Efficacy of 1-Year Cavacurmin® Therapy in Reducing Prostate Growth in Men Suffering from Lower Urinary Tract Symptoms
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Emberton, M.; Andriole, G.L.; De La Rosette, J.; Djavan, B.; Hoefner, K.; Vela Navarrete, R.; Nordling, J.; Roehrborn, C.; Schulman, C.; Teillac, P.; et al. Benign Prostatic Hyperplasia: A Progressive Disease of Aging Men. Urology 2003, 61, 267–273. [Google Scholar] [CrossRef]
- Martin, S.A.; Haren, M.T.; Marshall, V.R.; Lange, K.; Wittert, G.A. Members of the Florey Adelaide Male Ageing Study Prevalence and Factors Associated with Uncomplicated Storage and Voiding Lower Urinary Tract Symptoms in Community-Dwelling Australian Men. World J. Urol. 2011, 29, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Girman, C.J.; Jacobsen, S.J.; Guess, H.A.; Oesterling, J.E.; Chute, C.G.; Panser, L.A.; Lieber, M.M. Natural History of Prostatism: Relationship among Symptoms, Prostate Volume and Peak Urinary Flow Rate. J. Urol. 1995, 153, 1510–1515. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, S.J.; Guess, H.A.; Panser, L.; Girman, C.J.; Chute, C.G.; Oesterling, J.E.; Lieber, M.M. A Population-Based Study of Health Care⁁Seeking Behavior for Treatment of Urinary Symptoms: The Olmsted County Study of Urinary Symptoms and Health Status Among Men. Arch. Fam. Med. 1993, 2, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Gravas, S.; Cornu, J.; Gacci, M.; Gratzke, C.; Herrmann, T.R.W.; Mamoulakis, C.; Rieken, M.; Speakman, M.J.; Tikkinen, K.A.O. EAU Guidelines on Management of Non-Neurogenic Male Lower Urinary Tract Symptoms (LUTS), Incl. Benign Prostatic Obstruction (BPO). Available online: https://d56bochluxqnz.cloudfront.net/documents/full-guideline/EAU-Guidelines-on-Non-Neurogenic-Male-LUTS-2022.pdf (accessed on 30 August 2022).
- Djavan, B.; Chapple, C.; Milani, S.; Marberger, M. State of the Art on the Efficacy and Tolerability of Alpha1-Adrenoceptor Antagonists in Patients with Lower Urinary Tract Symptoms Suggestive of Benign Prostatic Hyperplasia. Urology 2004, 64, 1081–1088. [Google Scholar] [CrossRef]
- Naslund, M.J.; Miner, M. A Review of the Clinical Efficacy and Safety of 5alpha-Reductase Inhibitors for the Enlarged Prostate. Clin. Ther. 2007, 29, 17–25. [Google Scholar] [CrossRef]
- Plochocki, A.; King, B. Medical Treatment of Benign Prostatic Hyperplasia. Urol. Clin. N. Am. 2022, 49, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Madersbacher, S.; Ponholzer, A.; Berger, I.; Marszalek, M. Medical Management of BPH: Role of Plant Extracts. EAU EBU Updat. Ser. 2007, 5, 197–205. [Google Scholar] [CrossRef]
- Csikós, E.; Horváth, A.; Ács, K.; Papp, N.; Balázs, V.L.; Dolenc, M.S.; Kenda, M.; Kočevar Glavač, N.; Nagy, M.; Protti, M.; et al. Treatment of Benign Prostatic Hyperplasia by Natural Drugs. Molecules 2021, 26, 7141. [Google Scholar] [CrossRef]
- Aaron, L.; Franco, O.E.; Hayward, S.W. Review of Prostate Anatomy and Embryology and the Etiology of Benign Prostatic Hyperplasia. Urol. Clin. N. Am. 2016, 43, 279–288. [Google Scholar] [CrossRef]
