Use of NEedle Versus suRFACE Recording Electrodes for Detection of Intraoperative Motor Warnings: A Non-Inferiority Trial. The NERFACE Study Part II
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients
2.3. Objectives
2.4. Muscle-Recorded Transcranial Electrical Stimulation Motor Evoked Potentials
2.5. Anaesthesia
2.6. Data Collection
2.7. Sample Size Calculation
2.8. Statistical Analysis
3. Results
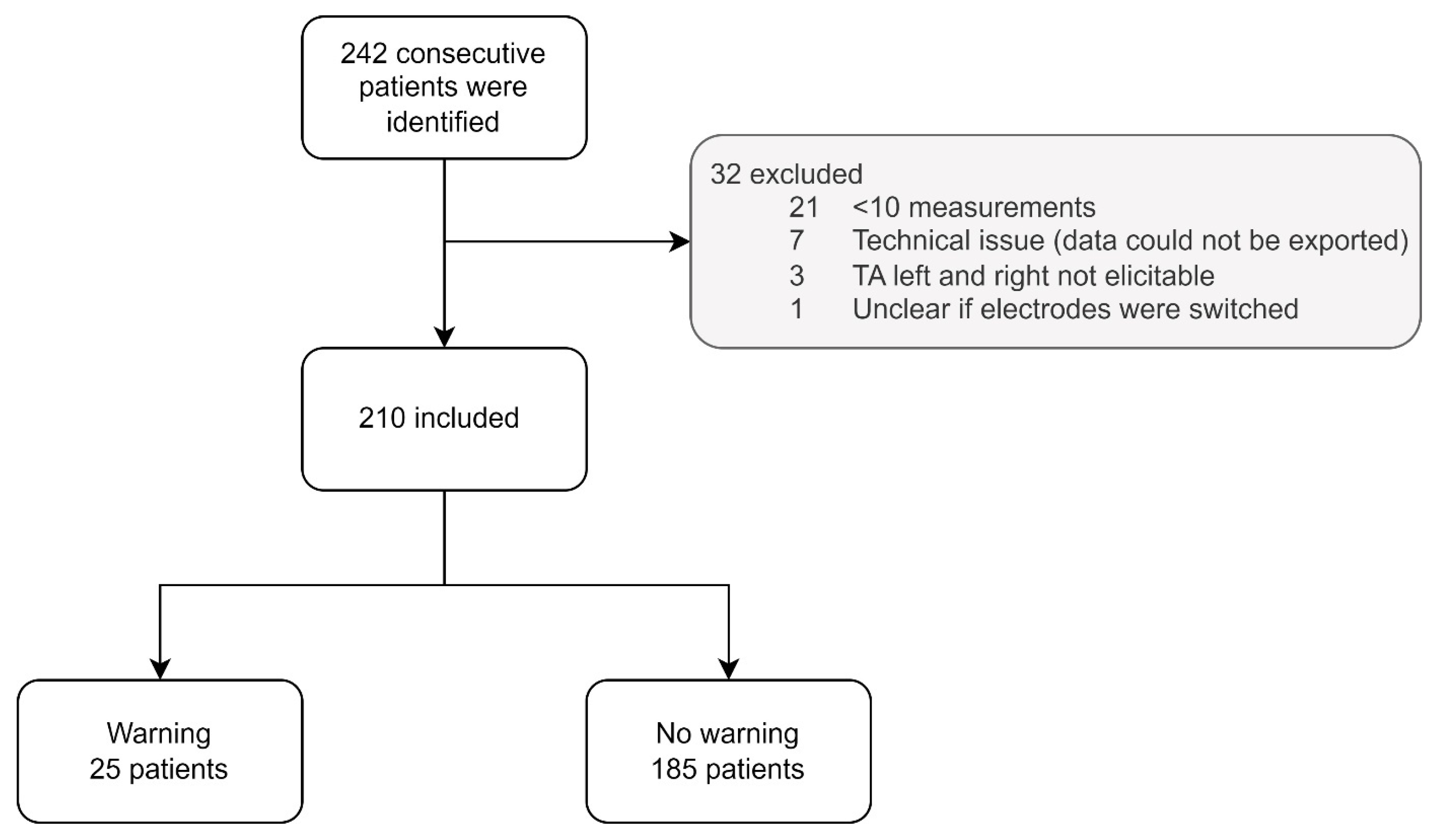
3.1. Patients
3.2. Primary Outcome
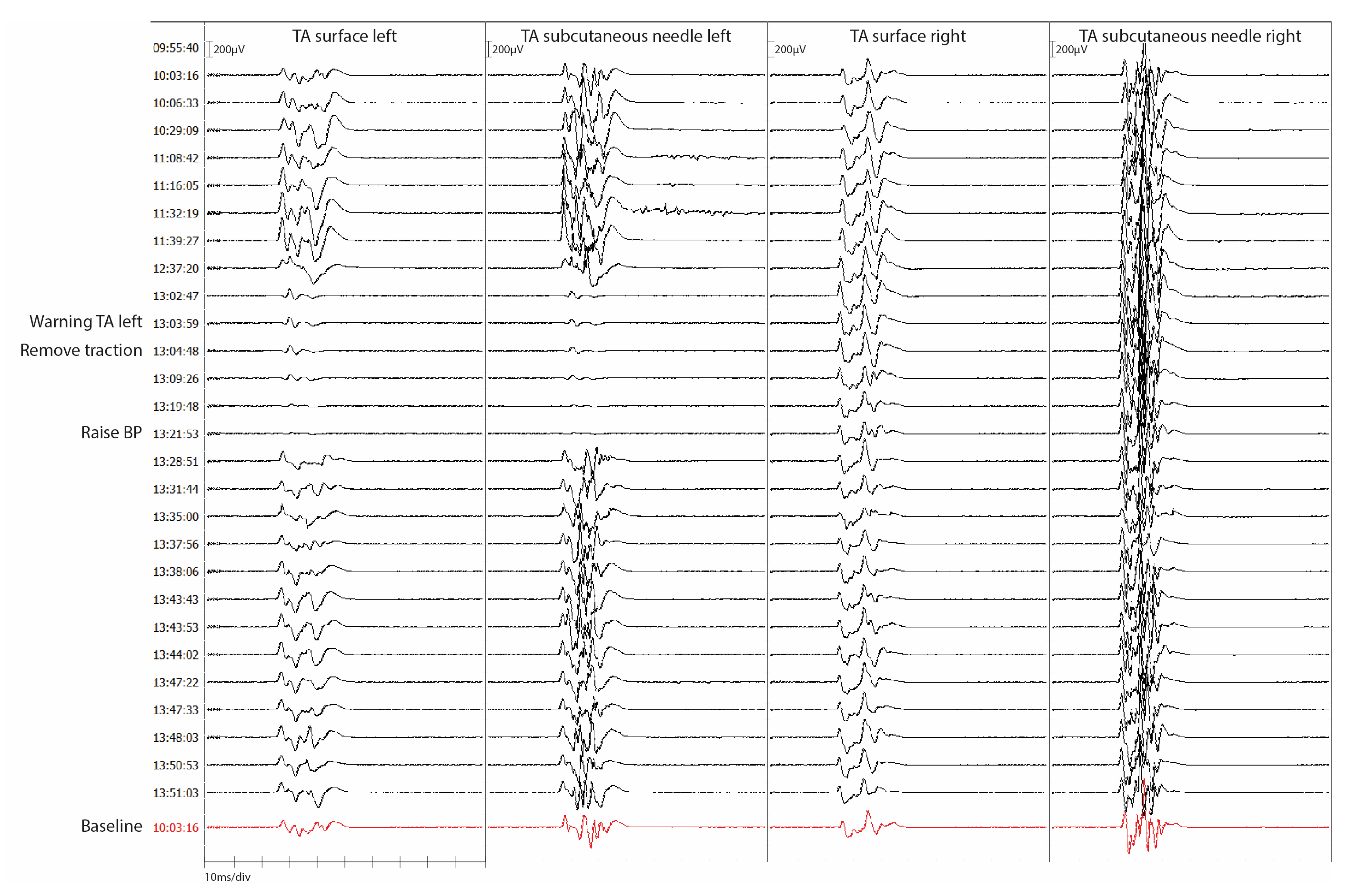
3.3. Secondary Outcome
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Langeloo, D.-D.; Journée, H.-L.; de Kleuver, M.; Grotenhuis, J.A. Criteria for Transcranial Electrical Motor Evoked Potential Monitoring during Spinal Deformity Surgery. Clin. Neurophysiol. 2007, 37, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Hausmann, O.N.; Min, K.; Boos, N.; Ruetsch, Y.A.; Erni, T.; Curt, A. Transcranial Electrical Stimulation: Significance of Fast versus Slow Charge Delivery for Intra-Operative Monitoring. Clin. Neurophysiol. 2002, 113, 1532–1535. [Google Scholar] [CrossRef]
- van Hal, C.; Hoebink, E.; Polak, H.E.; Racz, I.; de Kleuver, M.; Journee, H.L. Optimum Interpulse Interval for Transcranial Electrical Train Stimulation to Elicit Motor Evoked Potentials of Maximal Amplitude in Both Upper and Lower Extremity Target Muscles. Clin. Neurophysiol. 2013, 124, 2054–2059. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, D.B.; Skinner, S.; Shils, J.; Yingling, C. Intraoperative Motor Evoked Potential Monitoring—A Position Statement by the American Society of Neurophysiological Monitoring. Clin. Neurophysiol. 2013, 124, 2291–2316. [Google Scholar] [CrossRef] [PubMed]
- Fehlings, M.G.; Brodke, D.S.; Norvell, D.C.; Dettori, J.R. The Evidence for Intraoperative Neurophysiological Monitoring in Spine Surgery. Spine 2010, 35, S37–S46. [Google Scholar] [CrossRef]
