Establishment and Validation of Predictive Model of Tophus in Gout Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Subjects
2.1.2. Inclusion Criteria
2.1.3. Exclusion Standards
2.2. Methods
2.2.1. Grouping Methods and Diagnosis of Tophi
2.2.2. Study Indicators
2.2.3. Construction and Evaluation of Predictive Models
3. Statistical Analysis
4. Results
4.1. Comparison of Baseline Data
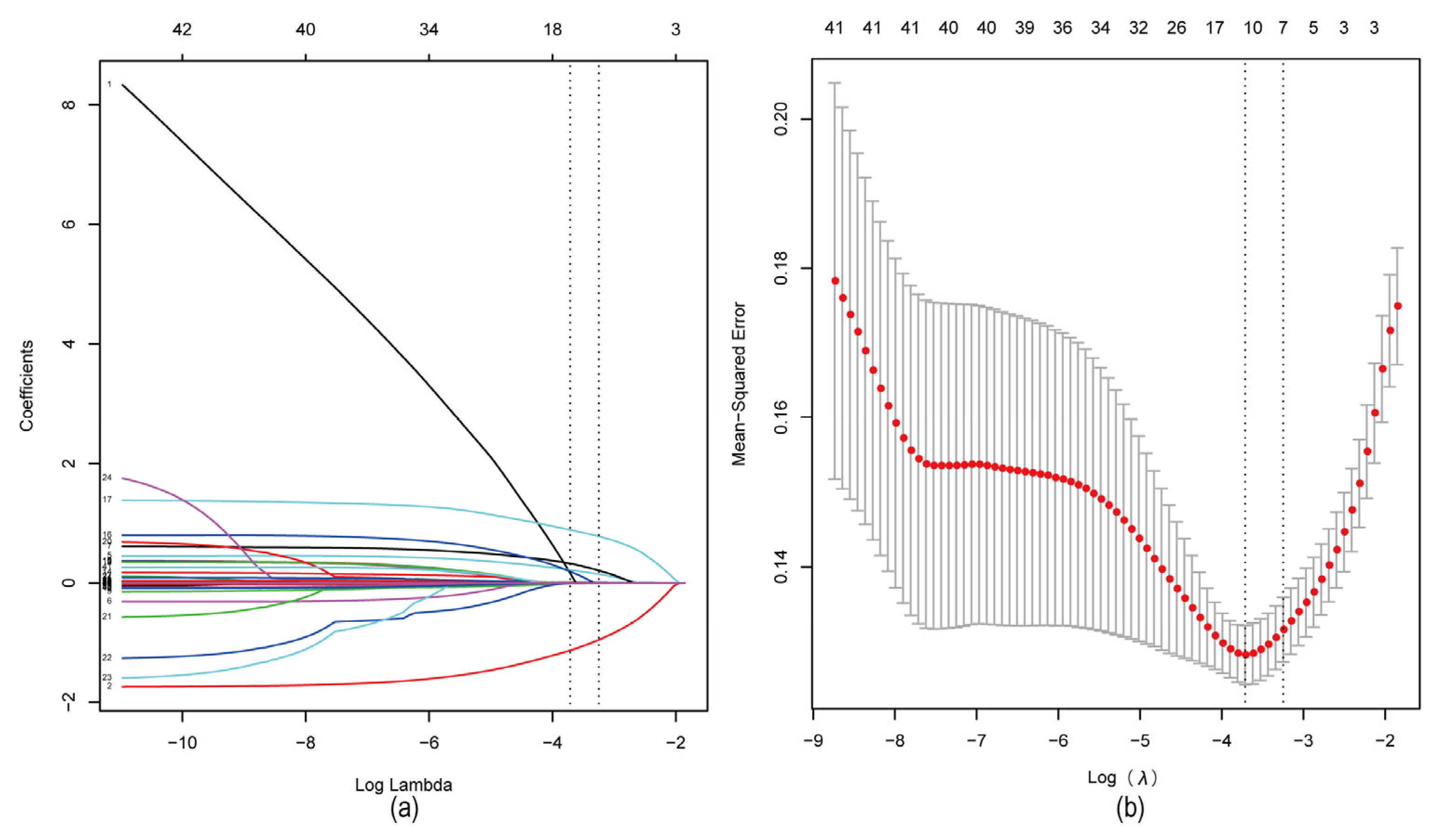
4.2. Screening of Characteristic Factors for Risk of Tophi in Gout Patients
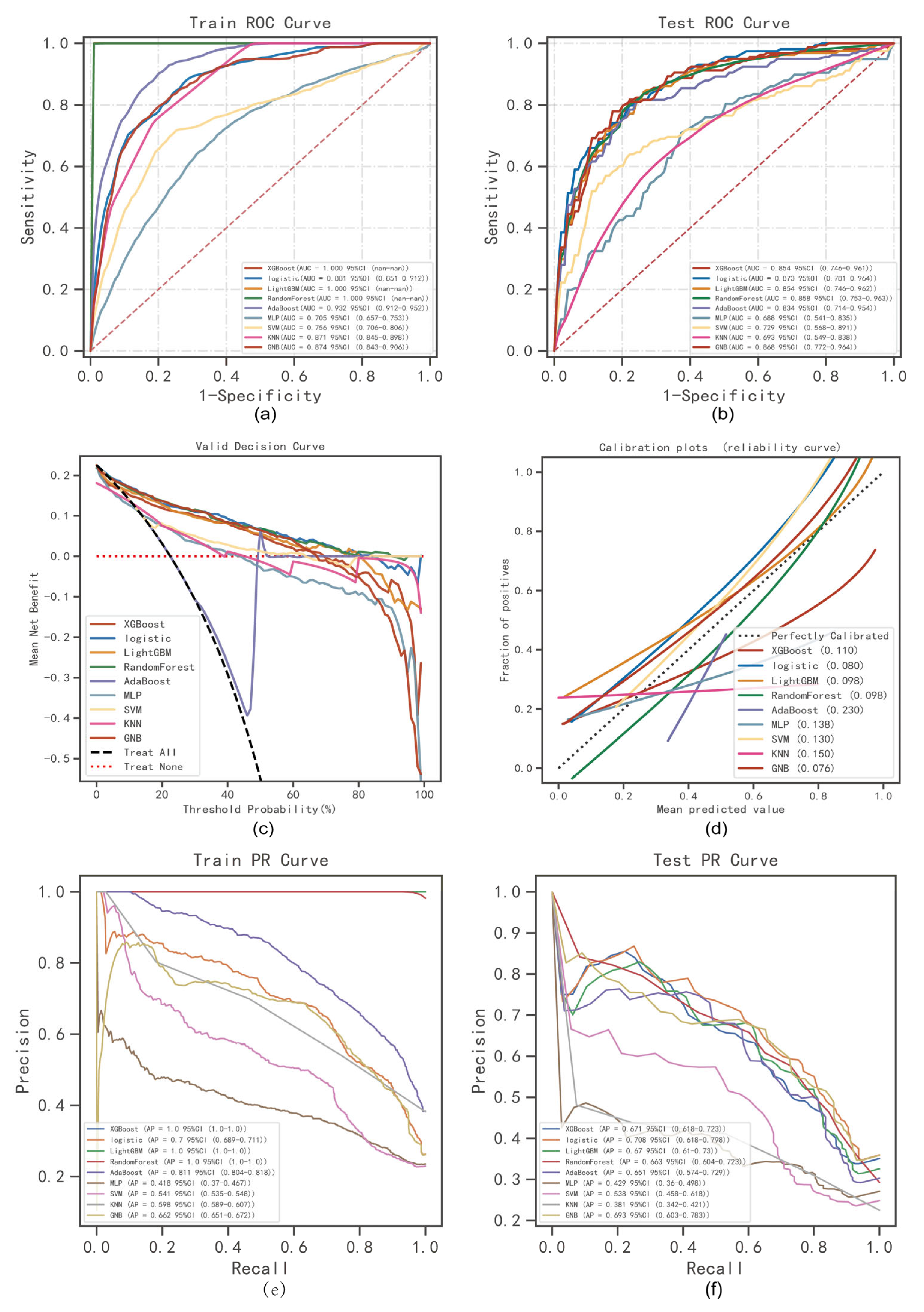
4.3. Comprehensive Analysis of Classified Multi-Model
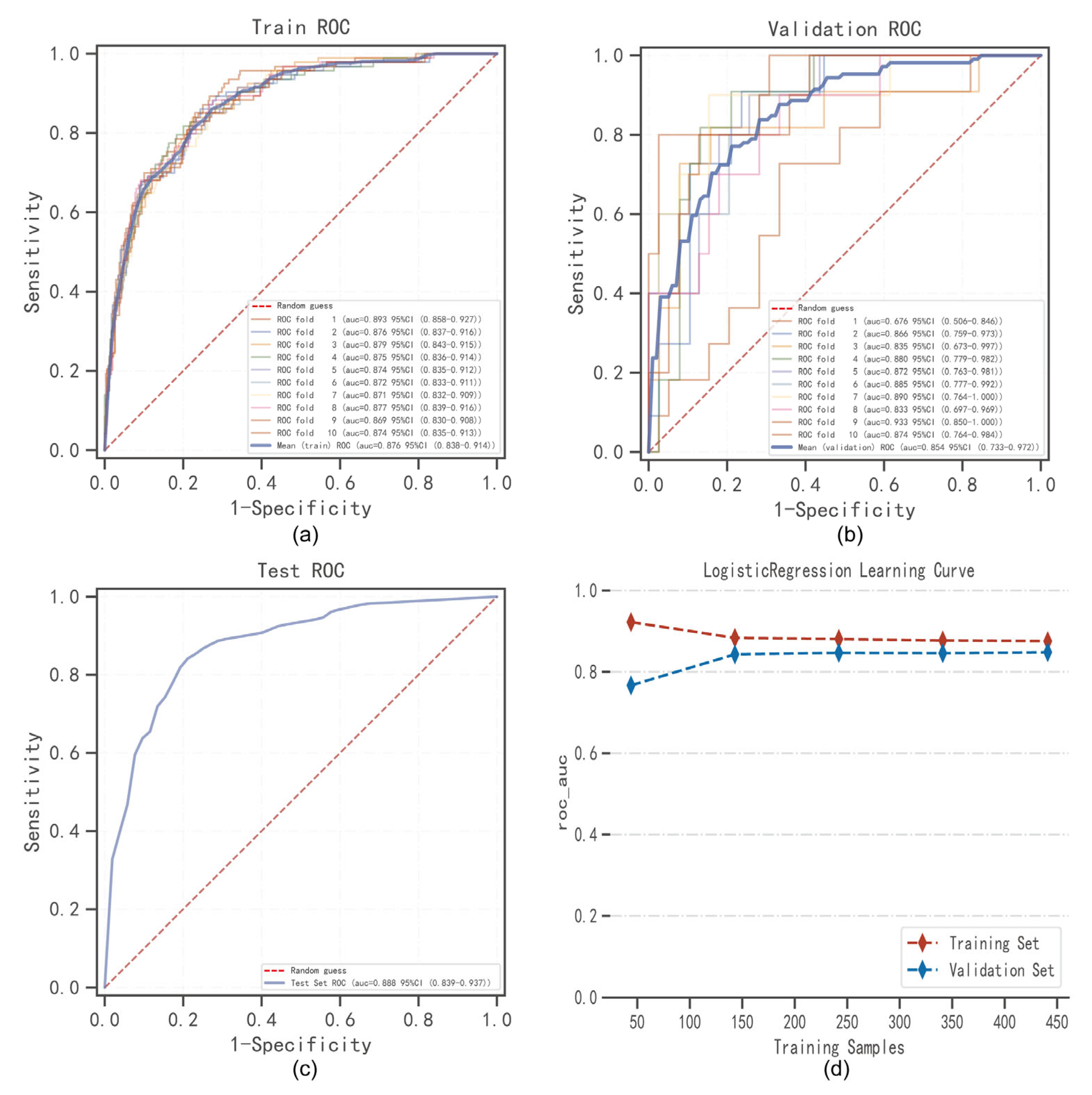
4.4. The Best Model Building and Evaluation
4.5. The SHAP to Model Interpretation
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dalbeth, N.; Gosling, A.L.; Gaffo, A.; Abhishek, A. Gout. Lancet 2021, 397, 1843–1855. [Google Scholar] [CrossRef]
- Singh, J.A. The impact of gout on patient’s lives: A study of African-American and Caucasian men and women with gout. Arthritis Res. Ther. 2014, 16, R132. [Google Scholar] [CrossRef]
- Dalbeth, N.; Stamp, L. Hyperuricaemia and gout: Time for a new staging system? Ann. Rheum. Dis. 2014, 73, 1598–1600. [Google Scholar] [CrossRef]
- Grassi, W.; De Angelis, R. Clinical features of gout. Reumatismo 2012, 63, 238–245. [Google Scholar] [CrossRef]
- Aati, O.; Taylor, W.J.; Horne, A.; Dalbeth, N. Toward development of a Tophus Impact Questionnaire: A qualitative study exploring the experience of people with tophaceous gout. J. Clin. Rheumatol. 2014, 20, 251–255. [Google Scholar] [CrossRef]
- Khanna, P.P.; Nuki, G.; Bardin, T.; Tausche, A.K.; Forsythe, A.; Goren, A.; Vietri, J.; Khanna, D. Tophi and frequent gout flares are associated with impairments to quality of life, productivity, and increased healthcare resource use: Results from a cross-sectional survey. Health Qual. Life Outcomes 2012, 10, 117. [Google Scholar] [CrossRef]
- Lu, B.; Lu, Q.; Huang, B.; Li, C.; Zheng, F.; Wang, P. Risk factors of ultrasound-detected tophi in patients with gout. Clin. Rheumatol. 2020, 39, 1953–1960. [Google Scholar] [CrossRef]
- Ebstein, E.; Forien, M.; Norkuviene, E.; Richette, P.; Mouterde, G.; Daien, C.; Ea, H.K.; Brière, C.; Lioté, F.; Petraitis, M.; et al. Ultrasound evaluation in follow-up of urate-lowering therapy in gout: The USEFUL study. Rheumatology 2019, 58, 410–417. [Google Scholar] [CrossRef]
- Sriranganathan, M.K.; Vinik, O.; Pardo Pardo, J.; Bombardier, C.; Edwards, C.J. Interventions for tophi in gout. Cochrane Database Syst. Rev. 2021, 8, Cd010069. [Google Scholar] [CrossRef]
- Schlesinger, N. Difficult-to-treat gouty arthritis: A disease warranting better management. Drugs 2011, 71, 1413–1439. [Google Scholar] [CrossRef]
