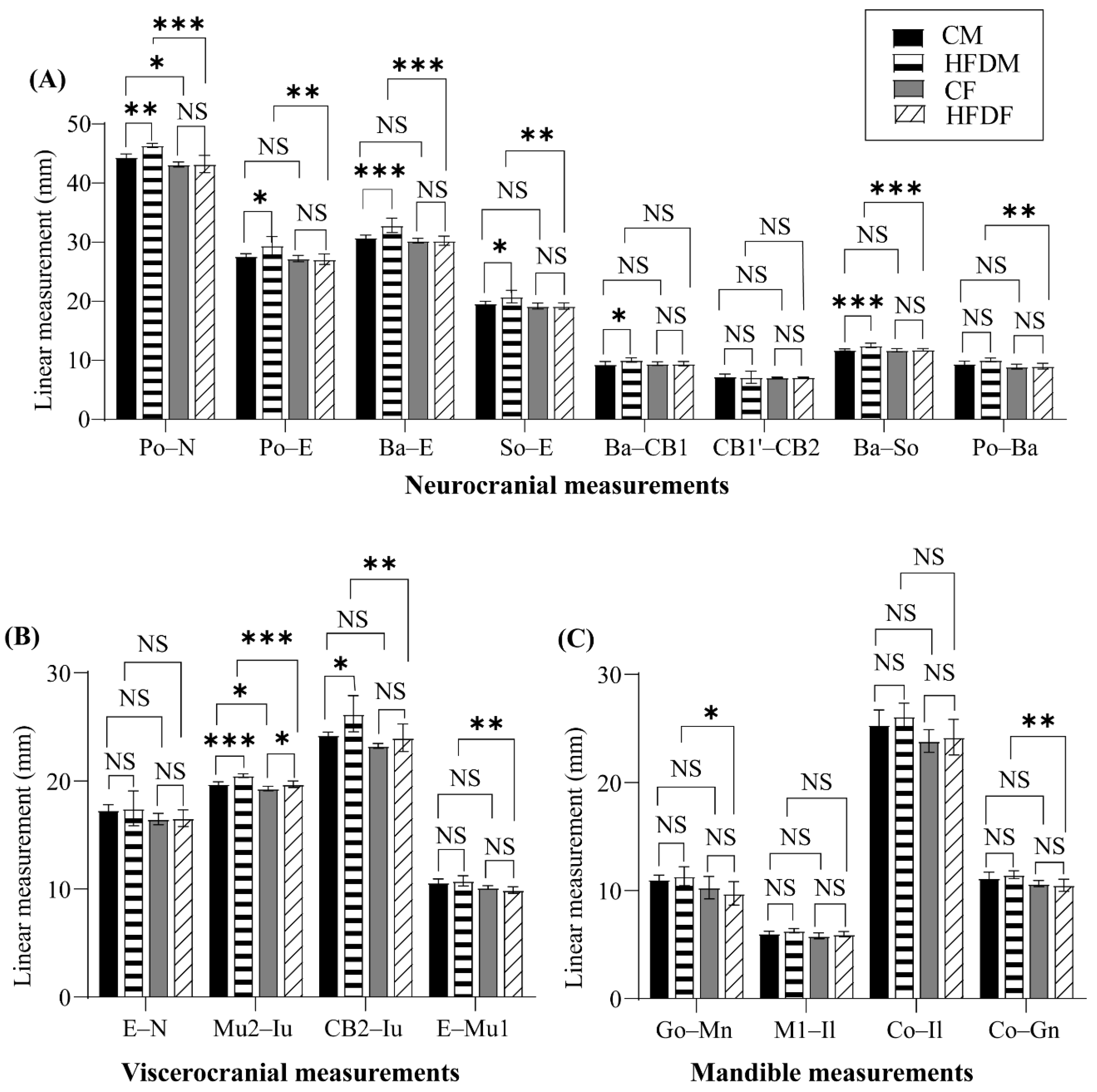
3.2. Changes in the Cephalometric Parameters
3.2.1. Changes in the Neurocranium
3.2.2. Changes in the Viscerocranium
3.2.3. Changes in the Mandible
3.2.4. Changes in the Dental Morphology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation on Obesity; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Bayol, S.A.; Farrington, S.J.; Stickland, N.C. A maternal ‘junk food’ diet in pregnancy and lactation promotes an exacerbated taste for ‘junk food’ and a greater propensity for obesity in rat offspring. Br. J. Nutr. 2007, 98, 843–851. [Google Scholar] [CrossRef] [PubMed]
- de Almeida Faria, J.; Duque-Guimarães, D.; Carpenter, A.A.; Loche, E.; Ozanne, S.E. A post-weaning obesogenic diet exacerbates the detrimental effects of maternal obesity on offspring insulin signaling in adipose tissue. Sci. Rep. 2017, 7, 44949. [Google Scholar] [CrossRef] [PubMed]
- Lanham, S.A.; Roberts, C.; Hollingworth, T.; Sreekumar, R.; Elahi, M.M.; Cagampang, F.R.; Hanson, M.A.; Oreffo, R.O. Maternal high-fat diet: Effects on offspring bone structure. Osteoporos. Int. 2010, 21, 1703–1714. [Google Scholar] [CrossRef] [PubMed]
- Loche, E.; Blackmore, H.L.; Carpenter, A.A.; Beeson, J.H.; Pinnock, A.; Ashmore, T.J.; Aiken, C.E.; de Almeida-Faria, J.; Schoonejans, J.M.; Giussani, D.A.; et al. Maternal diet-induced obesity programmes cardiac dysfunction in male mice independently of post-weaning diet. Cardiovasc. Res. 2017, 114, 1372–1384. [Google Scholar] [CrossRef] [PubMed]
- Birch, L.L.; Fisher, J.O. Development of eating behaviors among children and adolescents. Pediatrics 1998, 101, 539–549. [Google Scholar] [CrossRef]
- Buckels, E.J.; Bolam, S.M.; Tay, M.L.; Matthews, B.G. The impact of maternal high-fat diet on bone microarchitecture in offspring. Front. Nutr. 2021, 8, 730037. [Google Scholar] [CrossRef]
- Petersen, S.B.; Rasmussen, M.A.; Olsen, S.F.; Vestergaard, P.; Mølgaard, C.; Halldorsson, T.I.; Strøm, M. Maternal dietary patterns during pregnancy in relation to offspring forearm fractures: Prospective study from the danish national birth cohort. Nutrients 2015, 7, 2382–2400. [Google Scholar] [CrossRef]
- Chen, J.-R.; Lazarenko, O.P.; Zhao, H.; Alund, A.W.; Shankar, K. Maternal obesity impairs skeletal development in adult offspring. J. Endocrinol. 2018, 239, 33–47. [Google Scholar] [CrossRef]