- Journée, H.L.; Berends, H.I.; Kruyt, M.C. The Percentage of Amplitude Decrease Warning Criteria for Transcranial MEP Monitoring. J. Clin. Neurophysiol. 2017, 34, 22–31. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, D.B. Overview on Criteria for MEP Monitoring. J. Clin. Neurophysiol. 2017, 34, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Deletis, V. Intraoperative Neurophysiology and Methodologies Used to Monitor the Functional Integrity of the Motor System. In Neurophysiology in Neurosurgery; Academic Press: Cambridge, MA, USA, 2002; pp. 25–51. [Google Scholar]
- Langeloo, D.D.; Lelivelt, A.; Louis Journée, H.; Slappendel, R.; de Kleuver, M. Transcranial Electrical Motor-Evoked Potential Monitoring During Surgery for Spinal Deformity. Spine 2003, 28, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Gadella, M.C.; Dulfer, S.E.; Absalom, A.R.; Lange, F.; Scholtens-Henzen, C.H.M.; Groen, R.J.M.; Wapstra, F.H.; Faber, C.; Tamási, K.; Sahinovic, M.M.; et al. Comparing Motor-Evoked Potential Characteristics of NEedle versus SuRFACE Recording Electrodes during Spinal Cord Monitoring; The NERFACE Study Part I. J. Clin. Med. 2023, 12, 1404. [Google Scholar] [CrossRef]
- Kim, S.-M.; Yang, H.; Park, S.-B.; Han, S.G.; Park, K.-W.; Yoon, S.H.; Hyun, S.-J.; Kim, H.-J.; Park, K.S.; Lee, K.-W. Pattern-Specific Changes and Discordant Prognostic Values of Individual Leg-Muscle Motor Evoked Potentials during Spinal Surgery. Clin. Neurophysiol. 2012, 123, 1465–1470. [Google Scholar] [CrossRef] [PubMed]
- Compston, A. Aids to the Investigation of Peripheral Nerve Injuries. Medical Research Council: Nerve Injuries Research Committee. His Majesty’s Stationery Office: 1942; pp. 48 (iii) and 74 Figures and 7 Diagrams; with Aids to the Examination of the Peripheral Nervous System. By Michael O’Brien for the Guarantors of Brain. Saunders Elsevier: 2010; pp. [8] 64 and 94 Figures. Brain 2010, 133, 2838–2844. [Google Scholar] [PubMed]
- Fay, M.P. Confidence Intervals That Match Fisher’s Exact or Blaker’s Exact Tests. Biostatistics 2010, 11, 373. [Google Scholar] [CrossRef]
- Walsh, M.; Srinathan, S.K.; McAuley, D.F.; Mrkobrada, M.; Levine, O.; Ribic, C.; Molnar, A.O.; Dattani, N.D.; Burke, A.; Guyatt, G.; et al. The Statistical Significance of Randomized Controlled Trial Results Is Frequently Fragile: A Case for a Fragility Index. J. Clin. Epidemiol. 2014, 67, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Skinner, S.A.; Holdefer, R.N. Intraoperative Neuromonitoring Alerts That Reverse with Intervention: Treatment Paradox and What to Do about It. J. Clin. Neurophysiol. 2014, 31, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.B. The Environment and Disease: Association or Causation? J. R. Soc. Med. 1965, 58, 295–300. [Google Scholar] [CrossRef]