- Mandell, B.F.; Yeo, A.E.; Lipsky, P.E. Tophus resolution in patients with chronic refractory gout who have persistent urate-lowering responses to pegloticase. Arthritis Res. Ther. 2018, 20, 286. [Google Scholar] [CrossRef]
- Doscher, M.; Beutel, B.G.; Lovy, A.; Alba, B.; Melone, C. En Masse Excision and Curettage for Periarticular Gouty Tophi of the Hands. Eplasty 2022, 22, e25. [Google Scholar]
- Greener, J.G.; Kandathil, S.M.; Moffat, L.; Jones, D.T. A guide to machine learning for biologists. Nat. Rev. Mol. Cell Biol. 2022, 23, 40–55. [Google Scholar] [CrossRef]
- Handelman, G.S.; Kok, H.K.; Chandra, R.V.; Razavi, A.H.; Lee, M.J.; Asadi, H. eDoctor: Machine learning and the future of medicine. J. Intern. Med. 2018, 284, 603–619. [Google Scholar] [CrossRef]
- Moons, K.G.; Royston, P.; Vergouwe, Y.; Grobbee, D.E.; Altman, D.G. Prognosis and prognostic research: What, why, and how? BMJ 2009, 338, b375. [Google Scholar] [CrossRef]
- Adams, S.T.; Leveson, S.H. Clinical prediction rules. BMJ 2012, 344, d8312. [Google Scholar] [CrossRef]
- Churpek, M.M.; Yuen, T.C.; Winslow, C.; Meltzer, D.O.; Kattan, M.W.; Edelson, D.P. Multicenter Comparison of Machine Learning Methods and Conventional Regression for Predicting Clinical Deterioration on the Wards. Crit. Care Med. 2016, 44, 368–374. [Google Scholar] [CrossRef]
- Shortliffe, E.H.; Sepúlveda, M.J. Clinical Decision Support in the Era of Artificial Intelligence. JAMA 2018, 320, 2199–2200. [Google Scholar] [CrossRef]
- Vayena, E.; Blasimme, A.; Cohen, I.G. Machine learning in medicine: Addressing ethical challenges. PLoS Med. 2018, 15, e1002689. [Google Scholar] [CrossRef]
- Lundberg, S.M.; Erion, G.; Chen, H.; DeGrave, A.; Prutkin, J.M.; Nair, B.; Katz, R.; Himmelfarb, J.; Bansal, N.; Lee, S.I. From Local Explanations to Global Understanding with Explainable AI for Trees. Nat. Mach. Intell. 2020, 2, 56–67. [Google Scholar] [CrossRef]
- Obuchowski, N.A.; Bullen, J.A. Receiver operating characteristic (ROC) curves: Review of methods with applications in diagnostic medicine. Phys. Med. Biol. 2018, 63, 07tr01. [Google Scholar] [CrossRef]
- Vickers, A.J.; Elkin, E.B. Decision curve analysis: A novel method for evaluating prediction models. Med. Decis. Mak. 2006, 26, 565–574. [Google Scholar] [CrossRef]
- Fenlon, C.; O’Grady, L.; Doherty, M.L.; Dunnion, J. A discussion of calibration techniques for evaluating binary and categorical predictive models. Prev. Vet. Med. 2018, 149, 107–114. [Google Scholar] [CrossRef]
- Li, W.; Guo, Q. Plotting receiver operating characteristic and precision-recall curves from presence and background data. Ecol. Evol. 2021, 11, 10192–10206. [Google Scholar] [CrossRef]
