Management of Hyperthyroidism during Pregnancy: A Systematic Literature Review
Abstract
1. Introduction
2. Materials and Methods
- Literature published in the English language during the study period (1 January 2010–31 December 2021);
- Publications that corresponded to the term search (“hyperthyroidism in pregnancy”);
- Publications which included all causes of hyperthyroidism alongside pregnancy;
- Publications which included diagnosed hyperthyroidism before or during pregnancy;
- Publications which included management of hyperthyroidism complications;
- Publications which included management of iodine deficiency in pregnancy.
- Only the abstract was accessible;
- Published aside from the mentioned period (1 January 2010–31 December 2021);
- Not published in the English language;
- Did not use the mentioned topic;
- Was identified as a preclinical study;
- Has been restricted to the pediatric or neonatal population.
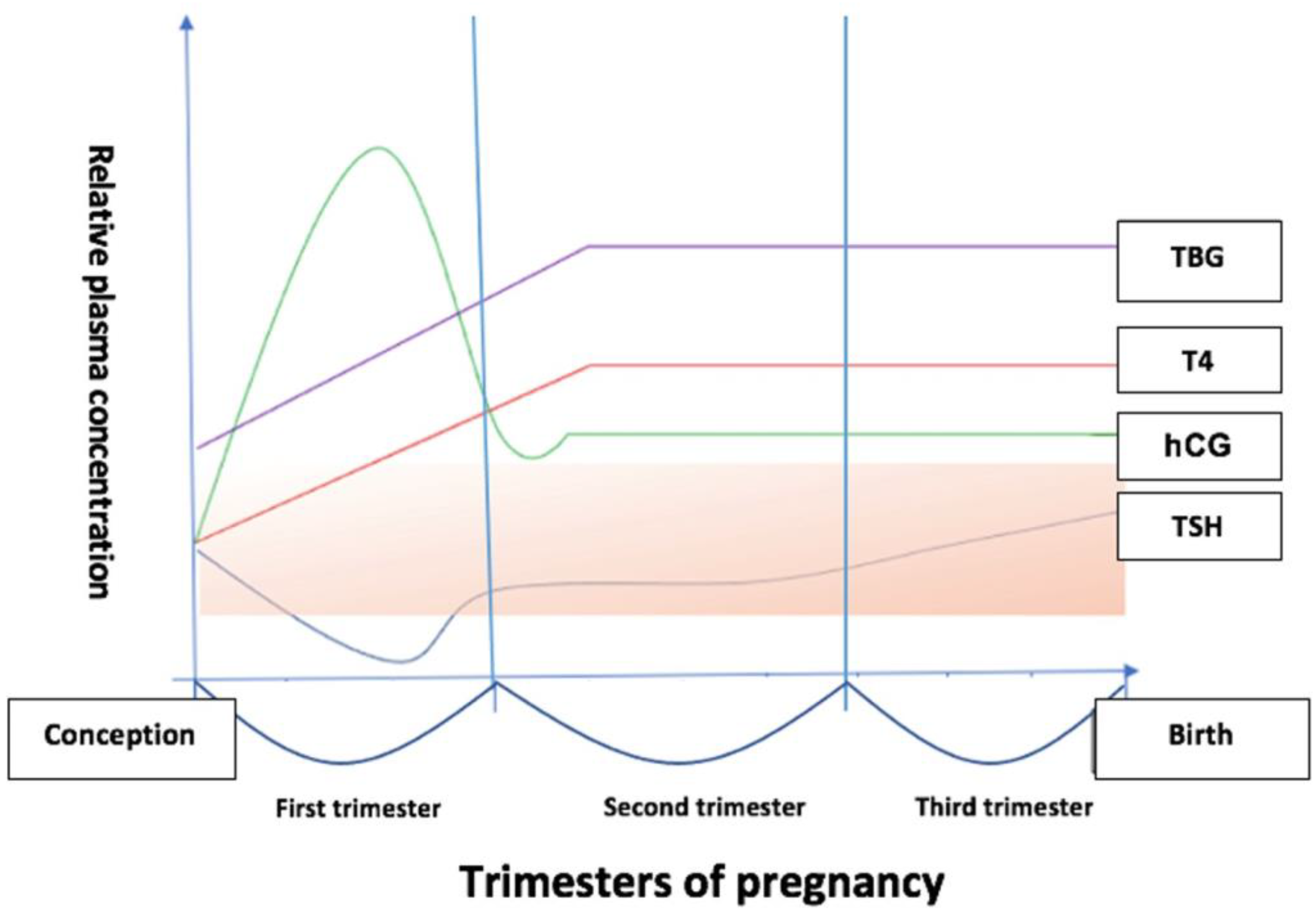
3. Changes in Thyroid Function during Pregnancy
4. Thyroid Hormones Trimester-Specific Reference Range
5. Thyroid Screening in Pregnancy
6. Hyperthyroidism Treatment in Pregnancy
7. Maternal and Fetal Adverse Events of Untreated/Uncontrolled Hyperthyroidism during Pregnancy
8. Iodine Intake in Pregnancy
9. Future Directions
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sorah, K.; Alderson, T.L. Hyperthyroidism in Pregnancy; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Azizi, F.; Amouzegar, A. Management of hyperthyroidism during pregnancy and lactation. Eur. J. Endocrinol. 2011, 164, 871–876. [Google Scholar] [CrossRef] [PubMed]
- De Cherney, A.H.; Nathan, L.; Laufer, N.; Roman, A.; Goodwin, M.; Nathan, L.; Roman, A.S. Current Diagnosis & Treatment: Obstetrics & Gynecology, 11th ed.; McGraw-Hill/Medical: New York, NY, USA, 2019. [Google Scholar]
- Ross, D.S. Overview of Thyroid Disease in Pregnancy. 2014. Available online: https://www.uptodate.com/contents/overview-of-thyroid-disease-and-pregnancy (accessed on 9 February 2022).
- Ngo, L.; Hale, T. HYPERthyroidism in Pregnancy. 2014. Available online: https://www.infantrisk.com/content/hyperthyroidism-pregnancy (accessed on 9 February 2022).
- Ross, D.S. Hypothyroidism during Pregnancy: Clinical Manifestations, Diagnosis, and Causes. 2012. Available online: https://www.uptodate.com/contents/hypothyroidism-during-pregnancy-clinical-manifestations-diagnosis-and-treatment (accessed on 9 February 2022).
- Marx, H.; Amin, P.; Lazarus, J.H. Hyperthyroidism and pregnancy. BMJ 2008, 336, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Stagnaro-Green, A. Maternal thyroid disease and preterm delivery. J. Endocrinol. Metab. 2008, 94, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Alamdari, S.; Azizi, F.; Delshad, H.; Sarvghadi, F.; Amouzegar, A.; Mehran, L. Management of Hyperthyroidism in Pregnancy: Comparison of Recommendations of America Thyroid Association and Endocrine Society. J. Thyroid Res. 2013, 6, 878467. [Google Scholar] [CrossRef] [PubMed]