- Tignanelli, C.J.; Napolitano, L.M. The Fragility Index in Randomized Clinical Trials as a Means of Optimizing Patient Care. JAMA Surg. 2019, 154, 74–79. [Google Scholar] [CrossRef]
- Sala, F.; Palandri, G.; Basso, E.; Lanteri, P.; Deletis, V.; Faccioli, F.; Bricolo, A. Motor Evoked Potential Monitoring Improves Outcome after Surgery for Intramedullary Spinal Cord Tumors: A Historical Control Study. Neurosurgery 2006, 58, 1129–1143. [Google Scholar] [CrossRef]
- Kothbauer, K.F. Motor Evoked Potential Monitoring for Intramedullary Spinal Cord Tumor Surgery. In Neurophysiology in Neurosurgery: A Modern Intraoperative Approach; Deletis, V., Shills, J.L., Eds.; Academic Press: San Diego, CA, USA, 2002; pp. 73–92. ISBN 0-12-209036-5. [Google Scholar]
- Ulkatan, S.; Neuwirth, M.; Bitan, F.; Minardi, C.; Kokoszka, A.; Deletis, V. Monitoring of Scoliosis Surgery with Epidurally Recorded Motor Evoked Potentials (D Wave) Revealed False Results. Clin. Neurophysiol. 2006, 117, 2093–2101. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Deng, Y.L.; Sui, W.Y.; Yang, J.F.; Xu, J.; Huang, Z.F.; Yang, J.L. Intraoperative Neuromonitoring Auxiliary Significance of DNEP for MEP-Positive Event During Severe Spinal Deformity Surgery. Clin. Spine Surg. 2022, 35, E167–E174. [Google Scholar] [CrossRef] [PubMed]

| Warning Criteria mTc-MEP Amplitude | |
|---|---|
| Scoliosis surgery Neurosurgical surgeries Vascular surgeries | ≥80% decrease in mTc-MEP amplitude ≥50% decrease in mTc-MEP amplitude ≥50% decrease in mTc-MEP amplitude |
| Patient Characteristics | Patients (n = 210) | |
|---|---|---|
| Median age at surgery in years (IQR) | 19 (15, 51) | |
| Female N (%) | 135 (64.3) | |
| Diagnosis N (%) | ||
| Orthopaedic surgery | 123 (58.6) | |
| Idiopathic scoliosis | 83 (39.5) | |
| Congenital scoliosis | 3 (1.4) | |
| Neuromuscular scoliosis | 19 (9.0) | |
