Current Understanding and New Advances in the Surgical Management of Reparable Rotator Cuff Tears: A Scoping Review
Abstract
1. Introduction
2. Pathoanatomy and Pathogenesis
3. Imaging
3.1. Magnetic Resonance Imaging (MRI) versus MR Arthrography (MRA)
3.2. Assessment of Muscle Quality
4. Surgical Repair of Rotator Cuff Tears
4.1. Indications and Timing
4.2. Fixation Techniques
4.3. Suture Anchor Systems and Materials
4.4. Concomitant Acromioplasty
4.5. Augmentation
5. Postoperative Rehabilitation
5.1. Immobilization Versus Nonimmobilization
5.2. Sling Versus Abduction Brace
6. Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Camenzind, R.S.; Lafosse, L.; Lafosse, T. Pseudoparalysis and pseudoparesis of the shoulder. Obere Extremität 2021, 16, 237–246. [Google Scholar] [CrossRef]
- Picavet, H.S.J.; Schouten, J.S.A.G. Musculoskeletal pain in the Netherlands: Prevalences, consequences and risk groups, the DMC(3)-study. Pain 2003, 102, 167–178. [Google Scholar] [CrossRef]
- Minagawa, H.; Yamamoto, N.; Abe, H.; Fukuda, M.; Seki, N.; Kikuchi, K.; Kijima, H.; Itoi, E. Prevalence of symptomatic and asymptomatic rotator cuff tears in the general population: From mass-screening in one village. J. Orthop. 2013, 10, 8–12. [Google Scholar] [CrossRef]
- Yamamoto, A.; Takagishi, K.; Osawa, T.; Yanagawa, T.; Nakajima, D.; Shitara, H.; Kobayashi, T. Prevalence and risk factors of a rotator cuff tear in the general population. J. Shoulder Elb. Surg. 2010, 19, 116–120. [Google Scholar] [CrossRef]
- Teunis, T.; Lubberts, B.; Reilly, B.T.; Ring, D. A systematic review and pooled analysis of the prevalence of rotator cuff disease with increasing age. J. Shoulder Elb. Surg. 2014, 23, 1913–1921. [Google Scholar] [CrossRef]
- Lo, C.N.; Leung, B.P.L.; Sanders, G.; Li, M.W.M.; Ngai, S.P.C. The Major Pain Source of Rotator Cuff-Related Shoulder Pain: A Narrative Review on Current Evidence. Musculoskeletal Care. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/msc.1719 (accessed on 26 January 2023).
- Leong, H.; Fu, S.; He, X.; Oh, J.; Yamamoto, N.; Yung, S. Risk factors for rotator cuff tendinopathy: A systematic review and meta-analysis. J. Rehabilitation Med. 2019, 51, 627–637. [Google Scholar] [CrossRef]
- Moor, B.; Röthlisberger, M.; Müller, D.A.; Zumstein, M.; Bouaicha, S.; Ehlinger, M.; Gerber, C. Age, trauma and the critical shoulder angle accurately predict supraspinatus tendon tears. Orthop. Traumatol. Surg. Res. 2014, 100, 489–494. [Google Scholar] [CrossRef]
- Pandey, V.; Jaap Willems, W. Rotator cuff tear: A detailed update. Asia Pac. J. Sport. Med. Arthrosc. Rehabil. Technol. 2015, 2, 1–14. [Google Scholar] [CrossRef]
- Bigliani, L.U.; Ticker, J.B.; Flatow, E.L.; Soslowsky, L.J.; Mow, V.C. The relationship of acromial architecture to rotator cuff disease. Clin. Sports Med. 1991, 10, 823–838. [Google Scholar] [CrossRef]
- Neer, C.S. Anterior acromioplasty for the chronic impingement syndrome in the shoulder: A preliminary report. J. Bone Jt. Surg. 1972, 54, 41–50. [Google Scholar] [CrossRef]
- A Rockwood, C.; Lyons, F.R. Shoulder impingement syndrome: Diagnosis, radiographic evaluation, and treatment with a modified Neer acromioplasty. J. Bone Jt. Surg. 1993, 75, 409–424. [Google Scholar] [CrossRef]
- Cheng, C.; Chen, B.; Xu, H.; Zhang, Z.; Xu, W. Efficacy of concomitant acromioplasty in the treatment of rotator cuff tears: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0207306. [Google Scholar] [CrossRef]
- Beard, D.J.; Rees, J.L.; Cook, J.A.; Rombach, I.; Cooper, C.; Merritt, N.; Shirkey, B.A.; Donovan, J.L.; Gwilym, S.; Savulescu, J.; et al. Arthroscopic subacromial decompression for subacromial shoulder pain (CSAW): A multicentre, pragmatic, parallel group, placebo-controlled, three-group, randomised surgical trial. Lancet 2018, 391, 329–338. [Google Scholar] [CrossRef]
- Gerber, C.; Snedeker, J.G.; Baumgartner, D.; Viehöfer, A.F. Supraspinatus tendon load during abduction is dependent on the size of the critical shoulder angle: A biomechanical analysis. J. Orthop. Res. 2014, 32, 952–957. [Google Scholar] [CrossRef]
- Nyffeler, R.W.; Werner, C.M.L.; Sukthankar, A.; Schmid, M.R.; Gerber, C. Association of a large lateral extension of the acromion with rotator cuff tears. J. Bone Jt. Surg. Am. 2006, 88, 800–805. [Google Scholar]
- Moor, B.K.; Bouaicha, S.; Rothenfluh, D.A.; Sukthankar, A.; Gerber, C. Is there an association between the individual anatomy of the scapula and the development of rotator cuff tears or osteoarthritis of the glenohumeral joint? A radiological study of the critical shoulder angle. Bone Jt. J. 2013, 95, 935–941. [Google Scholar] [CrossRef]