- Mestman, J.H. Hyperthyroidism in pregnancy. Best Prat. Res. Clin. Endocrinol. Metab. 2004, 18, 67–88. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.E.; Lucas, M.J.; Hankins, G.D.; Roark, M.L.; Cunningham, F.G. Thyrotoxicosis complicating pregnancy. Am. J. Obstet. Gynecol. 1989, 160, 63–70. [Google Scholar] [CrossRef]
- Cignini, P.; Cafa, E.V.; Giorlandino, C.; Capriglione, S.; Spata, A.; Dugo, N. Thyroid physiology and common diseases in pregnancy: Review of literature. J. Prenat. Med. 2012, 6, 64–71. [Google Scholar] [PubMed]
- Rivkees, S.A.; Mandel, S.J. Thyroid Disease in Pregnancy. Horm. Res. Paediatr. 2011, 76 (Suppl. S1), 91–96. [Google Scholar] [CrossRef] [PubMed]
- Moleti, M.; Trimarchi, F.; Vermiglio, F. Thyroid Physiology in Pregnancy. Endocr. Pract. Endocr. Pract. 2014, 20, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yu, B.; Huang, R.; Cao, F.; Zhu, Z.; Sun, D.; Zhou, H. Assessment of thyroid function during pregnancy: The advantage of self-sequential longitudinal reference intervals. Arch. Med. Sci. 2011, 7, 679–684. [Google Scholar] [CrossRef]
- De Groot, L.; Abalovich, M.; Alexander, E.K.; Amino, N.; Barbour, L.; Cobin, R.H.; Eastmen, C.J.; Lazarus, J.H.; Luton, D.; Mandel, S.J.; et al. Management of thyroid dysfunction during pregnancy and postpartum: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2012, 97, 2543–2565. [Google Scholar] [CrossRef] [PubMed]
- Stagnaro- Green, A.; Abalovich, M.; Alexander, E.; Azizi, F.; Mestman, J.; Negro, R.; Nixon, A.; Pearce, E.N.; Soldin, O.P.; Sullivan, S.; et al. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease pregnancy and postpartum. Thyroid 2011, 21, 1081–1125. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, A.; Sadiq, F.; Zaki, S.; Batool, H.; Ibrahim, M.; Khurram, M.; Awan, U.A.; Saeed, K.; Afzal, M.S. Trimester-specific reference ranges for thyroid hormones of pregnant females at tertiary care hospitals in Lahore, Pakistan. BMC Pregnancy Childbirth 2021, 21, 717. [Google Scholar] [CrossRef] [PubMed]
- Negro, R.; Stagnaro-Green, A. Diagnosis and management of subclinical hypothyroidism in pregnancy. BMJ 2014, 349, 4929. [Google Scholar] [CrossRef]
- Kostecka-Matyja, M.; Fedorowicz, A.; Bar-Andziak, E.; Bednarczuk, T.; Buziak-Bereza, M.; Dumnicka, P.; Gorska, M.; Krasnodebska, M.; Niedzwiedzka, B.; Pach, D.; et al. Reference Values for TSH and Free Thyroid Hormones in Healthy Pregnant Women in Poland: A prospectice, multicenter Study. Eur. Thyroid J. 2017, 6, 82–88. [Google Scholar] [CrossRef]
- Khalid, A.S.; Marchocki, Z.; Hayes, K.; Lutomski, J.E.; Joyce, C.; Stapleton, M.; O’Mullane, J.; O’Donoghue, K. Establishing trimester-specific maternal thyroid function reference intervals. Ann. Clin. Biochem. 2014, 51 Pt 2, 277–283. [Google Scholar] [CrossRef]