| Syndromic scoliosis | 15 (7.1) | |
| Kyphosis | 3 (1.4) | |
| Neurosurgical surgery | 67 (31.9) | |
| Extradural spinal tumour | 2 (1.0) | |
| Intradural extramedullary tumour | 22 (10.5) | |
| Intradural intramedullary tumour | 15 (7.1) | |
| Intradural cauda equina tumour | 9 (4.3) | |
| Tethered spinal cord | 3 (1.4) | |
| ATSCH | 4 (1.9) | |
| Thoracic HNP | 6 (2.9) | |
| Transdural approach | 3 (1.4) | |
| Extradural approach | 3 (1.4) | |
| Cervical HNP (extradural approach) | 1 (0.5) | |
| Extradural spinal nerve root tumour | 3 (1.4) | |
| Transdural approach | 2 (1.0) | |
| Extradural approach | 1 (0.5) | |
| Degenerative spine instability (extradural approach) | 1 (0.5) | |
| Trauma thoracic spinal cord compression (extradural approach) | 1 (0.5) | |
| Endovascular thoracic/abdominal aortic aneurysm repair | 20 (9.5) | |
| Mean surgery time in minutes (SD) | 334 (111) | |
| Elicitability N patients (%) | ||
| TA subcutaneous needle left | 206 (98.1) | |
| TA surface left | 206 (98.1) | |
| TA subcutaneous needle right | 208 (99.0) | |
| TA surface right | 208 (99.0) | |
| Preoperative TA strength N (%) | Left | Right |
| MRC 0 | 1 (0.5) | 0 (0.0) |
| MRC 1 | 1 (0.5) | 0 (0.0) |
| MRC 2 | 2 (1.0) | 2 (1.0) |
| MRC 3 | 1 (0.5) | 4 (1.9) |
| MRC 4 | 8 (3.8) | 6 (2.9) |
| MRC 5 | 187 (89.0) | 187 (89.0) |
| MRC < 5 * | 10 (4.8) | 11 (5.2) |
| Postoperative TA strength N (%) ** | Left | Right |
| MRC 0 | 2 (1.0) | 1 (0.5) |
| MRC 1 | 1 (0.5) | 0 (0.0) |
| MRC 2 | 1 (0.5) | 3 (1.4) |
| MRC 3 | 3 (1.4) | 4 (1.9) |
| MRC 4 | 11 (5.3) | 11 (5.3) |
| MRC 5 | 183 (87.6) | 182 (87.1) |
| MRC < 5 * | 8 (3.8) | 8 (3.8) |
| mTc-MEP Warnings Tibialis Anterior Muscles (n = 210) | |||
|---|---|---|---|
| Subcutaneous needle electrode | |||
| +warning | −no warning | ||
| Surface electrode | +warning | 25 | 0 |
| −no warning | 0 | 185 | |
| Patient | Age | Sex | Diagnosis | mTc-MEP Warning: TAL, TAR or Both | Subsequent Action | Reversible | MRC TAL Pre-op | MRC TAL Post-op | MRC TAR Pre-op | MRC TAR Post-op | New Postoperative Deficits | D-Wave Measured | D-Wave Warning | D-Wave Reversible |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 57 | F | Intradural Meningioma Th6 | Both | None | Yes | 5 | 5 | 5 | 5 | No | Yes | No | |