- Viehöfer, A.F.; Gerber, C.; Favre, P.; Bachmann, E.; Snedeker, J.G. A larger critical shoulder angle requires more rotator cuff activity to preserve joint stability. J. Orthop. Res. 2015, 34, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Beeler, S.; Hasler, A.; Getzmann, J.; Weigelt, L.; Meyer, D.C.; Gerber, C. Acromial roof in patients with concentric osteoarthritis and massive rotator cuff tears: Multiplanar analysis of 115 computed tomography scans. J. Shoulder Elb. Surg. 2018, 27, 1866–1876. [Google Scholar] [CrossRef] [PubMed]
- Borbas, P.; Hartmann, R.; Ehrmann, C.; Ernstbrunner, L.; Wieser, K.; Bouaicha, S. Acromial Morphology and Its Relation to the Glenoid Is Associated with Different Partial Rotator Cuff Tear Patterns. J. Clin. Med. 2022, 12, 233. [Google Scholar] [CrossRef]
- Goutallier, D.; Postel, J.M.; Van Driessche, S.; Voisin, M.C. Histological lesions of supraspinatus tendons in full thickness tears of the rotator cuff. Rev. Chir. Orthop. Reparatrice Appar Mot 2005, 91, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Gumina, S.; Di Giorgio, G.; Bertino, A.; Della Rocca, C.; Sardella, B.; Postacchini, F. Inflammatory infiltrate of the edges of a torn rotator cuff. Int. Orthop. 2006, 30, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Löhr, J.F.; Uhthoff, H.K. Epidemiology and pathophysiology of rotator cuff tears. Orthopade 2007, 36, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Oliva, F.; Via, A.G.; Maffulli, N. Physiopathology of intratendinous calcific deposition. BMC Med. 2012, 10, 95. [Google Scholar] [CrossRef] [PubMed]
- Keener, J.D.; Patterson, B.M.; Orvets, N.; Chamberlain, A.M. Degenerative Rotator Cuff Tears: Refining Surgical Indications Based on Natural History Data. J. Am. Acad. Orthop. Surg. 2019, 27, 156–165. [Google Scholar] [CrossRef]
- Lohr, J.F.; Uhthoff, H.K. The Microvascular Pattern of the Supraspinatus Tendon. Clin. Orthop. Relat. Res. 1990, 254, 35–38. [Google Scholar] [CrossRef]
- Keener, J.D.; Hsu, J.E.; Steger-May, K.; Teefey, S.A.; Chamberlain, A.M.; Yamaguchi, K. Patterns of tear progression for asymptomatic degenerative rotator cuff tears. J. Shoulder Elb. Surg. 2015, 24, 1845–1851. [Google Scholar] [CrossRef]
- Patel, A.H.; Savoie, F.H.; O’Brien, M.J. Current concepts and expert practice report: Augmentation of rotator cuff repairs. J. Clin. Orthop. Trauma 2021, 19, 118–124. [Google Scholar] [CrossRef]
- Tashjian, R.Z.; Farnham, J.M.; Albright, F.S.; Teerlink, C.C.; A Cannon-Albright, L. Evidence for an Inherited Predisposition Contributing to the Risk for Rotator Cuff Disease. J. Bone Jt. Surg. 2009, 91, 1136–1142. [Google Scholar] [CrossRef]
- Oliva, F.; Osti, L.; Padulo, J.; Maffulli, N. Epidemiology of the rotator cuff tears: A new incidence related to thyroid disease. Muscle Ligaments Tendons J. 2019, 04, 309–314. [Google Scholar] [CrossRef]
- Oliva, F.; Berardi, A.C.; Misiti, S.; Verga Falzacappa, C.; Iacone, A.; Maffulli, N. Thyroid hormones enhance growth and counteract apoptosis in human tenocytes isolated from rotator cuff tendons. Cell Death Dis. 2013, 4, e705. [Google Scholar] [CrossRef]
- Snedeker, J.G.; Gautieri, A. The role of collagen crosslinks in ageing and diabetes-the good, the bad, and the ugly. Muscle Ligaments Tendons J. 2019, 4, 303–308. [Google Scholar] [CrossRef]
- Serpi, F.; Albano, D.; Rapisarda, S.; Chianca, V.; Sconfienza, L.M.; Messina, C. Shoulder ultrasound: Current concepts and future perspectives. J. Ultrason. 2021, 21, e154–e161. [Google Scholar] [CrossRef]
- Roy, J.-S.; Braën, C.; Leblond, J.; Desmeules, F.; Dionne, C.; MacDermid, J.C.; Bureau, N.J.; Fremont, P. Diagnostic accuracy of ultrasonography, MRI and MR arthrography in the characterisation of rotator cuff disorders: A systematic review and meta-analysis. Br. J. Sports Med. 2015, 49, 1316–1328. [Google Scholar] [CrossRef]
- Aminzadeh, B.; Najafi, S.; Moradi, A.; Abbasi, B.; Farrokh, D.; Emadzadeh, M. Evaluation of Diagnostic Precision of Ultrasound for Rotator Cuff Disorders in Patients with Shoulder Pain. Arch. Bone Jt. Surg. 2020, 8, 689–695. [Google Scholar] [PubMed]
- Gottsegen, C.J.; Merkle, A.N.; Bencardino, J.T.; Gyftopoulos, S. Advanced MRI Techniques of the Shoulder Joint: Current Applications in Clinical Practice. Am. J. Roentgenol. 2017, 209, 544–551. [Google Scholar] [CrossRef]
- Honda, H.; Morihara, T.; Arai, Y.; Horii, M.; Ito, H.; Furukawa, R.; Kida, Y.; Sukenari, T.; Ikoma, K.; Oda, R.; et al. Clinical application of radial magnetic resonance imaging for evaluation of rotator cuff tear. Orthop. Traumatol. Surg. Res. 2015, 101, 715–719. [Google Scholar] [CrossRef] [PubMed]