- Springer, D.; Bartos, V.; Zima, T. Reference intervals for thyroid markers in early pregnancy determined by 7 different analytical systems. Scand. J. Clin. Lab. Investig. 2014, 74, 95–101. [Google Scholar] [CrossRef]
- Negro, R.; Stagnaro-Green, A. Clinical aspects of hyperthyroidism, hypothyroidism and thyroid screening in pregnancy. Endocr. Pract. 2014, 20, 597–607. [Google Scholar] [CrossRef]
- Gao, X.; Li, Y.; Li, J.; Liu, A.; Sun, W.; Teng, W.; Shan, Z. Gestational TSH an FT4 Reference Intervals in Chinese Women: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2018, 9, 432. [Google Scholar] [CrossRef]
- Craig, W.Y.; Allan, W.C.; Kloza, E.M.; Pulkkinen, A.J.; Waisbren, S.; Spratt, D.I.; Palomaki, G.E.; Neveux, L.M.; Haddow, J.E. Mid-gestational maternal free thyroxine concentration and offspring neurocognitive development at age two years. J. Clin. Endocrinol. Metab. 2012, 97, E22–E28. [Google Scholar] [CrossRef]
- Carney, L.A.; Quinlan, J.D.; West, J.M. Thyroid Disease in Pregnancy. Am. Fam. Physician 2014, 89, 273–278. [Google Scholar] [PubMed]
- Chang, D.L.F.; Pearce, E.N. Screening for Maternal Thyroid Dysfunction in Pregnancy: A Review of the Clinical Evidence and Current Guidelines. J. Thyroid Res. 2013, 2013, 851326. [Google Scholar] [CrossRef] [PubMed]
- Okosieme, O.E.; Lazarus, J.H. Important consideration in the management of Graves’ disease in pregnant women. Expert Rev. Clin. Immunol. 2015, 11, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Bahn, R.S.; Burch, H.B.; Cooper, D.S.; Garber, J.R.; Greenlee, M.C.; Klein, I.; Laurberg, P.; McDougall, I.R.; Montori, V.M.; Rivkees, S.A.; et al. Hyperthyroidism and other causes of thyreotoxicosis: Management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Endocr. Pract. 2011, 17, 456–520. [Google Scholar] [CrossRef] [PubMed]
- Moleti, M.; Di Mauro, M.; Sturniolo, G.; Russo, M.; Vermiglio, F. Hyperthyroidism in the pregnant woman: Maternal and fetal aspects. J. Clin. Transl. Endocrinol. 2019, 16, 100190. [Google Scholar] [CrossRef] [PubMed]
- Vissenberg, R.; Van den Boogaard, E.; Van Wely, M.; Van der Post, J.A.; Fliers, E.; Bisschop, P.M.; Goddijn, M. Treatment of thyroid disorders before conception and in early pregnancy. A systematic review. Hum. Reprod. Update 2012, 4, 360–373. [Google Scholar] [CrossRef] [PubMed]
- Hackmon, R.; Blichowski, M.; Koren, G. The safety of methimazole and propylthiouracil in pregnancy: A systematic review. J. Obst. Gynaecol. Can. 2012, 34, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Xirasagar, S.; Lin, C.C.; Wang, L.H.; Kou, Y.R.; Lin, H.C. Risk of adverse perinatal outcomes with antithyroid treatment during pregnancy: A nationwide population-based study. BJOG 2011, 118, 1365–1373. [Google Scholar] [CrossRef]