- Belkin, M.; Hsu, D.; Ma, S.; Mandal, S. Reconciling modern machine-learning practice and the classical bias-variance trade-off. Proc. Natl. Acad. Sci. USA 2019, 116, 15849–15854. [Google Scholar] [CrossRef]
- Lundberg, S.M.; Lee, S.I. A unified approach to interpreting model predictions. In Advances in Neural Information Processing Systems 30, Proceedings of the 31st International Conference on Neural Information Processing Systems, Long Beach, CA, USA, 4–9 December 2017; MIT Press: Cambridge, MA, USA, 2017; pp. 4765–4774. [Google Scholar]
- Yin, R.; Li, L.; Zhang, G.; Cui, Y.; Zhang, L.; Zhang, Q.; Fu, T.; Cao, H.; Li, L.; Gu, Z. Rate of adherence to urate-lowering therapy among patients with gout: A systematic review and meta-analysis. BMJ Open 2018, 8, e017542. [Google Scholar] [CrossRef]
- Scheepers, L.; van Onna, M.; Stehouwer, C.D.A.; Singh, J.A.; Arts, I.C.W.; Boonen, A. Medication adherence among patients with gout: A systematic review and meta-analysis. Semin. Arthritis Rheum. 2018, 47, 689–702. [Google Scholar] [CrossRef]
- McGowan, B.; Bennett, K.; Silke, C.; Whelan, B. Adherence and persistence to urate-lowering therapies in the Irish setting. Clin. Rheumatol. 2016, 35, 715–721. [Google Scholar] [CrossRef]
- Perez-Ruiz, F.; Perez-Herrero, N.; Richette, P.; Stack, A.G. High Rate of Adherence to Urate-Lowering Treatment in Patients with Gout: Who’s to Blame? Rheumatol. Ther. 2020, 7, 1011–1019. [Google Scholar] [CrossRef]
- Sauerbrei, W.; Royston, P.; Binder, H. Selection of important variables and determination of functional form for continuous predictors in multivariable model building. Stat. Med. 2007, 26, 5512–5528. [Google Scholar] [CrossRef]
- Pourhoseingholi, M.A.; Baghestani, A.R.; Vahedi, M. How to control confounding effects by statistical analysis. Gastroenterol. Hepatol. Bed Bench 2012, 5, 79–83. [Google Scholar]
- Muschelli, J. ROC and AUC with a Binary Predictor: A Potentially Misleading Metric. J. Classif. 2020, 37, 696–708. [Google Scholar] [CrossRef]
- Leyva, F.; Anker, S.; Swan, J.W.; Godsland, I.F.; Wingrove, C.S.; Chua, T.P.; Stevenson, J.C.; Coats, A.J. Serum uric acid as an index of impaired oxidative metabolism in chronic heart failure. Eur. Heart J. 1997, 18, 858–865. [Google Scholar] [CrossRef]
- Sakai, H.; Tsutamoto, T.; Tsutsui, T.; Tanaka, T.; Ishikawa, C.; Horie, M. Serum level of uric acid, partly secreted from the failing heart, is a prognostic marker in patients with congestive heart failure. Circ. J. 2006, 70, 1006–1011. [Google Scholar] [CrossRef]