- Shibayama, Y.; Hirose, T.; Sugi, A.; Mizushima, E.; Watanabe, Y.; Tomii, R.; Iba, K.; Yamashita, T. Diagnostic accuracy of magnetic resonance imaging for partial tears of the long head of the biceps tendon in patients with rotator cuff tears. JSES Int. 2022, 6, 638–642. [Google Scholar] [CrossRef]
- Matsushita, R.; Yokoya, S.; Negi, H.; Matsubara, N.; Akiyama, Y.; Adachi, N. Evaluation of subscapularis tendon tears of the anterosuperior aspect using radial-sequence magnetic resonance imaging. JSES Int. 2021, 6, 97–103. [Google Scholar] [CrossRef]
- Groarke, P.; Jagernauth, S.; E Peters, S.; Manzanero, S.; O’Connell, P.; Cowderoy, G.; Gilpin, D.; Hope, B.; Marchant, D.; Cutbush, K.; et al. Correlation of magnetic resonance and arthroscopy in the diagnosis of shoulder injury. ANZ J. Surg. 2021, 91, 2145–2152. [Google Scholar] [CrossRef]
- Magee, T. 3-T MRI of the shoulder: Is MR arthrography necessary? AJR Am. J. Roentgenol. 2009, 192, 86–92. [Google Scholar] [CrossRef]
- Meyer, D.C.; Wieser, K.; Farshad, M.; Gerber, C. Retraction of Supraspinatus Muscle and Tendon as Predictors of Success of Rotator Cuff Repair. Am. J. Sports Med. 2012, 40, 2242–2247. [Google Scholar] [CrossRef]
- Gladstone, J.N.; Bishop, J.Y.; Lo, I.K.; Flatow, E.L. Fatty Infiltration and Atrophy of the Rotator Cuff do not Improve after Rotator Cuff Repair and Correlate with Poor Functional Outcome. Am. J. Sports Med. 2007, 35, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, B.; Weishaupt, D.; Zanetti, M.; Hodler, J.; Gerber, C. Fatty degeneration of the muscles of the rotator cuff: Assessment by computed tomography versus magnetic resonance imaging. J. Shoulder Elb. Surg. 1999, 8, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Goutallier, D.; Postel, J.M.; Bernageau, J.; Lavau, L.; Voisin, M.C. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin. Orthop. Relat. Res. 1994, 304, 78–83. [Google Scholar] [CrossRef]
- Werthel, J.-D.; de Casson, F.B.; Walch, G.; Gaudin, P.; Moroder, P.; Sanchez-Sotelo, J.; Chaoui, J.; Burdin, V. Three-dimensional muscle loss assessment: A novel computed tomography–based quantitative method to evaluate rotator cuff muscle fatty infiltration. J. Shoulder Elb. Surg. 2021, 31, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, F.; Böhm, D.; Eden, L.; Schmalzl, J.; Meffert, R.H.; Köstler, H.; Weng, A.M.; Ziegler, D. Comparing the MRI-based Goutallier Classification to an experimental quantitative MR spectroscopic fat measurement of the supraspinatus muscle. BMC Musculoskelet. Disord. 2016, 17, 355. [Google Scholar] [CrossRef]
- Santago, A.C.; Vidt, M.E.; Tuohy, C.J.; Poehling, G.G.; Freehill, M.T.; Jordan, J.H.; Kraft, R.A.; Saul, K.R. Quantitative Analysis of Three-Dimensional Distribution and Clustering of Intramuscular Fat in Muscles of the Rotator Cuff. Ann. Biomed. Eng. 2015, 44, 2158–2167. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, T.; Tasaki, A.; Horiuchi, S.; Osakabe, C.; Ohde, S.; Saida, Y.; Yoshioka, H. Quantification of Fatty Degeneration Within the Supraspinatus Muscle by Using a 2-Point Dixon Method on 3-T MRI. Am. J. Roentgenol. 2015, 205, 116–122. [Google Scholar] [CrossRef]
- Gyftopoulos, S.; Beltran, L.S.; Gibbs, K.; Jazrawi, L.; Berman, P.; Babb, J.; Meislin, R. Rotator cuff tear shape characterization: A comparison of two-dimensional imaging and three-dimensional magnetic resonance reconstructions. J. Shoulder Elb. Surg. 2015, 25, 22–30. [Google Scholar] [CrossRef]
- Adam, J.R.; Nanjayan, S.K.T.; Monga, P. Management of rotator cuff tears-Key historical landmarks. J. Clin. Orthop. Trauma 2021, 18, 6–12. [Google Scholar] [CrossRef]
- Dang, A.; Davies, M. Rotator Cuff Disease: Treatment Options and Considerations. Sports Med. Arthrosc. Rev. 2018, 26, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Longo, U.G.; Risi Ambrogioni, L.; Candela, V.; Berton, A.; Carnevale, A.; Schena, E.; Denaro, V. Conservative versus surgical management for patients with rotator cuff tears: A systematic review and META-analysis. BMC Musculoskelet. Disord. 2021, 22, 50. [Google Scholar]
- Moosmayer, S.; Lund, G.; Seljom, U.S.; Haldorsen, B.; Svege, I.C.; Hennig, T.; Pripp, A.H.; Smith, H.-J. Tendon repair compared with physiotherapy in the treatment of rotator cuff tears: A randomized controlled study in 103 cases with a five-year follow-up. J. Bone Jt. Surg. Am. 2014, 96, 1504–1514. [Google Scholar] [CrossRef]
- Piper, C.C.; Hughes, A.J.; Ma, Y.; Wang, H.; Neviaser, A.S. Operative versus nonoperative treatment for the management of full-thickness rotator cuff tears: A systematic review and meta-analysis. J. Shoulder Elb. Surg. 2018, 27, 572–576. [Google Scholar] [CrossRef]