- Mestman, J.H. Hyperthyroidism in pregnancy. Curr. Opin. Endocrinol. Diabetes Obes. 2012, 19, 394–401. [Google Scholar] [CrossRef]
- Yoshihara, A.; Noh, J.; Yamaguchi, T.; Ohye, H.; Sato, S.; Sekiya, K.; Kosuga, Y.; Suzuki, M.; Kunii, Y.; Watanabe, N.; et al. Treatment of graves’ disease with antithyroid drugs in the first trimester of pregnancy and the prevalence of congenital malformation. J. Clin. Endocrinol Metab. 2012, 97, 2396–2403. [Google Scholar] [CrossRef]
- Negro, R.; Beck-Peccoz, P.; Chiovato, L.; Garofalo, P.; Gugliemi, R.; Papini, E.; Tonacchera, M.; Vermiglio, F.; Vitti, P.; Zini, M.; et al. Hyperthyroidism and pregnancy. An Italian Thyroid Association (AIT) and Italian Association of Clinical Endocrinologists (AME) joint statement for clinical practice. J. Endocrinol. Investig. 2011, 34, 225–231. [Google Scholar] [CrossRef]
- Taylor, P.N.; Vaidya, B. Side effects of antithyroid drugs and their impact on the choice of treatment for thyrotoxicosis in pregnancy. Eur. Thyroid J. 2012, 1, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Bowman, P.; Osborne, N.J.; Sturley, R.; Vaidya, B. Carbimazole embryopathy: Implications for the choice of antithyroid drugs in pregnancy. QJM 2012, 105, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Turunen, S.; Vääräsmäki, M.; Lahesmaa-Korpinen, A.; Leinonen, M.K.; Gissler, M.; Männistö, T.; Suvanto, E. Maternal hyperthyroidism and pregnancy outcome: A population-based cohort study. Clin. Endocrinol. 2020, 93, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Steegers, E.A.P.; Von Dadelszen, P.; Duvekot, J.J.; Pijnenborg, R. Pre-eclampsia. Lancet 2010, 376, 631–644. [Google Scholar] [CrossRef]
- Korevaar, T.I.M.; Steegers, E.A.P.; Chaker, L.; Medici, M.; Jaddoe, V.W.V.; Visser, T.J.; De Rijke, Y.B.; Peeters, R.P. The risk of preeclampsia according to high thyroid function in pregnancy differs by hCG concentration. J. Clin. Endocrinol. Metab. 2016, 101, 5037–5043. [Google Scholar] [CrossRef]
- Medici, M.; Korevaar, T.I.M.; Schalekamp-Timmermans, S.; Gaillard, R.; De Rijke, Y.B.; Visser, W.E.; Visser, W.; De Muinck Keizer-Schrama, S.M.P.F.; Hofman, A.; Hooijkaas, H.; et al. Maternal Early-Pregnancy Thyroid Function is associated with subsequent hypertensive disorders of Pregnancy: The Generation R study. J. Clin. Endocrinol. Metab. 2014, 99, E2591–E2598. [Google Scholar] [CrossRef]
- Saki, F.; Dabbaghmanesh, M.H.; Ghaemi, S.Z.; Forouhari, S.; Omrani, G.R.; Bakhshayeshkaram, M. Thyroid Function in Pregnancy and its Influences on Maternal and Fetal Outcomes. Int. J. Endocrinol. Metab. 2014, 12, e19378. [Google Scholar] [CrossRef]
- Krassas, G.; Karras, S.N.; Pontikides, N. Thyroid diseases during pregnancy: A number of important issues. Hormones 2015, 14, 59–69. [Google Scholar] [CrossRef]
- Sullivan, S.A. Hypothyroidism in pregnancy. Clin. Obstet. Gynecol. 2019, 62, 308–319. [Google Scholar] [CrossRef]