- Kushwaha, P.; Khambadkone, S.G.; Li, M.; Goodman, E.J.; Aravindan, N.; Riddle, R.C.; Tamashiro, K.L.K. Maternal high-fat diet induces long-lasting defects in bone structure in rat offspring through enhanced osteoclastogenesis. Calcif. Tissue Int. 2021, 108, 680–692. [Google Scholar] [CrossRef]
- Harasymowicz, N.S.; Choi, Y.-R.; Wu, C.-L.; Iannucci, L.; Tang, R.; Guilak, F. Intergenerational transmission of diet-induced obesity, metabolic imbalance, and osteoarthritis in mice. Arthritis Rheumatol. 2020, 72, 632–644. [Google Scholar] [CrossRef]
- Lanham, S.; Cagampang, F.R.; Oreffo, R.O.C. Maternal high fat diet affects offspring’s vitamin k-dependent proteins expression levels. PLoS ONE 2015, 10, e0138730. [Google Scholar] [CrossRef]
- Ursi, W.J.S.; Trotman, C.-A.; McNamara, J.A., Jr.; Behrents, R.G. Sexual dimorphism in normal craniofacial growth. Angle Orthod. 1993, 63, 47–56. [Google Scholar] [CrossRef]
- Roosenboom, J.; Hens, G.; Mattern, B.C.; Shriver, M.D.; Claes, P. Exploring the underlying genetics of craniofacial morphology through various sources of knowledge. BioMed Res. Int. 2016, 2016, 3054578. [Google Scholar] [CrossRef] [PubMed]
- Perrett, D.I.; Lee, K.J.; Penton-Voak, I.; Rowland, D.; Yoshikawa, S.; Burt, D.M.; Henzi, S.P.; Castles, D.L.; Akamatsu, S. Effects of sexual dimorphism on facial attractiveness. Nature 1998, 394, 884–887. [Google Scholar] [CrossRef] [PubMed]
- Hwang, L.-L.; Wang, C.-H.; Li, T.-L.; Chang, S.-D.; Lin, L.-C.; Chen, C.-P.; Chen, C.-T.; Liang, K.-C.; Ho, I.-K.; Yang, W.-S.; et al. Sex differences in high-fat diet-induced obesity, metabolic alterations and learning, and synaptic plasticity deficits in mice. Obesity 2010, 18, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.-P.; Ronveaux, C.C.; Knotts, T.A.; Rutkowsky, J.R.; Ramsey, J.J.; Raybould, H.E. Sex differences in response to short-term high fat diet in mice. Physiol. Behav. 2020, 221, 112894. [Google Scholar] [CrossRef] [PubMed]
- Freire-Regatillo, A.; Fernández-Gómez, M.J.; Díaz, F.; Barrios, V.; Sánchez-Jabonero, I.; Frago, L.M.; Argente, J.; García-Segura, L.M.; Chowen, J.A. Sex differences in the peripubertal response to a short-term, high-fat diet intake. J. Neuroendocrinol. 2020, 32, e12756. [Google Scholar] [CrossRef]
- Wilson, D.B.; Finta, L.A. Early development of the brain and spinal cord in dysraphic mice. Anat. Embryol. 1980, 160, 315–326. [Google Scholar] [CrossRef]
- Cerf, M.E.; Louw, J.; Herrera, E. High fat diet exposure during fetal life enhances plasma and hepatic omega-6 fatty acid profiles in fetal wistar rats. Nutrients 2015, 7, 7231–7241. [Google Scholar] [CrossRef]