| 2 | 12 | F | Syndromic scoliosis: SMs | Both | Autologous blood transfusion, decrease of NE concentration * | Yes | 5 | 5 | 5 | 5 | No | No | ||
| 3 | 52 | M | HNP C6-7 (extradural) | Both | None (warning after small dura laceration, spontaneous recovery) | Yes | 5 | 5 | 5 | 5 | No | No | ||
| 4 | 85 | M | Endovascular aneurysm repair | Both | Removal femoral artery sheaths | Yes | 5 | 5 | 5 | 5 | No | No | ||
| 5 | 17 | F | Idiopathic scoliosis | Both | Increase BP, decrease propofol | Yes | 5 | 5 | 5 | 5 | No | No | ||
| 6 | 13 | F | Aneurysmal bone cyst C5-C6 | Both | None | No | 5 | 5 | 5 | 5 | No | No | ||
| 7 | 33 | M | NF1, kyphosis | Both | Increase BP, decrease propofol concentration | No | 5 | 5 | 5 | 5 | No | Yes | Yes | No |
| 8 | 29 | F | ATSCH | Both | None | No | 5 | 5 | 5 | 5 | No | Yes | No | |
| 9 | 40 | F | ATSCH | Both | None | No | 5 | 5 | 5 | 5 | No | Yes | Yes | No |
| 10 | 30 | F | ATSCH | TAL | None | Yes | 5 | 5 | 5 | 5 | No | Yes | No | |
| 11 | 68 | F | Endovascular aneurysm repair | TAL | Removal femoral artery sheath | Yes | 5 | 5 | 5 | 5 | No | No | ||
| 12 | 13 | M | Neuromuscular scoliosis: SMA | TAR | Removal hooks, decrease traction | Yes | <5 | <5 | <5 | <5 | No | No | ||
| 13 | 16 | M | Neuromuscular scoliosis: CP | TAR | Removal rod | Yes | <5 | <5 | <5 | <5 | No | No | ||
| 14 | 74 | M | Endovascular aneurysm repair | TAR | Removal femoral artery sheath | Yes | 5 | 5 | 5 | 5 | No | No | ||
| 15 | 72 | F | Endovascular aneurysm repair | TAR | Removal femoral artery sheath | Yes | 5 | 5 | 5 | 5 | No | No | ||
| 16 | 63 | M | Intramedullary lipoma Th1-4 | Both | None | TAL yes, TAR no | 5 | 5 | 5 | 4 | Transient | Yes *** | ||
| 17 | 73 | F | Spinal ependymoma C2-Th2 | Both | Increase BP | No | 5 | 3 | 5 | 0 | Transient | Yes **** | ||
| 18 | 50 | F | Ependymoma Th8-Th10 | Both | None | No | 5 | 4 | 5 | 4 | Transient | Yes | No | |
| 19 | 15 | F | Congenital scoliosis | TAL | None (warning after mechanical replacement of a nerve root) | Yes | 5 | 4 | 5 | 5 | Transient | No | ||
| 20 | 65 | F | HNP Th11-12 (extradural) | TAR (TAL not elicitable) | Increase BP | No | 5 | 5 | 5 | 4 | Transient | No | ||