- Luewan, S.; Chakkabut, P.; Tongsong, T. Outcomes of pregnancy complicated with hyperthyroidism: A cohort study. Arch. Gynecol. Obstet. 2011, 283, 243–247. [Google Scholar] [CrossRef]
- Andersen, S.L.; Olsen, J.; Wu, C.S.; Laurberg, P. Spontaneous abortion, stillbirth and hyperthyroidism: A danish population-based study. Eur. Thyroid J. 2014, 3, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.C.; Rivkees, S.A.; Chandra, M.; Gonzalez, J.R.; Korelitz, J.J.; Kuzniewicz, M.W. Gestational thyrotoxicosis, antithyroid drug use and neonatal outcomes within an integrated healthcare delivery system. Thyroid 2015, 25, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Xiaowen, Z.; Baomin, C.; Aihua, L.; Yingying, Z.; Weiping, T.; Zhongyan, S. The effect of subclinical maternal thyroid dysfunction and autoimmunity on intrauterine growth restriction. Medicine 2016, 95, e3677. [Google Scholar] [CrossRef] [PubMed]
- Vrijkotte, T.G.M.; Hrudey, E.J.; Twickler, M.B. Early Maternal Thyroid Function During Gestation is Associated with Fetal Growth, particularly in male newborns. J. Clin. Endocrinol. Metab. 2017, 102, 1059–1066. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Sasso, E.B.; Barton, L.; Mestamn, J.H. Graves’ hyperthyroidism in pregnancy: A clinical review. Clin. Diabetes Endocrinol. 2018, 4, 4. [Google Scholar] [CrossRef]
- Aiken, C.E.; Ozanne, S.E. Sex differences in developmental programming models. Reproduction 2013, 145, R1–R13. [Google Scholar] [CrossRef]
- Downes, K.L.; Grantz, K.L.; Shenassa, E.D. Maternal, labor, delivery and prinatal outcomes associated with placental abruption: A systematic review. Am. J. Perinatol. 2017, 34, 935–957. [Google Scholar] [CrossRef]
- Geng, X.; Chen, Y.; Li, S.; Wang, W.; Wu, W.; Sun, C.; Li, N.; Wang, L. Systematic review and meta-analysis on the influence of thyroid dysfunction in early pregnancy on pregnancy outcomes under ultrasound guidance. Ann. Palliat. Med. 2022, 11, 1001–1016. [Google Scholar] [CrossRef]
- Pillar, N.; Levy, A.; Holcberg, G.; Sheiner, E. Pregnancy and perinatal outcome in women with hyperthyroidism. Int. J. Gynaecol. Obstet. 2010, 108, 61–64. [Google Scholar] [CrossRef]
- Zimmermann, M.B. The effects of iodine deficiency in pregnancy and infancy. Paediatr. Perinat. Epidemiol. 2012, 26 (Suppl. S1), 108–117. [Google Scholar] [CrossRef]
- Schiller, T.; Agmon, A.; Ostrovsky, V.; Shefer, G.; Knobler, H.; Zornitzki, T. Moderate Iodine Deficiency is common in pregnancy but does not alter maternal and neonatal thyroid function tests. Front. Endocrinol. 2020, 11, 523319. [Google Scholar] [CrossRef] [PubMed]