- Abbassy, M.A.; Watari, I.; Soma, K. Effect of experimental diabetes on craniofacial growth in rats. Arch. Oral Biol. 2008, 53, 819–825. [Google Scholar] [CrossRef]
- Chałas, R.; Rudzka, O.; Wójcik-Chęcińska, I.; Vodanović, M. The impact of type 1 diabetes on the development of the craniofacial mineralised tissues (bones and teeth): Literature review. Folia Morphol. 2016, 75, 275–280. [Google Scholar] [CrossRef]
- Tomita, K.; Nakada, T.; Oshima, T.; Kawaguchi, R.; Oda, S. Serum levels of tau protein increase according to the severity of the injury in DAI rat model. F1000Research 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Goto, S.; Fujita, Y.; Hotta, M.; Sugiyama, A.; Maki, K. Influence of differences in the hardness and calcium content of diets on the growth of craniofacial bone in rats. Angle Orthod. 2015, 85, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Botero-González, D.; Pustovrh, M.C.; Ortiz, M.; Herrera-Rubio, A.M. Effects of an obesogenic diet on craniofacial morphology in rats. J. Int. Dent. Med. Res. 2021, 14, 5–11. [Google Scholar]
- Kim, H.Y. Statistical notes for clinical researchers: Evaluation of measurement error 2: Dahlberg’s error, Bland-Altman method, and Kappa coefficient. Restor. Dent. Endod. 2013, 38, 182–185. [Google Scholar] [CrossRef]
- Fujita, T.; Ohtani, J.; Shigekawa, M.; Kawata, T.; Kaku, M.; Kohno, S.; Tsutsui, K.; Tenjo, K.; Motokawa, M.; Tohma, Y.; et al. Effects of sex hormone disturbances on craniofacial growth in newborn mice. J. Dent. Res. 2004, 83, 250–254. [Google Scholar] [CrossRef]
- DeMoss, D.L.; Wright, G.L. Sex and strain differences in whole skeletal development in the rat. Calcif. Tissue Int. 1998, 62, 153–157. [Google Scholar] [CrossRef]
- Varghese, M.; Griffin, C.; Abrishami, S.; Eter, L.; Lanzetta, N.; Hak, L.; Clemente, J.; Agarwal, D.; Lerner, A.; Westerhoff, M.; et al. Sex hormones regulate metainflammation in diet-induced obesity in mice. J. Biol. Chem. 2021, 297, 101229. [Google Scholar] [CrossRef]
- Yagasaki, Y.; Yamaguchi, T.; Watahiki, J.; Konishi, M.; Katoh, H.; Maki, K. The role of craniofacial growth in leptin deficient (ob/ob) mice. Orthod. Craniofacial Res. 2003, 6, 233–241. [Google Scholar] [CrossRef]
- Achilleos, A.; Trainor, P.A. Chapter Sixteen—Mouse models of rare craniofacial disorders. In Current Topics in Developmental Biology; Chai, Y., Ed.; Academic Press: Cambridge, MA, USA, 2015; Volume 115, pp. 413–458. [Google Scholar]