- Moosmayer, S.; Tariq, R.; Stiris, M.; Smith, H.J. The natural history of asymptomatic rotator cuff tears: A three-year follow-up of fifty cases. J. Bone Jt. Surg. Am. 2013, 95, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Galatz, L.M.; Ball, C.M.; Teefey, S.A.; Middleton, W.D.; Yamaguchi, K. The Outcome and Repair Integrity of Completely Arthroscopically Repaired Large and Massive Rotator Cuff Tears. J. Bone Jt. Surg. 2004, 86, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Grubhofer, F.; Martinez, A.R.M.; Ernstbrunner, L.; Haberli, J.; Selig, M.E.; Yi, K.; Warner, J.J. Speed of recovery of the most commonly performed shoulder surgeries. JSES Int. 2021, 5, 776–781. [Google Scholar] [CrossRef]
- Collin, P.; Matsumura, N.; Lädermann, A.; Denard, P.J.; Walch, G. Relationship between massive chronic rotator cuff tear pattern and loss of active shoulder range of motion. J. Shoulder Elb. Surg. 2014, 23, 1195–1202. [Google Scholar] [CrossRef]
- Wirth, B.; Weinhold, L.; Müller-Rath, R. Surgical reconstruction of small and medium rotator cuff tears shows superior long-term results. Obere Extremität 2021, 16, 298–301. [Google Scholar] [CrossRef]
- Hantes, M.E.; Karidakis, G.K.; Vlychou, M.; Varitimidis, S.; Dailiana, Z.; Malizos, K.N. A comparison of early versus delayed repair of traumatic rotator cuff tears. Knee Surgery, Sports Traumatol. Arthrosc. 2011, 19, 1766–1770. [Google Scholar] [CrossRef]
- Mukovozov, I.; Byun, S.; Farrokhyar, F.; Wong, I. Time to surgery in acute rotator cufftear: A systematic review. Bone Jt. Res. 2013, 2, 122–128. [Google Scholar] [CrossRef] [PubMed]
- van der List, J.P.; Kok, L.M.; Alta, T.D.W.; van der List, M.P.J.; van Noort, A. Role of Delay Between Injury and Surgery on the Outcomes of Rotator Cuff Repair: A Systematic Review and Meta-analysis. Am. J. Sports Med. 2022, 31, 3635465211069560. [Google Scholar] [CrossRef] [PubMed]
- Meyer, D.C.; Zimmermann, S.M.; Wieser, K.; Bensler, S.; Gerber, C.; Germann, M. Lengthening of the subscapularis tendon as a sign of partial tearing in continuity. J. Shoulder Elb. Surg. 2015, 25, 31–37. [Google Scholar] [CrossRef]
- Burkhart, S.S.; Athanasiou, K.; Wirth, M.A. Margin convergence: A method of reducing strain in massive rotator cuff tears. Arthrosc. J. Arthrosc. Relat. Surg. 1996, 12, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Burkhart, S.S. The principle of margin convergence in rotator cuff repair as a means of strain reduction at the tear margin. Ann. Biomed. Eng. 2004, 32, 166–170. [Google Scholar] [CrossRef]
- Burkhart, S.S.; Danaceau, S.M.; Pearce, C.E. Arthroscopic rotator cuff repair: Analysis of results by tear size and by repair technique-margin convergence versus direct tendon-to-bone repair. Arthroscopy 2001, 17, 905–912. [Google Scholar] [CrossRef]
- Kim, K.C.; Shin, H.D.; Cha, S.M.; Kim, J.H. Repair Integrity and Functional Outcomes for Arthroscopic Margin Convergence of Rotator Cuff Tears. J. Bone Jt. Surg. 2013, 95, 536–541. [Google Scholar] [CrossRef]
- Baumgarten, K.M. Patient-Determined Outcomes After Arthroscopic Margin Convergence Rotator Cuff Repair. Arthrosc. Sports Med. Rehabil. 2020, 2, e517–e522. [Google Scholar] [CrossRef]
- Kurnaz, R.; Ergün, S.; Aşçı, M.; Akgün, U.; Güneş, T. Mid-term clinical and radiological outcomes of arthroscopic repair of isolated and combined subscapularis tears: A single-center experience. Acta Orthop. Et Traumatol. Turc. 2021, 55, 473–479. [Google Scholar] [CrossRef]
- Seppel, G.; Plath, J.E.; Völk, C.; Seiberl, W.; Buchmann, S.; Waldt, S.; Imhoff, A.B.; Braun, S. Long-term Results After Arthroscopic Repair of Isolated Subscapularis Tears. Am. J. Sports Med. 2016, 45, 759–766. [Google Scholar] [CrossRef]
- Wirth, B.; Kunz, S.; Schwyzer, H.-K.; Flury, M.; Lenz, M.; Audigé, L. Repair of Lafosse I subscapularis lesions brings no benefit in anterosuperior rotator cuff reconstruction. Knee Surgery, Sports Traumatol. Arthrosc. 2019, 27, 4021–4031. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, Q.; Liu, B. Repair of Lafosse I subscapularis injury adds no additional value in anterosuperior rotator cuff injury. BMC Musculoskelet. Disord. 2021, 22, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Shukla, D.R.; Sánchez-Sotelo, J. Subscapularis tears: Hidden and forgotten no more. JSES Open Access 2018, 2, 74–83. [Google Scholar] [CrossRef]
- Ek, E.T.; Perret, M.C.; Borbas, P. Arthroscopic Knotless Repair of Complete Full-Thickness Tears of the Subscapularis Tendon Through a Single Portal. Arthrosc. Tech. 2020, 9, e439–e443. [Google Scholar] [CrossRef] [PubMed]