- Von Lueder, T.G.; Girerd, N.; Atar, D.; Agewall, S.; Lamiral, Z.; Kanbay, M.; Pitt, B.; Dickstein, K.; Zannad, F.; Rossignol, P. Serum uric acid is associated with mortality and heart failure hospitalizations in patients with complicated myocardial infarction: Findings from the High-Risk Myocardial Infarction Database Initiative. Eur. J. Heart Fail. 2015, 17, 1144–1151. [Google Scholar] [CrossRef]
- Otaki, Y.; Watanabe, T.; Kinoshita, D.; Yokoyama, M.; Takahashi, T.; Toshima, T.; Sugai, T.; Murase, T.; Nakamura, T.; Nishiyama, S.; et al. Association of plasma xanthine oxidoreductase activity with severity and clinical outcome in patients with chronic heart failure. Int. J. Cardiol. 2017, 228, 151–157. [Google Scholar] [CrossRef]
- Enomoto, A.; Kimura, H.; Chairoungdua, A.; Shigeta, Y.; Jutabha, P.; Cha, S.H.; Hosoyamada, M.; Takeda, M.; Sekine, T.; Igarashi, T.; et al. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature 2002, 417, 447–452. [Google Scholar] [CrossRef]
- Ritter, F.; Franzeck, F.; Geisshardt, J.; Walker, U.A.; Osthoff, M. Gout Arthritis During Admission for Decompensated Heart Failure-A Descriptive Analysis of Risk Factors, Treatment and Prognosis. Front. Med. 2022, 9, 789414. [Google Scholar] [CrossRef]
- Furuhashi, M.; Mori, K.; Tanaka, M.; Maeda, T.; Matsumoto, M.; Murase, T.; Nakamura, T.; Koyama, M.; Moniwa, N.; Ohnishi, H.; et al. Unexpected high plasma xanthine oxidoreductase activity in female subjects with low levels of uric acid. Endocr. J. 2018, 65, 1083–1092. [Google Scholar] [CrossRef]
- Perl-Treves, D.; Addadi, L. A structural approach to pathological crystallizations. Gout: The possible role of albumin in sodium urate crystallization. Proc. R. Soc. Lond. B Biol. Sci. 1988, 235, 145–159. [Google Scholar] [CrossRef]
- Gelber, A.C. Among the Company That Gout Keeps, Is Cancer on the List? J. Rheumatol. 2021, 48, 1358–1360. [Google Scholar] [CrossRef]
- Leung, N.; Yip, K.; Pillinger, M.H.; Toprover, M. Lowering and Raising Serum Urate Levels: Off-Label Effects of Commonly Used Medications. Mayo Clin. Proc. 2022, 97, 1345–1362. [Google Scholar] [CrossRef]
- Ma, L.; Sun, R.; Jia, Z.; Zou, Y.; Xin, Y.; Cheng, X.; Liu, T.; Cui, L.; Liu, Z.; Wu, X.; et al. Clinical characteristics associated with subcutaneous tophi formation in Chinese gout patients: A retrospective study. Clin. Rheumatol. 2018, 37, 1359–1365. [Google Scholar] [CrossRef]
- Sun, C.; Qi, X.; Tian, Y.; Gao, L.; Jin, H.; Guo, H. Risk factors for the formation of double-contour sign and tophi in gout. J. Orthop. Surg. Res. 2019, 14, 239. [Google Scholar] [CrossRef]
- Si, K.; Wei, C.; Xu, L.; Lv, W.; Dong, B.; Wang, Z.; Huang, Y.; Chen, Y.; Wang, Y. Association between serum free fatty acid levels and tophus in patients with gout: A cross-sectional study. Clin. Exp. Rheumatol. 2022. [Google Scholar] [CrossRef]