- Tan, S.S.; Morriss-Kay, G. The development and distribution of the cranial neural crest in the rat embryo. Cell Tissue Res. 1985, 240, 403–416. [Google Scholar] [CrossRef]
- Fernandes, R.M.; Abreu, A.V.; Silva, R.B.; Silva, D.F.; Martinez, G.L.; Babinski, M.A.; Ramos, C.F. Maternal malnutrition during lactation reduces skull growth in weaned rat pups: Experimental and morphometric investigation. Anat. Sci. Int. 2008, 83, 123–130. [Google Scholar] [CrossRef]
- Nagata, M.; Ohashi, Y.; Ozawa, H. A histochemical study of the development of premaxilla and maxilla during secondary palate formation in the mouse embryo. Arch. Histol. Cytol. 1991, 54, 267–278. [Google Scholar] [CrossRef]
- Mendes-da-Silva, C.; Giriko, C.; Mennitti, L.V.; Hosoume, L.F.; Souto Tdos, S.; Silva, A.V. Maternal high-fat diet during pregnancy or lactation changes the somatic and neurological development of the offspring. Arq. De Neuro-Psiquiatr. 2014, 72, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Khaire, A.; Wadhwani, N.; Madiwale, S.; Joshi, S. Maternal fats and pregnancy complications: Implications for long-term health. Prostaglandins Leukot. Essent. Fat. Acids 2020, 157, 102098. [Google Scholar] [CrossRef] [PubMed]
- Herrera, E.; Amusquivar, E.; López-Soldado, I.; Ortega, H. Maternal lipid metabolism and placental lipid transfer. Horm. Res. 2006, 65 (Suppl. S3), 59–64. [Google Scholar] [CrossRef] [PubMed]
- Gallou-Kabani, C.; Gabory, A.; Tost, J.; Karimi, M.; Mayeur, S.; Lesage, J.; Boudadi, E.; Gross, M.-S.; Taurelle, J.; Vigé, A.; et al. Sex- and Diet-Specific Changes of Imprinted Gene Expression and DNA Methylation in Mouse Placenta under a High-Fat Diet. PLoS ONE 2010, 5, e14398. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. What is the placenta? Am. J. Obstet. Gynecol. 2015, 213, S6.e1–S6.e4. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.N.; Woollett, L.A.; Barbour, N.; Prasad, P.D.; Powell, T.L.; Jansson, T. High-fat diet before and during pregnancy causes marked up-regulation of placental nutrient transport and fetal overgrowth in C57/BL6 mice. FASEB J. 2009, 23, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Neeley, W.W.; Gonzales, D.A. Obesity in adolescence: Implications in orthodontic treatment. Am. J. Orthod. Dentofac. Orthop. 2007, 131, 581–588. [Google Scholar] [CrossRef]