- Lo, I.K.; Burkhart, S.S. The comma sign: An arthroscopic guide to the torn subscapularis tendon. Arthrosc. J. Arthrosc. Relat. Surg. 2003, 19, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Gröger, F.; Hackl, M.; Buess, E. Arthroscopic Suture-Bridge Repair of the Subscapularis Tendon—“Inside and Outside the Box” With Preservation of the Comma Sign. Arthrosc. Tech. 2021, 11, e31–e36. [Google Scholar] [CrossRef]
- Hohmann, E.; König, A.; Kat, C.-J.; Glatt, V.; Tetsworth, K.; Keough, N. Single- versus double-row repair for full-thickness rotator cuff tears using suture anchors. A systematic review and meta-analysis of basic biomechanical studies. Eur. J. Orthop. Surg. Traumatol. 2017, 28, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Park, M.C.; ElAttrache, N.S.; Tibone, J.E.; Ahmad, C.S.; Jun, B.-J.; Lee, T.Q. Part I: Footprint contact characteristics for a transosseous-equivalent rotator cuff repair technique compared with a double-row repair technique. J. Shoulder Elb. Surg. 2007, 16, 461–468. [Google Scholar] [CrossRef]
- Wieser, K.; Rahm, S.; Farshad, M.; Ek, E.T.; Gerber, C.; Meyer, D.C. Stitch positioning influences the suture hold in supraspinatus tendon repair. Knee Surgery, Sports Traumatol. Arthrosc. 2012, 21, 1587–1592. [Google Scholar] [CrossRef]
- Zumstein, M.A.; Raniga, S.; Labrinidis, A.; Eng, K.; Bain, G.I.; Moor, B.K. Optimal Lateral Row Anchor Positioning in Posterior-Superior Transosseous Equivalent Rotator Cuff Repair: A Micro-Computed Tomography Study. Orthop. J. Sports Med. 2016, 4, 2325967116671305. [Google Scholar] [CrossRef]
- Park, M.C.; Cadet, E.R.; Levine, W.N.; Bigliani, L.U.; Ahmad, C.S. Tendon-to-Bone Pressure Distributions at a Repaired Rotator Cuff Footprint Using Transosseous Suture and Suture Anchor Fixation Techniques. Am. J. Sports Med. 2005, 33, 1154–1159. [Google Scholar] [CrossRef]
- Kim, D.-H.; Jeon, J.-H.; Choi, B.-C.; Cho, C.-H. Knot impingement after arthroscopic rotator cuff repair mimicking infection: A case report. World J. Clin. Cases 2022, 10, 5097–5102. [Google Scholar] [CrossRef]
- McCormick, F.; Wilson, H.; Gupta, A.; Bruce, B.; Harris, J.; Abrams, G.; Hussey, K.; Cole, B.J. Single-row, double-row, and transosseous equivalent techniques for isolated supraspinatus tendon tears with minimal atrophy: A retrospective comparative outcome and radiographic analysis at minimum 2-year followup. Int. J. Shoulder Surg. 2014, 8, 15–20. [Google Scholar] [CrossRef]
- Franceschi, F.; Ruzzini, L.; Longo, U.G.; Martina, F.M.; Zobel, B.B.; Maffulli, N.; Denaro, V. Equivalent clinical results of arthroscopic single-row and double-row suture anchor repair for rotator cuff tears: A randomized controlled trial. Am. J. Sports Med. 2007, 35, 1254–1260. [Google Scholar] [CrossRef]
- Ponugoti, N.; Raghu, A.; Colaco, H.B.; Magill, H. A comparison of simple and complex single-row versus transosseous-equivalent double-row repair techniques for full-thickness rotator cuff tears: A systematic review and meta-analysis. JSES Int. 2021, 6, 70–78. [Google Scholar] [CrossRef]
- Lapner, P.; Henry, P.; Athwal, G.S.; Moktar, J.; McNeil, D.; MacDonald, P. Treatment of rotator cuff tears: A systematic review and meta-analysis. J. Shoulder Elb. Surg. 2021, 31, e120–e129. [Google Scholar] [CrossRef] [PubMed]
- Millett, P.J.; Warth, R.J.; Dornan, G.J.; Lee, J.T.; Spiegl, U.J. Clinical and structural outcomes after arthroscopic single-row versus double-row rotator cuff repair: A systematic review and meta-analysis of level I randomized clinical trials. J. Shoulder Elb. Surg. 2014, 23, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ge, H.; Zhou, J.; Yuan, C.; Chen, K.; Cheng, B. Single-Row or Double-Row Fixation Technique for Full-Thickness Rotator Cuff Tears: A Meta-Analysis. PLoS ONE 2013, 8, e68515. [Google Scholar] [CrossRef] [PubMed]
- Storti, T.M.; Ribeiro, T.d.S.; Faria, R.S.S.; Simionatto, J.E.; Simionatto, C.; Paniago, A.F. Arthroscopic Repair of Rotator Cuff Injury: An Analysis of Function, Muscular Strength and Pain Between Single Row and Double Row Techniques. Rev. Bras. Ortop. (Sao Paulo) 2022, 57, 472–479. [Google Scholar] [PubMed]
- Borbas, P.; Wieser, K.; Grubhofer, F. Fixationssysteme und Techniken zur arthroskopischen Rotatorenmanschettenrekonstruktion: Evidenz im Jahr 2021. Arthroskopie 2021, 34, 185–191. [Google Scholar] [CrossRef]
- Cummins, C.A.; Murrell, G.A. Mode of failure for rotator cuff repair with suture anchors identified at revision surgery. J. Shoulder Elb. Surg. 2003, 12, 128–133. [Google Scholar] [CrossRef]
- Milewski, M.D.; Diduch, D.R.; Hart, J.M.; Tompkins, M.; Ma, S.-Y.; Gaskin, C.M. Bone Replacement of Fast-Absorbing Biocomposite Anchors in Arthroscopic Shoulder Labral Repairs. Am. J. Sports Med. 2012, 40, 1392–1401. [Google Scholar] [CrossRef]