- Liu, W.; Song, H.; Man, S.; Li, H.; Gao, S. Simple metabolic markers associated with tophaceous gout. Clin. Rheumatol. 2021, 40, 5047–5053. [Google Scholar] [CrossRef]
- Dalbeth, N.; Jones, G.; Terkeltaub, R.; Khanna, D.; Fung, M.; Baumgartner, S.; Perez-Ruiz, F. Efficacy and safety during extended treatment of lesinurad in combination with febuxostat in patients with tophaceous gout: CRYSTAL extension study. Arthritis Res. Ther. 2019, 21, 8. [Google Scholar] [CrossRef]
- Khanna, D.; Fitzgerald, J.D.; Khanna, P.P.; Bae, S.; Singh, M.K.; Neogi, T.; Pillinger, M.H.; Merill, J.; Lee, S.; Prakash, S.; et al. 2012 American College of Rheumatology guidelines for management of gout. Part 1: Systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res. 2012, 64, 1431–1446. [Google Scholar] [CrossRef]
- Ellmann, H.; Bayat, S.; Araujo, E.; Manger, B.; Kleyer, A.; Cavallaro, A.; Lell, M.; Schenker, H.; Simon, D.; Tascilar, K.; et al. Effects of Conventional Uric Acid-Lowering Therapy on Monosodium Urate Crystal Deposits. Arthritis Rheumatol. 2020, 72, 150–156. [Google Scholar] [CrossRef]
- Nielsen, S.M.; Zobbe, K.; Kristensen, L.E.; Christensen, R. Nutritional recommendations for gout: An update from clinical epidemiology. Autoimmun. Rev. 2018, 17, 1090–1096. [Google Scholar] [CrossRef]
- Waalen, J.; Buxbaum, J.N. Is older colder or colder older? The association of age with body temperature in 18,630 individuals. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 487–492. [Google Scholar] [CrossRef]
- Hoffmann, M.E.; Rodriguez, S.M.; Zeiss, D.M.; Wachsberg, K.N.; Kushner, R.F.; Landsberg, L.; Linsenmeier, R.A. 24-h core temperature in obese and lean men and women. Obesity 2012, 20, 1585–1590. [Google Scholar] [CrossRef]
- Loeb, J.N. The influence of temperature on the solubility of monosodium urate. Arthritis Rheum. 1972, 15, 189–192. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, Z.; Chao, Z.; Chao, Y.; Ma, L.; Cheng, X.; Wang, Y.; Li, C.; Chen, Y. Nonlinear relationship between serum uric acid and body mass index: A cross-sectional study of a general population in coastal China. J. Transl. Med. 2019, 17, 389. [Google Scholar] [CrossRef]
- Akazawa, N.; Kishi, M.; Hino, T.; Tsuji, R.; Tamura, K.; Hioka, A.; Moriyama, H. Higher Body Mass Index in Hospitalized Older Patients Is Related to Higher Muscle Quality. J. Nutr. Health Aging 2022, 26, 495–500. [Google Scholar] [CrossRef]
- Chen, Z.; Song, C.; Yao, Z.; Sun, J.; Liu, W. Associations between albumin, globulin, albumin to globulin ratio and muscle mass in adults: Results from the national health and nutrition examination survey 2011–2014. BMC Geriatr. 2022, 22, 383. [Google Scholar] [CrossRef]