- Sadeghianrizi, A.; Forsberg, C.M.; Marcus, C.; Dahllöf, G. Craniofacial development in obese adolescents. Eur. J. Orthod. 2005, 27, 550–555. [Google Scholar] [CrossRef]
- Chen, J.-R.; Zhang, J.; Lazarenko, O.P.; Kang, P.; Blackburn, M.L.; Ronis, M.J.J.; Badger, T.M.; Shankar, K. Inhibition of fetal bone development through epigenetic down-regulation of HoxA10 in obese rats fed high-fat diet. FASEB J. 2012, 26, 1131–1141. [Google Scholar] [CrossRef]
- Liang, C.; Oest, M.E.; Jones, J.C.; Prater, M.R. Gestational high saturated fat diet alters C57BL/6 mouse perinatal skeletal formation. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2009, 86, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Scott, G.R. Dental anthropology. Encycl. Hum. Biol. 1991, 2, 789–804. [Google Scholar]
- Imai, A.; Yamashita, A.; Ota, M. High-fat diet increases labial groove formation in maxillary incisors and is related to aging in C57BL/6 mice. J. Oral Biosci. 2019, 62. [Google Scholar] [CrossRef] [PubMed]
- Townsend, G.C.; Aldred, M.J.; Bartold, P.M. Genetic aspects of dental disorders. Aust. Dent. J. 1998, 43, 269–286. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Hosonuma, M.; Sugawara, D.; Azetsu, Y.; Karakawa, A.; Chatani, M.; Funatsu, T.; Takami, M.; Sakai, N. Cholesterol and fat in diet disrupt bone and tooth homeostasis in mice. Biomed. Pharmacother. 2022, 156, 113940. [Google Scholar] [CrossRef] [PubMed]

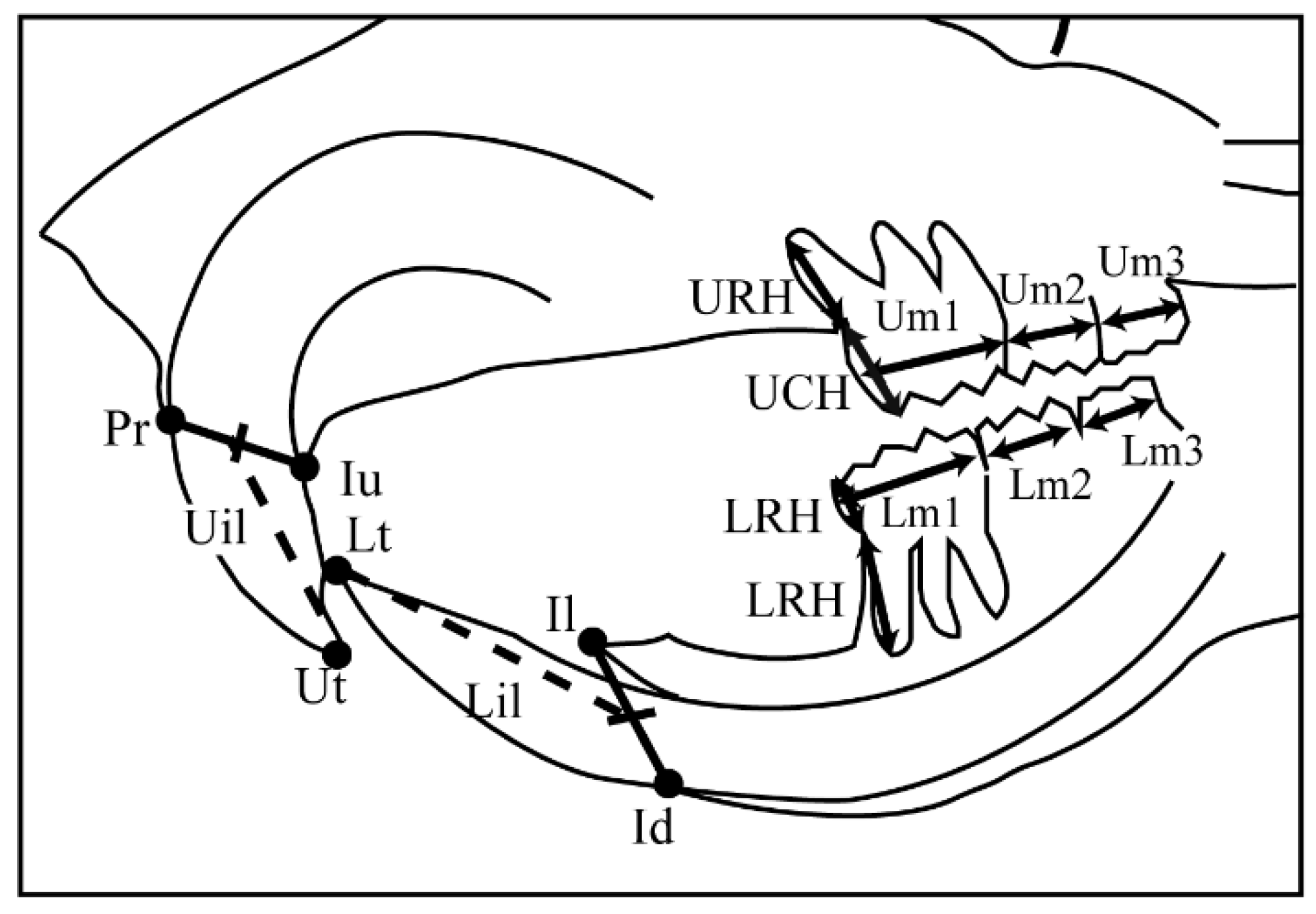

| Landmark | Definition |
|---|---|
| N | The most anterior point on the nasal bone |
| E | The intersection of the frontal bone and floor of the anterior cranial fossa |
| Po | The most posterior and superior point on the skull |
| Ba | The most posterior and inferior point on the occipital condyle |
| Co | The most posterior and superior point on the mandibular condyle |
| Go | The most posterior point on the mandibular ramus |
| Mn | The most concave portion of the concavity on the inferior border of the mandibular corpus |
| Gn | The most inferior point on the ramus that lies on a perpendicular bisector of the line Go–Mn |
| I1 | The most anterior and superior point on the alveolar bone of the mandibular incisor |
| So | The intersection of the most anterior tympanic bulla and the superior border of the sphenoid bone |
| CB1 | The most anterior point on the occipital bone at the spheno-occipital synchondrosis |
| CB1′ | The most posterior point on the sphenoid bone at the spheno-occipital synchondrosis |