- Tingart, M.J.; Apreleva, M.; Lehtinen, J.; Zurakowski, D.; Warner, J.J.P. Anchor design and bone mineral density affect the pull-out strength of suture anchors in rotator cuff repair: Which anchors are best to use in patients with low bone quality? Am. J. Sports Med. 2004, 32, 1466–1473. [Google Scholar] [CrossRef]
- Mazzocca, A.D.; Chowaniec, D.; Cote, M.P.; Fierra, J.; Apostolakos, J.; Nowak, M.; Arciero, R.A.; Beitzel, K. Biomechanical Evaluation of Classic Solid and Novel All-Soft Suture Anchors for Glenoid Labral Repair. Arthrosc. J. Arthrosc. Relat. Surg. 2012, 28, 642–648. [Google Scholar] [CrossRef]
- Bisson, L.J.; Manohar, L.M.; Wilkins, R.D.; Gurske-Deperio, J.; Ehrensberger, M.T. Influence of suture material on the biomechanical behavior of suture-tendon specimens: A controlled study in bovine rotator cuff. Am. J. Sports Med. 2008, 36, 907–912. [Google Scholar] [CrossRef]
- Gnandt, R.J.; Smith, J.L.; Nguyen-Ta, K.; McDonald, L.; LeClere, L.E. High-Tensile Strength Tape Versus High-Tensile Strength Suture: A Biomechanical Study. Arthrosc. J. Arthrosc. Relat. Surg. 2015, 32, 356–363. [Google Scholar] [CrossRef]
- Woodmass, J.M.; Al Khatib, L.; McRae, S.; Lapner, P.; Mascarenhas, R.; Neogi, D.; MacDonald, P.B. Arthroscopic Rotator Cuff Repair with and without Acromioplasty in the Treatment of Full-Thickness Rotator Cuff Tears: Long-Term Outcomes of a Multicenter, Randomized Controlled Trial. J. Bone Jt. Surg. Am. 2022, 104, 2101–2107. Available online: https://pubmed.ncbi.nlm.nih.gov/36476738/ (accessed on 30 January 2023). [CrossRef] [PubMed]
- Swindell, H.W.; Kang, H.P.; Mueller, J.D.; Heffernan, J.T.; Saltzman, B.M.; Ahmad, C.S.; Levine, W.N.; Weber, A.E.; Trofa, D.P. Rotator Cuff Repair With Acromioplasty Is Associated With an Increased Rate of Revision and Subsequent Procedures. Arthrosc. Sport. Med. Rehabil. 2022, 4, e2065–e2071. Available online: https://pubmed.ncbi.nlm.nih.gov/36579038/ (accessed on 30 January 2023). [CrossRef] [PubMed]
- Altchek, D.W.; Warren, R.F.; Wickiewicz, T.L.; Skyhar, M.J.; Ortiz, G.; Schwartz, E. Arthroscopic acromioplasty. Technique and results. J. Bone Jt. Surg. Am. 1990, 72, 1198–1207. [Google Scholar] [CrossRef]
- Gerber, C.; Catanzaro, S.; Betz, M.; Ernstbrunner, L. Arthroscopic Correction of the Critical Shoulder Angle Through Lateral Acromioplasty: A Safe Adjunct to Rotator Cuff Repair. Arthrosc. J. Arthrosc. Relat. Surg. 2017, 34, 771–780. [Google Scholar] [CrossRef]
- Barber, F.A.; Herbert, M.A.; Boothby, M.H. Ultimate tensile failure loads of a human dermal allograft rotator cuff augmentation. Arthroscopy 2008, 24, 20–24. [Google Scholar] [CrossRef]
- Jung, C.; Spreiter, G.; Audigé, L.; Ferguson, S.J.; Flury, M. Patch-augmented rotator cuff repair: Influence of the patch fixation technique on primary biomechanical stability. Arch. Orthop. Trauma Surg. 2016, 136, 609–616. [Google Scholar] [CrossRef]
- Kataoka, T.; Kokubu, T.; Muto, T.; Mifune, Y.; Inui, A.; Sakata, R.; Nishimoto, H.; Harada, Y.; Takase, F.; Ueda, Y.; et al. Rotator cuff tear healing process with graft augmentation of fascia lata in a rabbit model. J. Orthop. Surg. Res. 2018, 13, 200. [Google Scholar] [CrossRef] [PubMed]
- McCarron, J.A.; Milks, R.A.; Mesiha, M.; Aurora, A.; Walker, E.; Iannotti, J.P.; Derwin, K.A. Reinforced fascia patch limits cyclic gapping of rotator cuff repairs in a human cadaveric model. J. Shoulder Elb. Surg. 2012, 21, 1680–1686. [Google Scholar] [CrossRef]
- Milks, R.A.; Kolmodin, J.D.; Ricchetti, E.T.; Iannotti, J.P.; Derwin, K.A. Augmentation with a reinforced acellular fascia lata strip graft limits cyclic gapping of supraspinatus repairs in a human cadaveric model. J. Shoulder Elb. Surg. 2018, 27, 1105–1111. [Google Scholar] [CrossRef]
- Santoni, B.G.; McGilvray, K.C.; Lyons, A.S.; Bansal, M.; Turner, A.S.; MacGillivray, J.D.; Coleman, S.H.; Puttlitz, C.M. Biomechanical Analysis of an Ovine Rotator Cuff Repair via Porous Patch Augmentation in a Chronic Rupture Model. Am. J. Sports Med. 2010, 38, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Mehta, V.M.; Mandala, C.L.; Shriver, R.J.; Benson, M. Biomechanical Effects of Fiber Patch Augmentation on Rotator Cuff Repairs. Orthopedics 2020, 43, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.S.; Smith, R.D.J.; Nagra, N.; Wheway, K.; Watkins, B.; Snelling, S.; Dakin, S.G.; Carr, A.J. Rotator cuff repair with biological graft augmentation causes adverse tissue outcomes. Acta Orthop. 2020, 91, 782–788. [Google Scholar] [CrossRef]