- Ovadia, Y.S.; Arbelle, J.E.; Gefel, D.; Brik, H.; Wolf, T.; Nadler, V.; Hunziker, S.; Zimmermann, M.B.; Troen, A.M. First Israeli National Iodine Survey Demonstrates Iodine Deficiency Among School- Aged Children and Pregnant Women. Thyroid 2017, 27, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Martinez, S.; Riestra-Fernandez, M.; Martinez-Morillo, E.; Avello-Lano, N.; Delgado-Alvarez, E.; Menendez-Torre, E. Nutritional Iodine Status in Pregnant Women from Health Area IV in Asturias (Spain): Iodised Salt is enough. Nutrients 2021, 13, 1816. [Google Scholar] [CrossRef] [PubMed]
- WHO Secretariat; Andersson, M.; de Benoist, B.; Delange, F.; Zupan, J. Prevention and control of iodine deficiency in pregnant and lactating women and in children less than 2-years-old: Conclusions and recommendations of the technical consultation. Public Health Nutr. 2007, 10, 1606–1611. [Google Scholar] [CrossRef]
- Bath, S.C.; Walter, A.; Taylor, A.; Wright, J.; Rayman, M.P. Iodine deficiency in pregnant women living in the South East of the UK: The influence of diet and nutritional supplements on iodine status. Br. J. Nutr. 2014, 111, 1622–1631. [Google Scholar] [CrossRef]
- Alexander, E.K.; Pearce, E.N.; Brent, G.A.; Brown, R.S.; Chen, H.; Dosiou, C.; Grobman, W.A.; Laurberg, P.; Lazarus, J.J.; Mandel, S.J.; et al. 2017 Guidelines of the American thyroid association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid 2017, 27, 315–389. [Google Scholar] [CrossRef]
- Zimmermann, M.; Delange, F. Iodine supplementation of pregnant women in Europe: A review and recommendations. Eur. J. Clin. Nutr. 2004, 58, 979–984. [Google Scholar] [CrossRef]
- Bath, S.C.; Steer, C.D.; Golding, J.; Emmett, P.; Rayman, M.P. Effect of inadequate iodine status in UK pregnant women on cognitive outcomes in their children: Results from the Avon Longitudinal Study of Parents and Children (ALSPAC). Lancet 2013, 382, 331–337. [Google Scholar] [CrossRef]
- Murcia, M.; Espada, M.; Julvez, J.; Llop, S.; Lopez-Espinosa, M.-J.; Vioque, J.; Basterrechea, M.; Riaño, I.; González, L.; Alvarez-Pedrerol, M.; et al. Iodine intake from supplements and diet during pregnancy and child cognitive and motor development: The INMA mother and child cohort study. J. Epidemiol. Commun. Health 2018, 72, 216–222. [Google Scholar] [CrossRef]
- Candido, A.C.; Azevedo, F.M.; Machamba, A.A.L.; Pinto, C.A.; Lopes, S.O.; de Souza Macedo, M.; Vieira Ribeiro, S.A.; Priore, S.E.; do Carmo Castro Franceschini, S. Implications of iodine deficiency by gestational trimester: A systematic review. Arch. Endocrinol. Metab. 2020, 64, 507–513. [Google Scholar] [CrossRef]
- Xiao, Y.; Sun, H.; Li, C.; Li, Y.; Peng, S.; Fan, C.; Teng, W.; Shan, Z. Effect of iodine nutrition on pregnancy outcomes in an iodine-sufficient area in China. Biol. Trace Elem. Res. 2018, 182, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Charoenratana, C.; Leelapat, P.; Traisrisilp, K.; Tongsong, T. Maternal iodine insufficiency and adverse pregnancy outcomes. Matern. Child Nutr. 2016, 12, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Ghassabian, A.; Graaff, J.S.; Peeters, R.P.; Ross, H.A.; Jaddoe, V.W.; Hofman, A.; Verhulst, F.C.; White, T.; Tiemeier, T. Maternal urinary iodine concentration in pregnancy and children’s cognition: Results from a population-based birth cohort in an iodine-sufficient area. BMJ Open 2014, 4, e005520. [Google Scholar] [CrossRef] [PubMed]