| 21 | 20 | M | Congenital scoliosis | Both | Increase BP, removal temporary rod | No | 5 | 4 | 5 | 4 | Transient | No | ||
| 22 | 54 | F | HNP Th10-11 (transdural) | Both | Increase BP | No | 5 | 3 | 5 | 0 | TAL transient, TAR Permanent | No | ||
| 23 | 55 | M | Chondrosarcoma th7-9 | Both | None | No | 5 | 4 | 5 | 4 | Permanent | Yes | Yes | No |
| 24 | 48 | F | HNP Th8-9 (transdural) | TAL (TAR not elicitable) | Increase BP, irrigation spinal cord | No | 5 | 5 | 5 | 2 | TAL No, TAR Permanent | Yes | Yes | Yes |
| 25 | 12 | F | Neuromuscular scoliosis: SMA | Both | Decrease traction | TAL yes, TAR no | NA | 3 | NA | 3 | NA | No |
| Tibialis Anterior Left (n = 204) * | ||||
|---|---|---|---|---|
| New postoperative motor deficit | ||||
| Permanent | Transient | None | ||
| mTc-MEP result | Warning: complete loss | 1 | 2 | 1 |
| Warning: irreversible deterioration | 0 | 2 | 4 | |
| Reversible deterioration or loss | 0 | 1 | 8 | |
| No warning | 0 | 0 | 185 | |
| Tibialis Anterior Right (n = 206) ** | ||||
| New postoperative motor deficit | ||||
| Permanent | Transient | None | ||
| mTc-MEP result | Warning: complete loss | 2 | 4 | 1 |
| Warning: irreversible deterioration | 0 | 1 | 3 | |
| Reversible deterioration or loss | 0 | 0 | 9 | |
| No warning | 0 | 1 | 185 | |
| Tibialis Anterior Left * | ||||
|---|---|---|---|---|
| Predictive values of mTc-MEP warnings for new postoperative motor deficits % (CI) | ||||
| Without RW (n = 195) | RW as TP (n = 204) | RW as FP (n = 204) | RW after application of causality guidelines (n = 204) *** | |
| Sensitivity | 100.0 (47.8–100.0) | 100.0 (76.8–100.0) | 100 (47.8–100.0) | 100 (59.0–100.0) |
| Specificity | 97.4 (94.0–99.1) | 97.37 (94.0–99.1) | 93.0 (88.5–96.1) | 93.9 (89.6–96.8) |
| Positive predictive value | 50.0 (29.6–70.4) | 73.7 (54.1–86.9) | 26.3 (17.7–37.2) | 36.8 (25.2–50.2) |
| Negative predictive value | 100.0 (100.0–100.0) | 100.0 (100.0–100.0) | 100.0 (100.0–100.0) | 100.0 (100.0–100.0) |
| Tibialis Anterior Right ** | ||||
| Predictive values of mTc-MEP warnings for new postoperative motor deficits % (CI) | ||||