- Kippen, I.; Klinenberg, J.R.; Weinberger, A.; Wilcox, W.R. Factors affecting urate solubility in vitro. Ann. Rheum. Dis. 1974, 33, 313–317. [Google Scholar] [CrossRef]
- Katz, W.A.; Schubert, M. The interaction of monosodium urate with connective tissue components. J. Clin. Investig. 1970, 49, 1783–1789. [Google Scholar] [CrossRef]
- Romo, M.; López-Vicario, C.; Pérez-Romero, N.; Casulleras, M.; Martínez-Puchol, A.I.; Sánchez, B.; Flores-Costa, R.; Alcaraz-Quiles, J.; Duran-Güell, M.; Ibarzábal, A.; et al. Small fragments of hyaluronan are increased in individuals with obesity and contribute to low-grade inflammation through TLR-mediated activation of innate immune cells. Int. J. Obes. 2022, 46, 1960–1969. [Google Scholar] [CrossRef]
- Burt, H.M.; Dutt, Y.C. Growth of monosodium urate monohydrate crystals: Effect of cartilage and synovial fluid components on in vitro growth rates. Ann. Rheum. Dis. 1986, 45, 858–864. [Google Scholar] [CrossRef]

| Variable | Training Set (n = 491) | Testing Set (n = 211) | Z | P |
|---|---|---|---|---|
| annual attack frequency (times), n (%) | ||||
| <6 | 319 (64.969) | 136 (64.455) | 1.089 | 0.58 |
| 6–12 | 74 (15.071) | 27 (12.796) | ||
| >12 | 98 (19.959) | 48 (22.749) | ||
| history of smoking, n (%) | ||||
| no | 224 (45.621) | 96 (45.498) | 0.001 | 0.976 |
| yes | 267 (54.379) | 115 (54.502) | ||
| history of drinking, n (%) | ||||
| no | 92 (18.737) | 46 (21.801) | 1.219 | 0.544 |
| drinking every week < 70 g | 269 (54.786) | 107 (50.711) | ||
| drinking every week ≥ 70 g/ years of drinking ≥ 10 years | 130 (26.477) | 58 (27.488) | ||
| history of sugary diet, n (%) | ||||
| no | 326 (66.395) | 151 (71.564) | 1.81 | 0.178 |
| yes | 165 (33.605) | 60 (28.436) | ||
| history of high purine diet, n (%) | ||||
| no | 218 (44.399) | 80 (37.915) | 2.54 | 0.111 |
| yes | 273 (55.601) | 131 (62.085) | ||
| history of high altitude residence, n (%) | ||||
| no | 404 (82.281) | 177 (83.886) | 0.267 | 0.606 |
| yes | 87 (17.719) | 34 (16.114) | ||
| history of hypertension, n (%) | ||||
| no | 400 (81.466) | 159 (75.355) | 3.398 | 0.065 |
| yes | 91 (18.534) | 52 (24.645) | ||
| history of diabetes, n (%) | ||||
| no | 474 (96.538) | 202 (95.735) | 0.267 | 0.605 |
| yes | 17 (3.462) | 9 (4.265) | ||
| history of hyperlipidemia, n (%) | ||||
| no | 311 (63.469) | 131 (62.085) | 0.121 | 0.728 |
| yes | 179 (36.531) | 80 (37.915) | ||
| history of kidney stones, n (%) | ||||
| no | 376 (76.578) | 156 (73.934) | 0.562 | 0.453 |
| yes | 115 (23.422) | 55 (26.066) | ||
| history of kidney crystallization, n (%) | ||||