| CB2 | The most anterior point on the sphenoid bone at the spheno-basispheno synchondrosis |
| CB2′ | The most posterior point on the basisphenoid bone at the spheno-basispheno synchondrosis |
| M1 | The junction of the alveolar bone and the mesial surface of the first mandibular molar |
| Mu1 | The junction of the alveolar bone and the mesial surface of the first maxillary molar |
| Mu2 | The junction of the alveolar bone and the distal surface of the third maxillary molar |
| Iu | The most anterior and inferior point on the maxilla posterior to the maxillary incisors |
| Pr | The most anterior and inferior point on the alveolar process of the premaxilla |
| Id | The most anterior and inferior point on the alveolar process of the mandible |
| Ut | The most prominent point between the incisal edges of the upper incisors |
| Lt | The most prominent point between the incisal edges of the lower incisors |
| Neurocranium | |
| Po-N | Total skull length |
| Po–E | Cranial vault length |
| Ba–E | Total cranial base length |
| So–E | Anterior cranial base length |
| Ba–CB1 | Occipital bone length |
| CB1′–CB2 | Sphenoid bone length |
| Ba–So | Posterior cranial base length |
| Po–Ba | Posterior neurocranium height |
| Viscerocranium | |
| E–N | Nasal length |
| Mu2–Iu | Palate length |
| CB2–Iu | Midface length |
| E–Mu1 | Viscerocranial height |
| Mandible | |
| Go–Mn | Posterior corpus length |
| M1–Il | Anterior corpus length |
| Co–Il | Total mandibular length |
| Co–Gn | Ramus height |
| Dental | |
|---|---|
| Um1 | Maxillary first molar crown width |
| Um2 | Maxillary second molar crown width |
| Um3 | Maxillary third molar crown width |
| Lm1 | Mandibular first molar crown width |
| Lm2 | Mandibular second molar crown width |
| Lm3 | Mandibular third molar width |
| Uil | Maxillary incisor length |
| Lil | Mandibular incisor length |
| UCH | Maxillary first molar crown height |
| URH | Maxillary first molar mesial root length |
| LCH | Mandibular first molar crown height |
| LRH | Mandibular first molar mesial root length |
| Male | Female | Two-Way ANOVA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CM | HFDM | CF | HFDF | Effect (p-Value) | Interaction | ||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Food | Sex | (p-Value) | |
| Neurocranium | |||||||||||
| Po–N | 44.38 | 0.57 | 46.40 | 0.34 | 43.15 | 0.43 | 43.22 | 1.45 | 0.015 | <0.001 | 0.024 |
| Po–E | 27.65 | 0.41 | 29.42 | 1.55 | 27.23 | 0.55 | 27.12 | 0.90 | 0.047 | 0.002 | 0.026 |