- Lee, G.W.; Kim, J.Y.; Lee, H.W.; Yoon, J.H.; Noh, K.-C. Clinical and Anatomical Outcomes of Arthroscopic Repair of Large Rotator Cuff Tears with Allograft Patch Augmentation: A Prospective, Single-Blinded, Randomized Controlled Trial with a Long-term Follow-up. Clin. Orthop. Surg. 2022, 14, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Colbath, G.; Murray, A.; Siatkowski, S.; Pate, T.; Krussig, M.; Pill, S.; Hawkins, R.; Tokish, J.; Mercuri, J. Autograft Long Head Biceps Tendon Can Be Used as a Scaffold for Biologically Augmenting Rotator Cuff Repairs. Arthroscopy 2022, 38, 38–48. [Google Scholar] [CrossRef]
- Veen, E.J.; Stevens, M.; Diercks, R.L. Biceps Autograft Augmentation for Rotator Cuff Repair: A Systematic Review. Arthrosc. J. Arthrosc. Relat. Surg. 2018, 34, 1297–1305. [Google Scholar] [CrossRef]
- Klatte-Schulz, F.; Thiele, K.; Scheibel, M.; Duda, G.N.; Wildemann, B. Subacromial Bursa: A Neglected Tissue Is Gaining More and More Attention in Clinical and Experimental Research. Cells 2022, 11, 663. [Google Scholar] [CrossRef]
- Freislederer, F.; Dittrich, M.; Scheibel, M. Biological Augmentation With Subacromial Bursa in Arthroscopic Rotator Cuff Repair. Arthrosc. Tech. 2019, 8, e741–e747. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, D.N. Arthroscopic Biological Augmentation for Massive Rotator Cuff Tears: The Biceps-Cuff-Bursa Composite Repair. Arthrosc. Tech. 2021, 10, e2279–e2285. [Google Scholar] [CrossRef] [PubMed]
- Muench, L.N.; Uyeki, C.L.; Mancini, M.R.; Berthold, D.P.; McCarthy, M.B.; Mazzocca, A.D. Arthroscopic Rotator Cuff Repair Augmented with Autologous Subacromial Bursa Tissue, Concentrated Bone Marrow Aspirate, Platelet-Rich Plasma, Platelet-Poor Plasma, and Bovine Thrombin. Arthrosc. Tech. 2021, 10, e2053–e2059. [Google Scholar] [CrossRef]
- Muench, L.N.; Kia, C.; Jerliu, A.; Williams, A.A.; Berthold, D.P.; Cote, M.P.; McCarthy, M.B.; Arciero, R.; Mazzocca, A.D. Clinical Outcomes Following Biologically Enhanced Patch Augmentation Repair as a Salvage Procedure for Revision Massive Rotator Cuff Tears. Arthroscopy 2020, 36, 1542–1551. [Google Scholar] [CrossRef] [PubMed]
- Berthold, D.P.; Garvin, P.; Mancini, M.R.; Uyeki, C.L.; LeVasseur, M.R.; Mazzocca, A.D.; Voss, A. Arthroscopic rotator cuff repair with biologically enhanced patch augmentation. Oper. Orthop. Und Traumatol. 2021, 34, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Wellington, I.J.; Muench, L.N.; Hawthorne, B.C.; Uyeki, C.L.; Antonacci, C.L.; McCarthy, M.B.; Connors, J.P.; Kia, C.; Mazzocca, A.D.; Berthold, D.P. Clinical Outcomes following Biologically Enhanced Demineralized Bone Matrix Augmentation of Complex Rotator Cuff Repair. J. Clin. Med. 2022, 11, 2956. [Google Scholar] [CrossRef]
- Feldman, M.D. Editorial Commentary: Has the Pendulum Swung Yet Again? Allowing Early Active Motion After Arthroscopic Rotator Cuff Repair. Arthroscopy 2019, 35, 761–762. [Google Scholar] [CrossRef]
- Baldwin, M.J.; Nagra, N.S.; Merritt, N.; Rees, J.; Carr, A.J.; Rangan, A.; Thomas, M.; Beard, D.J.; Cooper, C.; Kottam, L.; et al. The use of a patch to augment rotator cuff surgery—A survey of UK shoulder and elbow surgeons. PLoS ONE 2020, 15, e0230235. [Google Scholar] [CrossRef]
- A Cook, J.; Baldwin, M.; Cooper, C.; Nagra, N.S.; Crocker, J.C.; Glaze, M.; Greenall, G.; Rangan, A.; Kottam, L.; Rees, J.L.; et al. Patch augmentation surgery for rotator cuff repair: The PARCS mixed-methods feasibility study. Health Technol. Assess. 2021, 25, 1–138. [Google Scholar] [CrossRef] [PubMed]
- Tirefort, J.; Schwitzguebel, A.J.; Collin, P.; Nowak, A.; Plomb-Holmes, C.; Lädermann, A. Postoperative Mobilization After Superior Rotator Cuff Repair: Sling Versus No Sling: A Randomized Prospective Study. J. Bone Jt. Surg. Am. 2019, 101, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Sheps, D.M.; Silveira, A.; Beaupre, L.; Styles-Tripp, F.; Balyk, R.; Lalani, A.; Glasgow, R.; Bergman, J.; Bouliane, M. Early Active Motion Versus Sling Immobilization After Arthroscopic Rotator Cuff Repair: A Randomized Controlled Trial. Arthrosc. J. Arthrosc. Relat. Surg. 2019, 35, 749–760e2. [Google Scholar] [CrossRef]
- Hollman, F.; Wolterbeek, N.; Zijl, J.A.; van Egeraat, S.P.; Wessel, R.N. Abduction Brace Versus Antirotation Sling After Arthroscopic Cuff Repair: The Effects on Pain and Function. Arthrosc. J. Arthrosc. Relat. Surg. 2017, 33, 1618–1626. [Google Scholar] [CrossRef] [PubMed]
- Ghandour, T.M.; Ibrahim, A.; Abdelrahman, A.A.; Elgammal, A.; Hammad, M.H. Does The Type of Shoulder Brace Affect Postoperative Pain and Clinical Outcome After Arthroscopic Rotator Cuff Repair? Arthroscopy 2019, 35, 1016–1023. [Google Scholar] [CrossRef]