| no | 441 (89.817) | 182 (86.256) | 1.874 | 0.171 |
| yes | 50 (10.183) | 29 (13.744) | ||
| family history of gout, n (%) | ||||
| no | 404 (82.281) | 181 (85.782) | 1.302 | 0.254 |
| yes | 87 (17.719) | 30 (14.218) | ||
| polyjoint involvement (joints), n (%) | ||||
| <3 | 275 (56.008) | 114 (54.028) | 0.234 | 0.628 |
| ≥3 | 216 (43.992) | 97 (45.972) | ||
| tophus, n (%) | ||||
| no | 387 (78.819) | 157 (74.408) | 1.646 | 0.199 |
| yes | 104 (21.181) | 54 (25.592) | ||
| sex, n (%) | ||||
| no | 8 (1.629) | 2 (0.948) | 0.488 | 0.485 |
| yes | 483 (98.371) | 209 (99.052) | ||
| compliance of ULT, n (%) | ||||
| MPR < 60% | 230 (46.843) | 96 (45.498) | 0.107 | 0.743 |
| MPR ≥ 60% | 261 (53.157) | 115 (54.502) | ||
| urine specific gravity, median [IQR] | 1.014 [1.011, 1.018] | 1.015 [1.011, 1.020] | −1.577 | 0.114 |
| Urine Ph, median [IQR] | 6.000 [5.500, 6.000] | 5.500 [5.500, 6.000] | 0.998 | 0.304 |
| CysC, median [IQR] | 1.010 [0.870, 1.210] | 1.020 [0.890, 1.210] | −1.031 | 0.303 |
| GLOB, median [IQR] | 32.400 [29.500, 35.100] | 31.500 [29.000, 35.000] | 1.293 | 0.196 |
| ALB, median [IQR] | 46.100 [43.600, 48.600] | 45.600 [43.500, 48.300] | 0.594 | 0.552 |
| AST, median [IQR] | 27.000 [22.000, 35.000] | 26.000 [20.000, 34.100] | 1.579 | 0.114 |
| ALT, median [IQR] | 32.000 [21.000, 49.600] | 31.000 [20.000, 47.000] | 0.961 | 0.337 |
| Crea, median [IQR] | 82.500 [73.700, 92.900] | 83.500 [74.700, 95.000] | −0.855 | 0.393 |
| Urea, median [IQR] | 4.740 [3.780, 5.700] | 4.700 [3.750, 5.840] | 0.022 | 0.983 |
| UA, median [IQR] | 527.100 [434.300, 595.900] | 507.000 [417.600, 593.300] | 1.053 | 0.293 |
| PDW, median [IQR] | 16.500 [16.200, 16.800] | 16.500 [16.100, 16.700] | 1.882 | 0.059 |
| PCT, median [IQR] | 0.231 [0.202, 0.272] | 0.238 [0.199, 0.273] | 0.107 | 0.915 |
| MPV, median [IQR] | 11.200 [10.100, 12.600] | 11.300 [10.200, 12.700] | −0.446 | 0.656 |
| PLT, median [IQR] | 204.000 [166.000, 249.000] | 206.000 [164.000, 245.000] | −0.027 | 0.978 |
| MCHC, median [IQR] | 331.000 [323.000, 337.000] | 330.000 [322.000, 339.000] | 0.04 | 0.968 |
| MCV, median [IQR] | 92.000 [89.700, 94.400] | 91.500 [89.300, 94.500] | 0.547 | 0.584 |
| HCT, median [IQR] | 0.462 [0.435, 0.485] | 0.456 [0.428, 0.479] | 1.844 | 0.065 |
| HGB, median [IQR] | 153.000 [143.000, 161.000] | 151.000 [139.000, 160.000] | 1.549 | 0.121 |
| RBC, median [IQR] | 5.040 [4.710, 5.350] | 4.990 [4.660, 5.340] | 1.139 | 0.255 |
| MO, median [IQR] | 0.440 [0.350, 0.580] | 0.450 [0.340, 0.580] | −0.138 | 0.891 |
| LY, median [IQR] | 1.930 [1.570, 2.410] | 2.000 [1.590, 2.530] | −0.721 | 0.471 |
| GR, median [IQR] | 4.550 [3.690, 6.230] | 4.600 [3.420, 6.420] | 0.135 | 0.893 |