| Ba–E | 30.77 | 0.40 | 32.86 | 1.20 | 30.26 | 0.40 | 30.26 | 0.77 | 0.003 | <0.001 | 0.003 |
| So–E | 19.66 | 0.33 | 20.79 | 1.07 | 19.20 | 0.49 | 19.17 | 0.54 | NS | 0.001 | 0.047 |
| Ba–CB1 | 9.35 | 0.48 | 10.06 | 0.37 | 9.44 | 0.31 | 9.44 | 0.39 | 0.04 | NS | 0.037 |
| CB1′–CB2 | 7.26 | 0.43 | 7.15 | 1.05 | 7.09 | 0.08 | 7.15 | 0.10 | NS | NS | NS |
| Ba–So | 11.78 | 0.16 | 12.52 | 0.35 | 11.71 | 0.27 | 11.79 | 0.23 | <0.001 | 0.001 | 0.005 |
| Po–Ba | 9.42 | 0.49 | 10.05 | 0.34 | 8.95 | 0.42 | 9.02 | 0.52 | NS | <0.001 | NS |
| Viscerocranium | |||||||||||
| E–N | 17.31 | 0.51 | 17.47 | 1.61 | 16.48 | 0.52 | 16.57 | 0.77 | NS | 0.04 | NS |
| Mu2–Iu | 19.73 | 0.21 | 20.52 | 0.17 | 19.32 | 0.20 | 19.71 | 0.30 | <0.001 | <0.001 | 0.042 |
| CB2–Iu | 24.27 | 0.28 | 26.22 | 1.67 | 23.25 | 0.23 | 24.01 | 1.27 | 0.005 | 0.001 | NS |
| E–Mu1 | 10.60 | 0.34 | 10.77 | 0.47 | 10.15 | 0.15 | 9.92 | 0.30 | NS | <0.001 | NS |
| Mandible | |||||||||||
| Go–Mn | 11.04 | 0.39 | 11.33 | 0.88 | 10.28 | 1.03 | 9.75 | 1.09 | NS | 0.004 | NS |
| M1–Il | 6.03 | 0.22 | 6.29 | 0.20 | 5.81 | 0.28 | 5.96 | 0.24 | 0.042 | 0.01 | NS |
| Co–Il | 25.35 | 1.35 | 26.13 | 1.21 | 23.86 | 1.05 | 24.21 | 1.65 | NS | 0.005 | NS |
| Co–Gn | 11.17 | 0.54 | 11.47 | 0.37 | 10.65 | 0.29 | 10.50 | 0.56 | NS | <0.001 | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serirukchutarungsee, S.; Watari, I.; Sangsuriyothai, P.; Akakura, M.; Ono, T. The Effect of Two-Generation Exposure to a High-Fat Diet on Craniofacial Morphology in Rats. J. Clin. Med. 2023, 12, 1903. https://doi.org/10.3390/jcm12051903
Serirukchutarungsee S, Watari I, Sangsuriyothai P, Akakura M, Ono T. The Effect of Two-Generation Exposure to a High-Fat Diet on Craniofacial Morphology in Rats. Journal of Clinical Medicine. 2023; 12(5):1903. https://doi.org/10.3390/jcm12051903
Chicago/Turabian StyleSerirukchutarungsee, Saranya, Ippei Watari, Pornchanok Sangsuriyothai, Masato Akakura, and Takashi Ono. 2023. "The Effect of Two-Generation Exposure to a High-Fat Diet on Craniofacial Morphology in Rats" Journal of Clinical Medicine 12, no. 5: 1903. https://doi.org/10.3390/jcm12051903
APA StyleSerirukchutarungsee, S., Watari, I., Sangsuriyothai, P., Akakura, M., & Ono, T. (2023). The Effect of Two-Generation Exposure to a High-Fat Diet on Craniofacial Morphology in Rats. Journal of Clinical Medicine, 12(5), 1903. https://doi.org/10.3390/jcm12051903