| Without RW (n = 197) | RW as TP (n = 206) | RW as FP (n = 206) | RW after application of causality guidelines (n = 206) *** | |
| Sensitivity | 87.5 (47.4–99.7) | 94.1 (71.3–99.9) | 87.5 (47.4–99.7) | 92.3 (64.0–99.8) |
| Specificity | 97.9 (94.7–99.4) | 97.9 (94.7–99.4) | 93.4 (89.0–96.5) | 95.9 (92.0–98.2) |
| Positive predictive value | 63.6 (39.1–82.7) | 80.0 (60.1–91.4) | 35.0 (23.0–49.2) | 60.0 (42.8–75.1) |
| Negative predictive value | 99.5 (96.7–99.9) | 99.5 (96.5–99.2) | 99.5 (88.9–96.2) | 99.5 (91.9–98.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dulfer, S.E.; Gadella, M.C.; Tamási, K.; Absalom, A.R.; Lange, F.; Scholtens-Henzen, C.H.M.; Faber, C.; Wapstra, F.H.; Groen, R.J.M.; Sahinovic, M.M.; et al. Use of NEedle Versus suRFACE Recording Electrodes for Detection of Intraoperative Motor Warnings: A Non-Inferiority Trial. The NERFACE Study Part II. J. Clin. Med. 2023, 12, 1753. https://doi.org/10.3390/jcm12051753
Dulfer SE, Gadella MC, Tamási K, Absalom AR, Lange F, Scholtens-Henzen CHM, Faber C, Wapstra FH, Groen RJM, Sahinovic MM, et al. Use of NEedle Versus suRFACE Recording Electrodes for Detection of Intraoperative Motor Warnings: A Non-Inferiority Trial. The NERFACE Study Part II. Journal of Clinical Medicine. 2023; 12(5):1753. https://doi.org/10.3390/jcm12051753
Chicago/Turabian StyleDulfer, Sebastiaan E., Maria C. Gadella, Katalin Tamási, Anthony R. Absalom, Fiete Lange, Carola H. M. Scholtens-Henzen, Christopher Faber, Frits H. Wapstra, Rob J. M. Groen, Marko M. Sahinovic, and et al. 2023. "Use of NEedle Versus suRFACE Recording Electrodes for Detection of Intraoperative Motor Warnings: A Non-Inferiority Trial. The NERFACE Study Part II" Journal of Clinical Medicine 12, no. 5: 1753. https://doi.org/10.3390/jcm12051753
APA StyleDulfer, S. E., Gadella, M. C., Tamási, K., Absalom, A. R., Lange, F., Scholtens-Henzen, C. H. M., Faber, C., Wapstra, F. H., Groen, R. J. M., Sahinovic, M. M., Ulkatan, S., & Drost, G. (2023). Use of NEedle Versus suRFACE Recording Electrodes for Detection of Intraoperative Motor Warnings: A Non-Inferiority Trial. The NERFACE Study Part II. Journal of Clinical Medicine, 12(5), 1753. https://doi.org/10.3390/jcm12051753