- Cuellar-Rufino, S.; Navarro-Meza, M.; Garcia-Solis, P.; Xochihua-Rosas, I.; Arroyo-Helguera, O. Iodine levels are associated with oxidative stress and antioxidant status in pregnant women with hypertensive disease. Nutr. Hosp. 2017, 34, 661–666. [Google Scholar] [CrossRef]
- Aguayo, A.; Grau, G.; Vela, A.; Aniel-Quiroga, A.; Espada, M.; Martul, P.; Castano, L.; Rica, I. Urinary iodine and thyroid function in a population of healthy pregnant women in the North of Spain. J. Trace Elem. Med. Biol. 2013, 27, 302–306. [Google Scholar] [CrossRef]
- Andersen, S.L.; Laurberg, P. Iodine supplementation in pregnancy and the dilemma of ambiguous recommendations. Eur. Thyroid J. 2016, 5, 35–43. [Google Scholar] [CrossRef]

| Physiological Changes | Effect |
|---|---|
| 1. Thyroid stimulation by hCG produced in trophoblastic tissue | Transiently increased fT4 and T3Decreased TSH levels |
| 2. Estrogen-induced in-crease in serum TBG | Increased total T4 and T3 |
| 3. Placental expression of D2 and D3 deiodinases | Increased peripheral iodothyronine metabolism |
| 4. (a) Increased renal Iode clearance; (b) Iode diversion to feto-placentar unit; (c) Increased iodide consumption for thyroid hormone synthesis | Reduced maternal iodide pool |
| Current Thyroid Therapy |
|---|
| Family history of autoimmune thyroid disease |
| Goiter |
Personal background of:
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petca, A.; Dimcea, D.A.-M.; Dumitrașcu, M.C.; Șandru, F.; Mehedințu, C.; Petca, R.-C. Management of Hyperthyroidism during Pregnancy: A Systematic Literature Review. J. Clin. Med. 2023, 12, 1811. https://doi.org/10.3390/jcm12051811
Petca A, Dimcea DA-M, Dumitrașcu MC, Șandru F, Mehedințu C, Petca R-C. Management of Hyperthyroidism during Pregnancy: A Systematic Literature Review. Journal of Clinical Medicine. 2023; 12(5):1811. https://doi.org/10.3390/jcm12051811
Chicago/Turabian StylePetca, Aida, Daiana Anne-Marie Dimcea, Mihai Cristian Dumitrașcu, Florica Șandru, Claudia Mehedințu, and Răzvan-Cosmin Petca. 2023. "Management of Hyperthyroidism during Pregnancy: A Systematic Literature Review" Journal of Clinical Medicine 12, no. 5: 1811. https://doi.org/10.3390/jcm12051811
APA StylePetca, A., Dimcea, D. A.-M., Dumitrașcu, M. C., Șandru, F., Mehedințu, C., & Petca, R.-C. (2023). Management of Hyperthyroidism during Pregnancy: A Systematic Literature Review. Journal of Clinical Medicine, 12(5), 1811. https://doi.org/10.3390/jcm12051811