| WBC, median [IQR] | 7.380 [6.190, 9.090] | 7.710 [6.090, 9.260] | −0.371 | 0.711 |
| ESR, median [IQR] | 13.000 [5.000, 26.000] | 13.000 [6.000, 29.000] | 0.159 | 0.874 |
| eGFR, median [IQR] | 61.149 [49.171, 72.686] | 58.768 [47.969, 70.451] | 1.541 | 0.123 |
| course of disease, median [IQR] | 48.000 [24.000, 96.000] | 60.000 [24.000, 108.000] | −1.171 | 0.241 |
| BMI, median [IQR] | 25.687 [23.459, 27.682] | 25.712 [23.437, 27.682] | −0.205 | 0.838 |
| Variable | R | SE | Z | p | OR (95% CI) |
|---|---|---|---|---|---|
| sex | 16.646 | 593.391 | 0.028 | 0.978 | 16,956,319.853 (-) |
| compliance of ULT | −1.53 | 0.251 | −6.104 | <0.001 | 0.217 (0.131–0.35) |
| annual attack frequency (>12 times) | 0.848 | 0.273 | 3.1 | 0.002 | 2.334 (1.365–3.996) |
| annual attack frequency (6–12 times) | −0.156 | 0.339 | −0.461 | 0.644 | 0.855 (0.434–1.642) |
| history of drinking (drinking ≥ 70 g per week/drinking years ≥ 10 years), | 0.819 | 0.33 | 2.481 | 0.013 | 2.268 (1.198–4.386) |
| history of drinking (drinking < 70 g per week) | −0.632 | 0.328 | −1.929 | 0.054 | 0.532 (0.281–1.017) |
| family history of gout | 0.824 | 0.291 | 2.83 | 0.005 | 2.279 (1.285–4.033) |
| polyjoint involvement | 1.288 | 0.256 | 5.028 | <0.001 | 3.624 (2.209–6.041) |
| course of disease | 0.009 | 0.002 | 4.858 | <0.001 | 1.009 (1.005–1.012) |
| BMI | −0.084 | 0.038 | −2.242 | 0.025 | 0.919 (0.853–0.989) |
| ESR | 0.013 | 0.006 | 2.371 | 0.018 | 1.013 (1.002–1.025) |
| eGFR | −0.024 | 0.007 | −3.333 | 0.001 | 0.977 (0.963–0.99) |
| UA | 0.001 | 0.001 | 1.515 | 0.13 | 1.001 (1–1.1003) |
| (Intercept) | −16.443 | 593.392 | −0.028 | 0.978 | 0 (-) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, T.; Guo, J.; Wang, P.; Zhang, Z.; Niu, S.; Zhang, Q.; Qing, Y. Establishment and Validation of Predictive Model of Tophus in Gout Patients. J. Clin. Med. 2023, 12, 1755. https://doi.org/10.3390/jcm12051755
Lei T, Guo J, Wang P, Zhang Z, Niu S, Zhang Q, Qing Y. Establishment and Validation of Predictive Model of Tophus in Gout Patients. Journal of Clinical Medicine. 2023; 12(5):1755. https://doi.org/10.3390/jcm12051755
Chicago/Turabian StyleLei, Tianyi, Jianwei Guo, Peng Wang, Zeng Zhang, Shaowei Niu, Quanbo Zhang, and Yufeng Qing. 2023. "Establishment and Validation of Predictive Model of Tophus in Gout Patients" Journal of Clinical Medicine 12, no. 5: 1755. https://doi.org/10.3390/jcm12051755
APA StyleLei, T., Guo, J., Wang, P., Zhang, Z., Niu, S., Zhang, Q., & Qing, Y. (2023). Establishment and Validation of Predictive Model of Tophus in Gout Patients. Journal of Clinical Medicine, 12(5), 1755. https://doi.org/10.3390/jcm12051755