- Longo, U.G.; Ambrogioni, L.R.; Berton, A.; Candela, V.; Migliorini, F.; Carnevale, A.; Schena, E.; Nazarian, A.; DeAngelis, J.; Denaro, V. Conservative versus accelerated rehabilitation after rotator cuff repair: A systematic review and meta-analysis. BMC Musculoskelet. Disord. 2021, 22, 637. [Google Scholar] [CrossRef] [PubMed]
- Grubhofer, F.; Ernstbrunner, L.; Gerber, C.; Hochreiter, B.; Schwihla, I.; Wieser, K.; Bouaicha, S. Effect of Abduction Brace Wearing Compliance on the Results of Arthroscopic Rotator Cuff Repair. JBJS Open Access 2022, 7, e2100148. [Google Scholar] [CrossRef]
- Grubhofer, F.; Gerber, C.; Meyer, D.C.; Wieser, K.; Ernstbrunner, L.; Catanzaro, S.; Bouaicha, S. Compliance with wearing an abduction brace after arthroscopic rotator cuff repair: A prospective, sensor-controlled study. Prosthet. Orthot. Int. 2019, 43, 440–446. [Google Scholar] [CrossRef]
- Schenk, P.; Bachmann, E.; Aichmair, A.; Götschi, T.; Gerber, C.; Meyer, D.C. Biomechanical and Clinical Evaluation of the Optimal Arm Position After Rotator Cuff Surgery With an Adjustable Abduction Brace. Orthopedics 2020, 44, e1–e6. [Google Scholar] [CrossRef]
- Pandey, V.; Madi, S.; Maddukuri, S.; Acharya, K.; Karegowda, L.H.; Willems, W.J. Does application of abduction brace after arthroscopic rotator cuff repair improve blood flow around posterosuperior rotator cuff and repair site, affecting pain levels and clinical and structural outcomes? A pilot randomized controlled trial. JSES Int. 2020, 4, 848–859. [Google Scholar] [CrossRef]
- Caldwell, G.L.; Warner, J.J.P.; Miller, M.D.; Boardman, D.; Towers, J.; Debski, R. Strength of Fixation with Transosseous Sutures in Rotator Cuff Repair. J. Bone Jt. Surg. 1997, 79, 1064–1068. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, H.L. Lesions of the musculotendinous cuff of the shoulder. The exposure and treatment of tears with retraction. 1944. Clin. Orthop. Relat. Res. 1994, 304, 3–9. [Google Scholar]
- McLaughlin, H.L. Rupture of the Rotator Cuff: JBJS. J. Bone Jt. Surg. Am. 1962, 44, 979–983. Available online: https://journals.lww.com/jbjsjournal/Citation/1962/44050/Rupture_of_the_Rotator_Cuff.10.aspx (accessed on 14 August 2022). [CrossRef]
- Longo, U.; Carnevale, A.; Massaroni, C.; Presti, D.L.; Berton, A.; Candela, V.; Schena, E.; Denaro, V. Personalized, Predictive, Participatory, Precision, and Preventive (P5) Medicine in Rotator Cuff Tears. J. Pers. Med. 2021, 11, 255. [Google Scholar] [CrossRef] [PubMed]
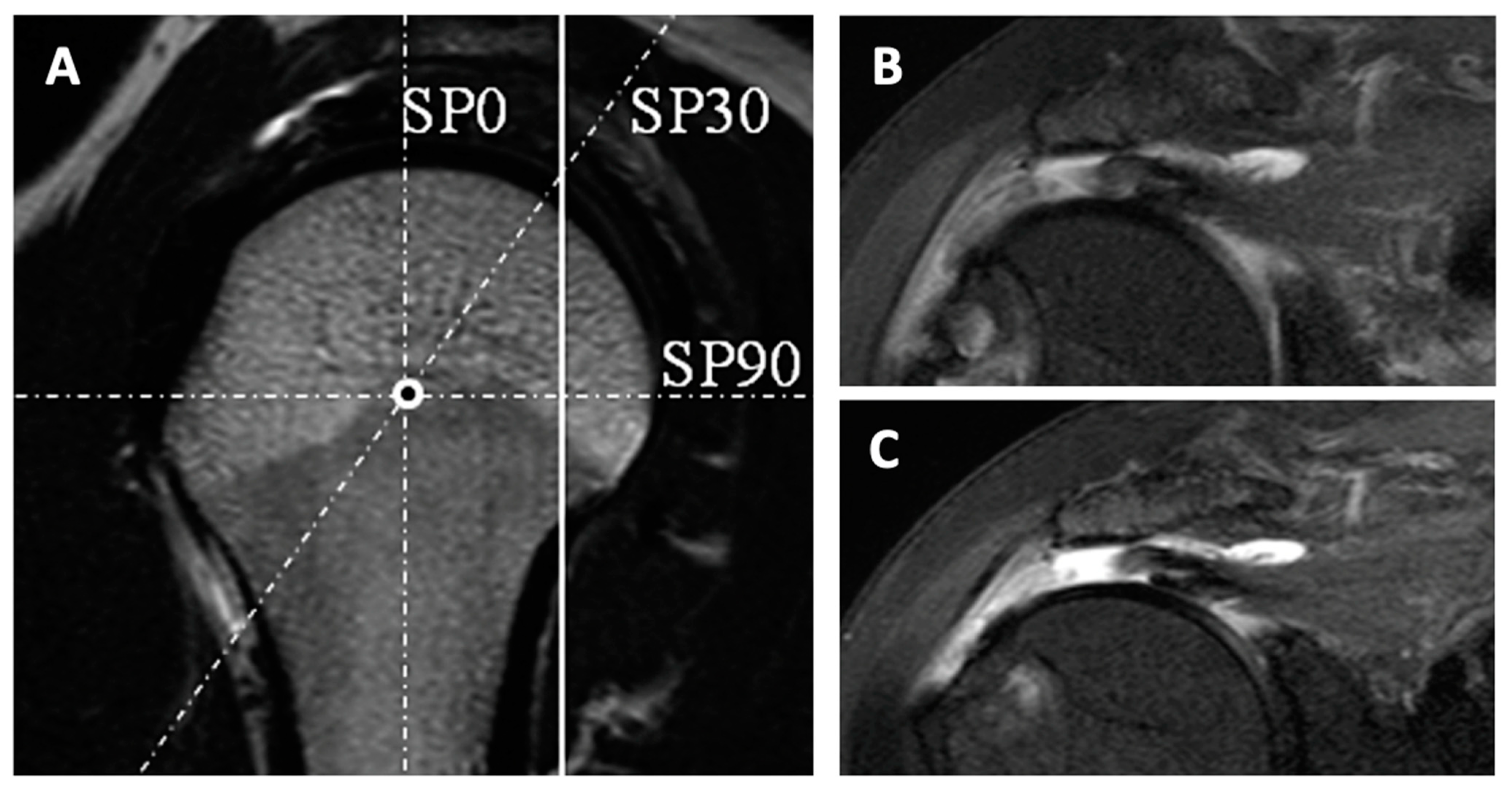
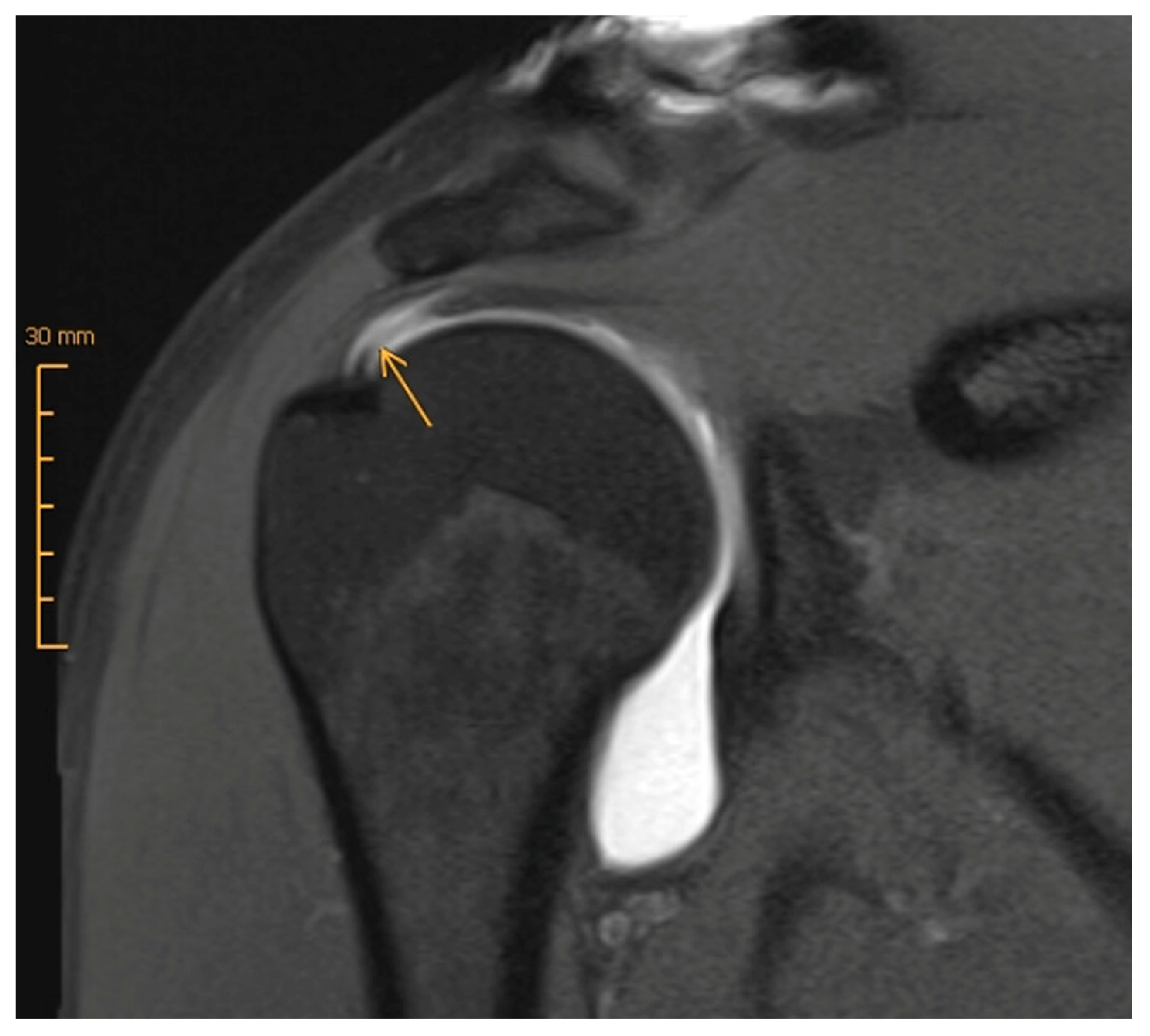
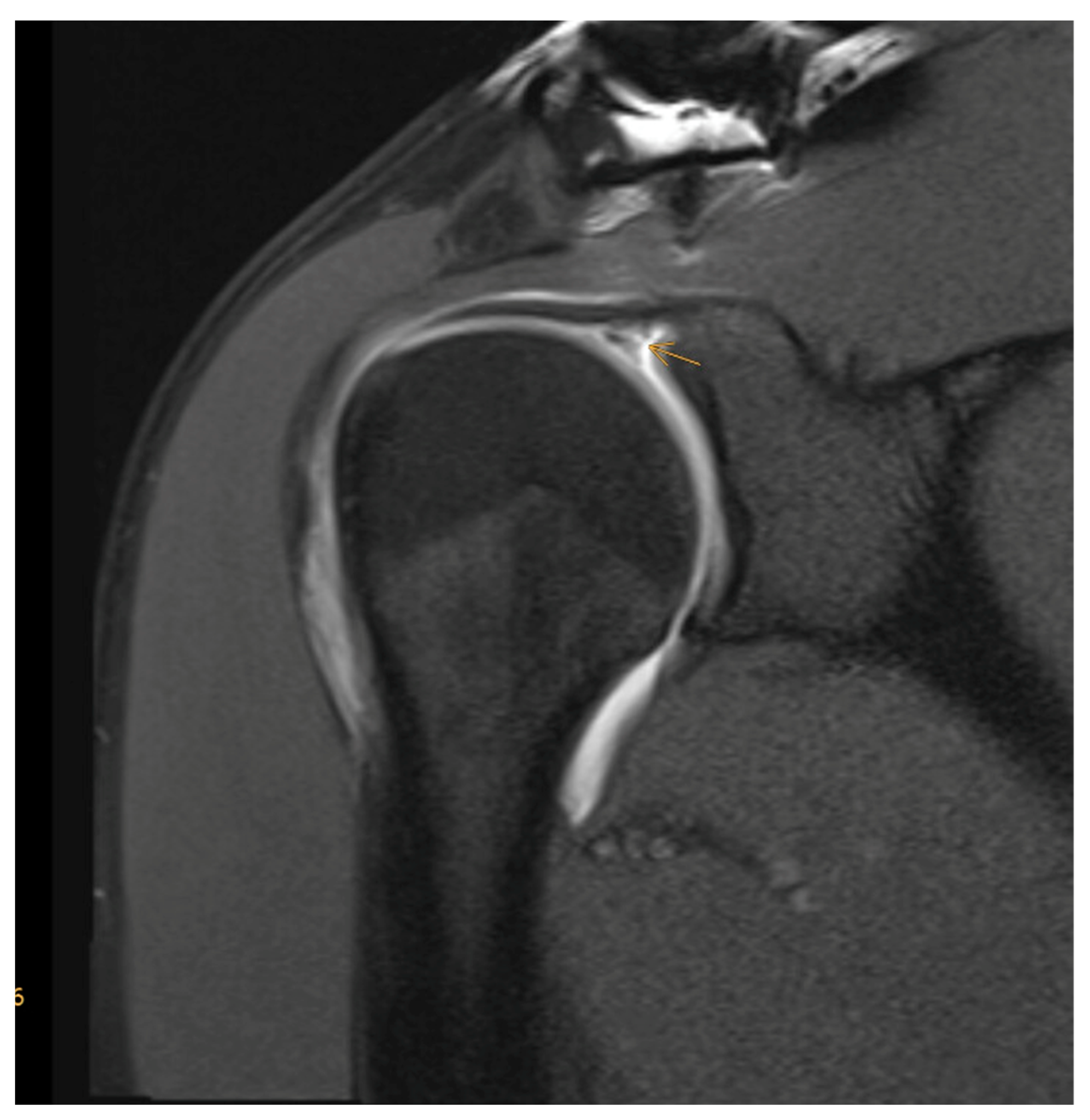
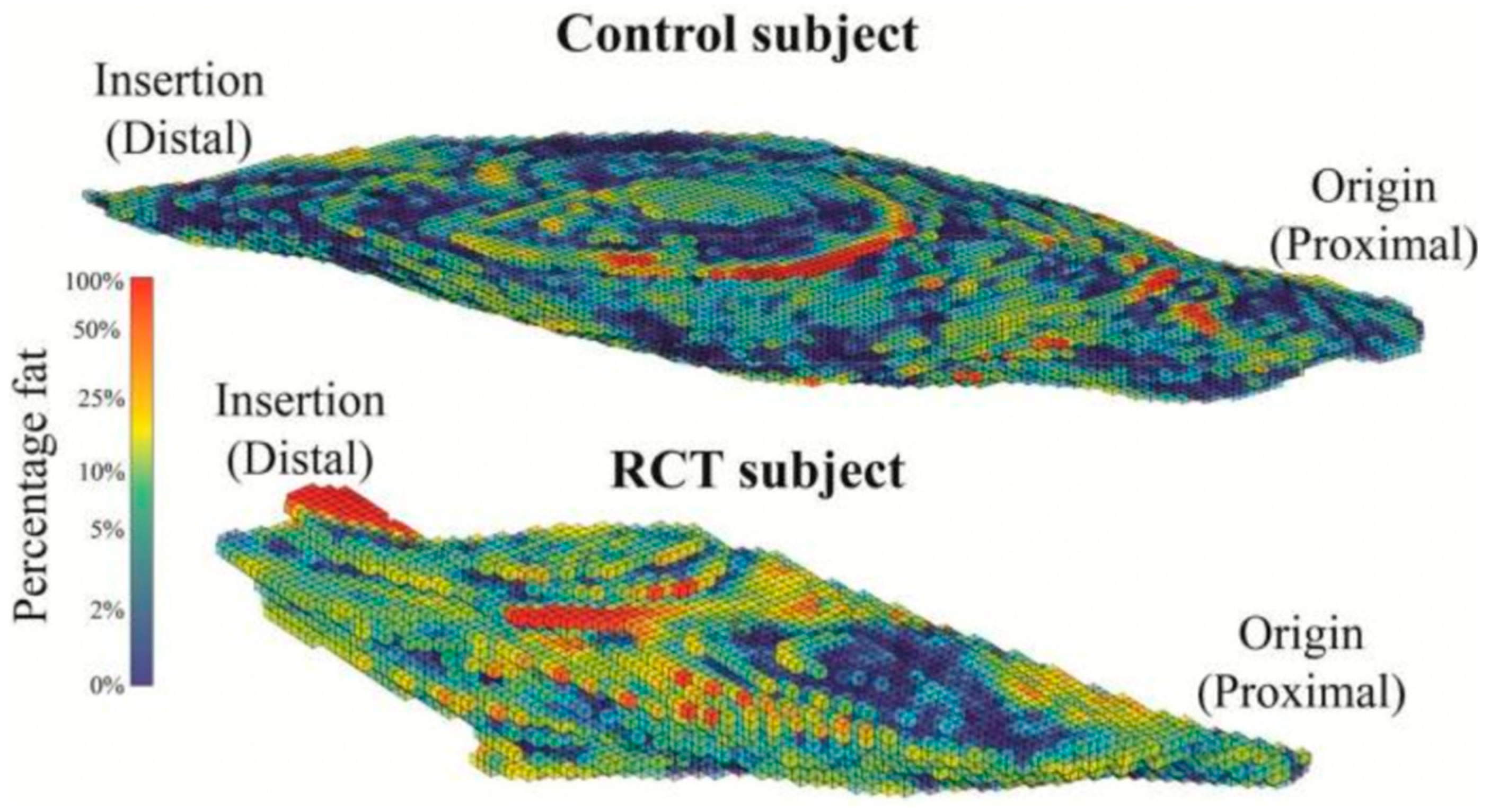
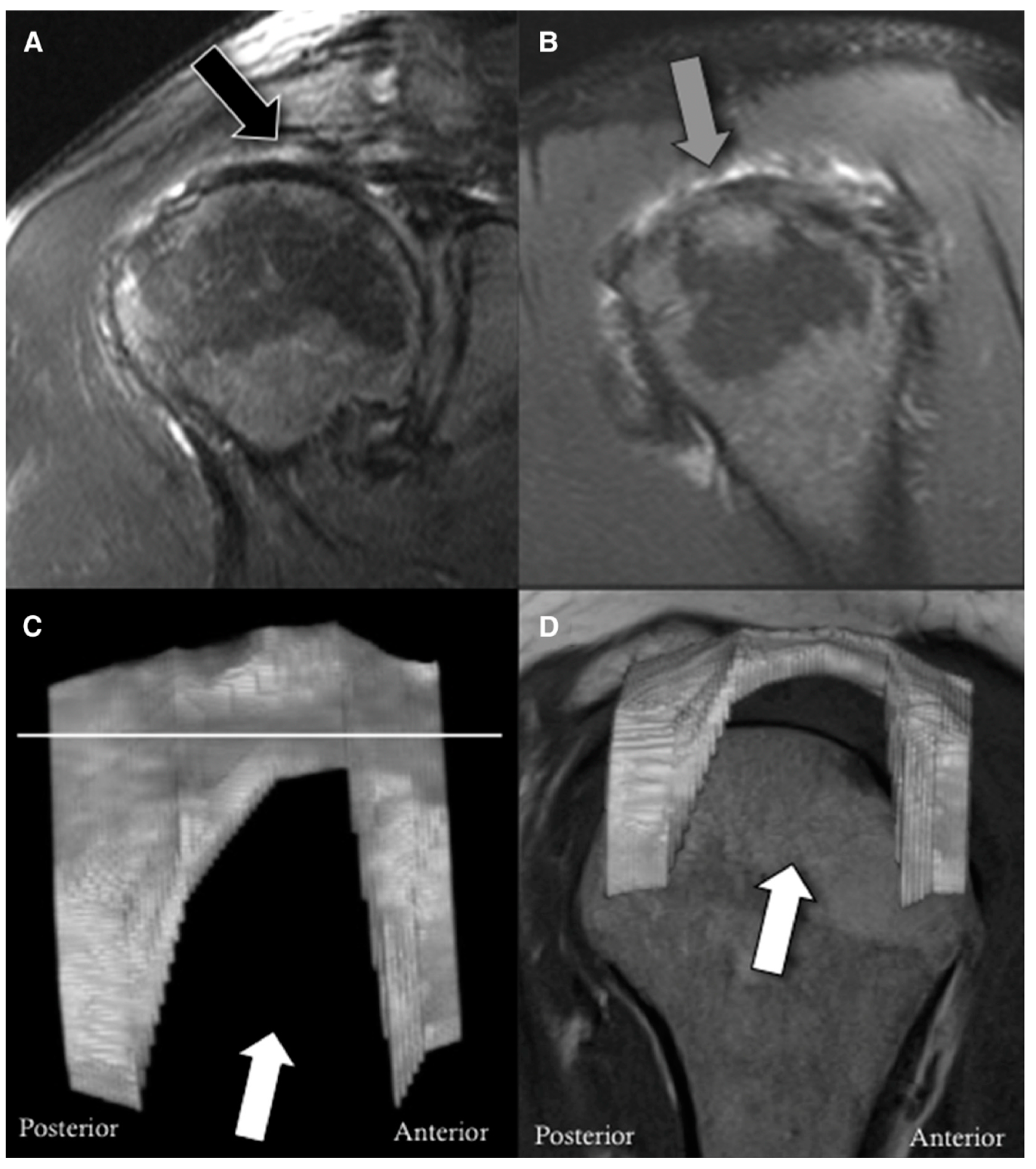
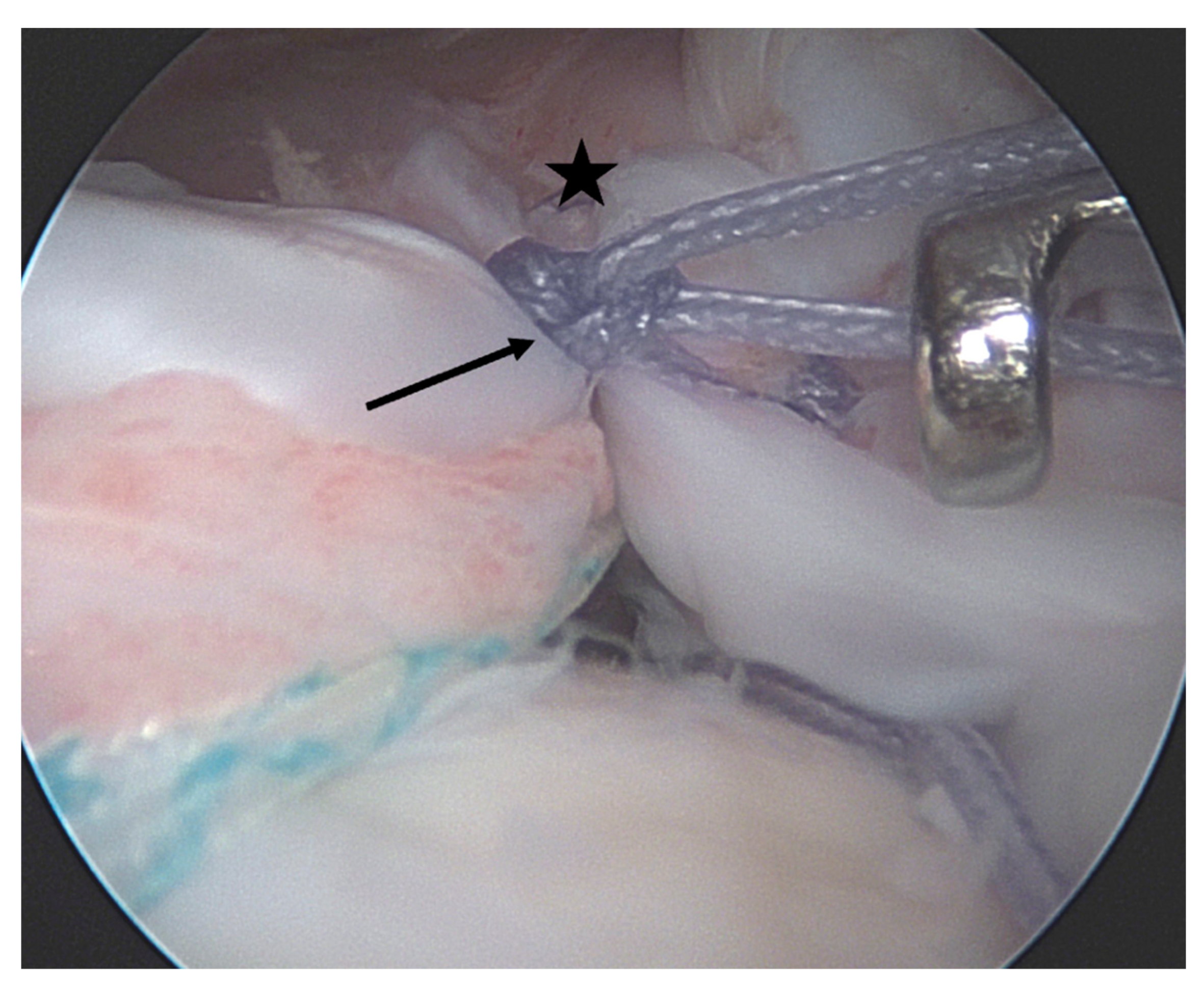
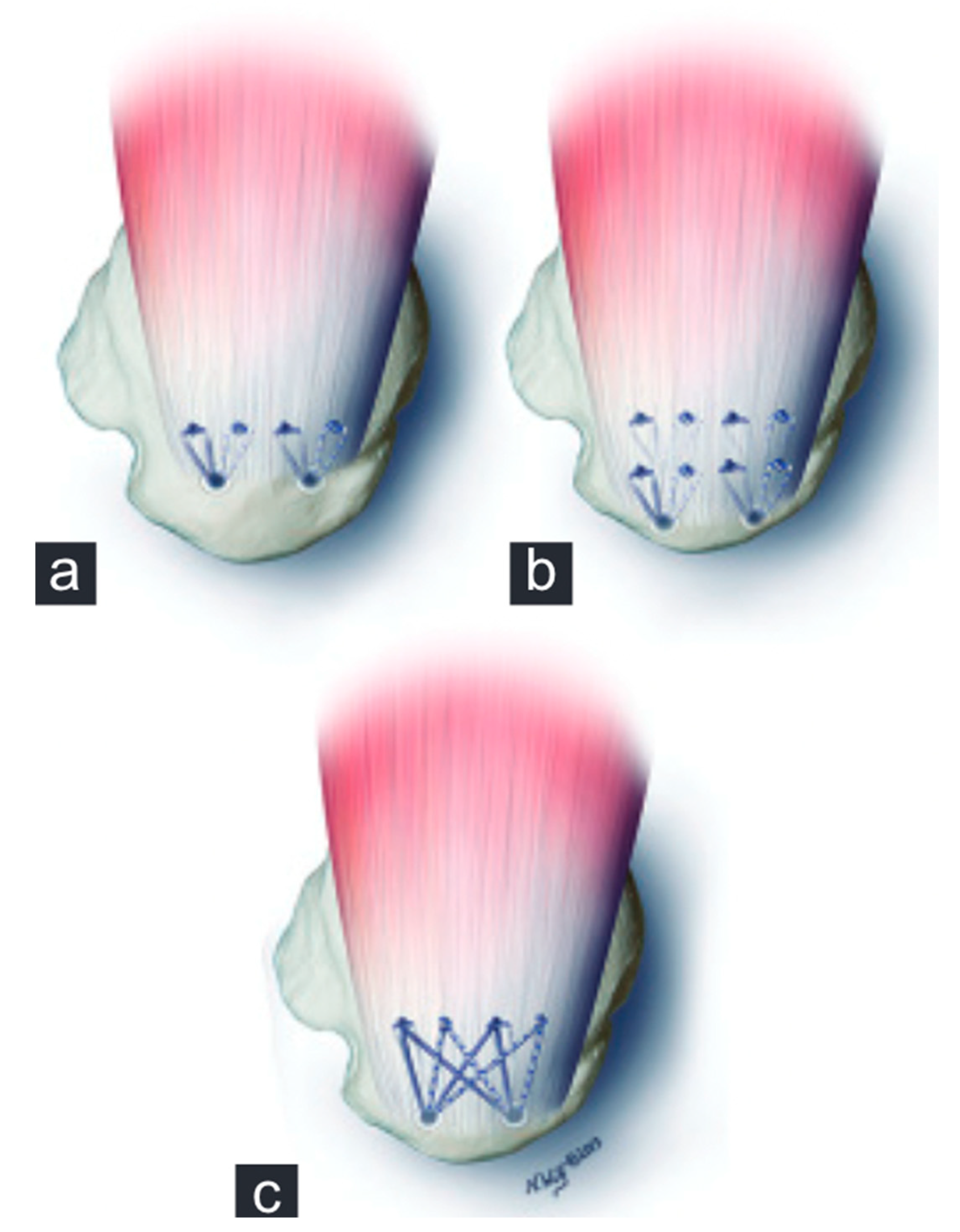
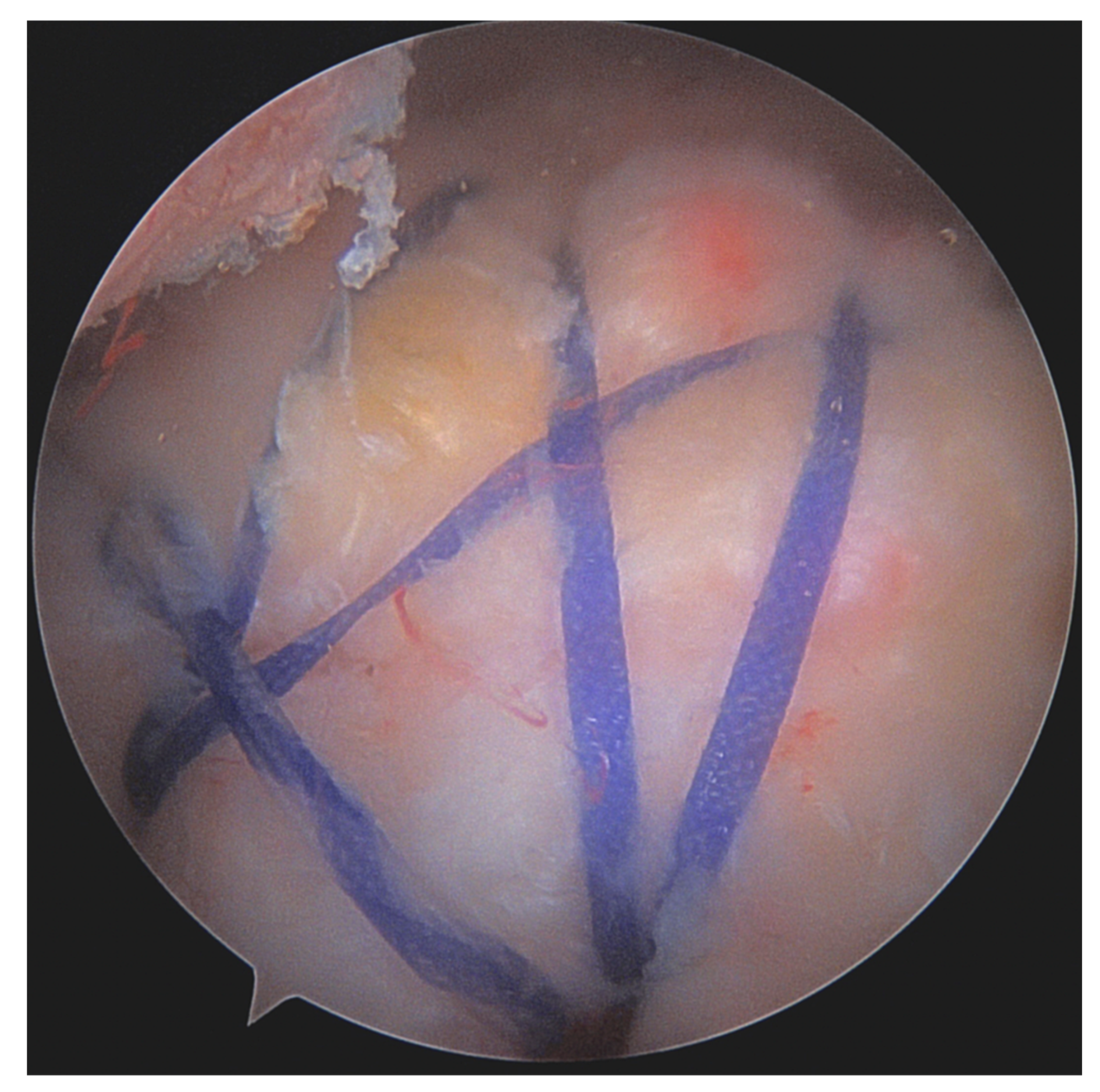
| Age <65 years |
| Small anteroposterior tear size |
| Little tendon retraction |
| No/minor fatty infiltration Long tendon stump (>15 mm) |
| Early intervention for traumatic tears |
| Imaging | Gold Standard: MRI |
| |
| Treatment Indication | Degenerative tears deteriorate slowly and can undergo a conservative treatment trial |
| |
| Traumatic rotator cuff tears should be addressed surgically soon after trauma. | |
| Fixation Technique | DR/suture bridge techniques are biomechanically superior to SR/transosseus techniques. |
| Modern anchor and suture materials facilitate stable fixation. | |
Consider mechanical and/or biological augmentation in
| |
| Postoperative Rehabilitation | Small/partial rotator cuff repairs: conventional broad arm sling |
| Medium-/large rotator cuff repairs (incl. infraspinatus): abduction brace |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eckers, F.; Loske, S.; Ek, E.T.; Müller, A.M. Current Understanding and New Advances in the Surgical Management of Reparable Rotator Cuff Tears: A Scoping Review. J. Clin. Med. 2023, 12, 1713. https://doi.org/10.3390/jcm12051713
Eckers F, Loske S, Ek ET, Müller AM. Current Understanding and New Advances in the Surgical Management of Reparable Rotator Cuff Tears: A Scoping Review. Journal of Clinical Medicine. 2023; 12(5):1713. https://doi.org/10.3390/jcm12051713
Chicago/Turabian StyleEckers, Franziska, Stefan Loske, Eugene T. Ek, and Andreas M. Müller. 2023. "Current Understanding and New Advances in the Surgical Management of Reparable Rotator Cuff Tears: A Scoping Review" Journal of Clinical Medicine 12, no. 5: 1713. https://doi.org/10.3390/jcm12051713
APA StyleEckers, F., Loske, S., Ek, E. T., & Müller, A. M. (2023). Current Understanding and New Advances in the Surgical Management of Reparable Rotator Cuff Tears: A Scoping Review. Journal of Clinical Medicine, 12(5), 1713. https://doi.org/10.3390/jcm12051713