- Fu, Y.-S.; Chen, T.-H.; Weng, L.; Huang, L.; Lai, D.; Weng, C.-F. Pharmacological Properties and Underlying Mechanisms of Curcumin and Prospects in Medicinal Potential. Biomed. Pharmacother. 2021, 141, 111888. [Google Scholar] [CrossRef]
- Zielińska, A.; Alves, H.; Marques, V.; Durazzo, A.; Lucarini, M.; Alves, T.F.; Morsink, M.; Willemen, N.; Eder, P.; Chaud, M.V.; et al. Properties, Extraction Methods, and Delivery Systems for Curcumin as a Natural Source of Beneficial Health Effects. Medicina 2020, 56, 336. [Google Scholar] [CrossRef]
- Kim, S.K.; Seok, H.; Park, H.J.; Jeon, H.S.; Kang, S.W.; Lee, B.-C.; Yi, J.; Song, S.Y.; Lee, S.H.; Kim, Y.O.; et al. Inhibitory Effect of Curcumin on Testosterone Induced Benign Prostatic Hyperplasia Rat Model. BMC Complement. Altern. Med. 2015, 15, 380. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Gan, Y.; Chen, X.; Zhang, B.; Chen, Z.; Liu, P.; Li, B.; Ru, F.; He, Y. Curcumin Attenuates Prostatic Hyperplasia Caused by Inflammation via Up-Regulation of Bone Morphogenetic Protein and Activin Membrane-Bound Inhibitor. Pharm. Biol. 2021, 59, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Wacker Biotech GmbH CAVACURMIN® Curcumin Complex. Available online: https://www.wacker.com/h/en-us/cyclodextrins-complexes/complexes/cavacurmin-curcumin-complex/p/000000112 (accessed on 31 August 2022).
- Moyad, M.A. Nutraceuticals and Phytotherapy in Men’s Health: Antioxidants, Pro-Oxidants, and a Novel Opportunity for Lifestyle Changes. Urol. Clin. N. Am. 2022, 49, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Levin, R.M.; Das, A.K. A Scientific Basis for the Therapeutic Effects of Pygeum Africanum and Serenoa Repens. Urol. Res. 2000, 28, 201–209. [Google Scholar] [CrossRef]
- Madersbacher, S.; Berger, I.; Ponholzer, A.; Marszalek, M. Plant Extracts: Sense or Nonsense? Curr. Opin. Urol. 2008, 18, 16–20. [Google Scholar] [CrossRef]
- Shao, J.-C.; Wang, Y.; Zhang, S.-W.; Luo, D.-K.; Chang, D.-G.; Wu, X.-Q.; Tang, M.; He, Z.-M. [Angiogenesis and regulatory factors in rats with BPH induced by testosterone]. Zhonghua Nan Ke Xue 2005, 11, 413–418. [Google Scholar] [PubMed]
- Cross, N.A.; Reid, S.V.; Harvey, A.J.; Jokonya, N.; Eaton, C.L. Opposing Actions of TGFbeta1 and FGF2 on Growth, Differentiation and Extracellular Matrix Accumulation in Prostatic Stromal Cells. Growth Factors 2006, 24, 233–241. [Google Scholar] [CrossRef]
- Kleinberg, D.L.; Ruan, W.; Yee, D.; Kovacs, K.T.; Vidal, S. Insulin-like Growth Factor (IGF)-I Controls Prostate Fibromuscular Development: IGF-I Inhibition Prevents Both Fibromuscular and Glandular Development in Eugonadal Mice. Endocrinology 2007, 148, 1080–1088. [Google Scholar] [CrossRef] [PubMed]
- Ide, H.; Tokiwa, S.; Sakamaki, K.; Nishio, K.; Isotani, S.; Muto, S.; Hama, T.; Masuda, H.; Horie, S. Combined Inhibitory Effects of Soy Isoflavones and Curcumin on the Production of Prostate-Specific Antigen. Prostate 2010, 70, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- McLaren, I.D.; Jerde, T.J.; Bushman, W. Role of Interleukins, IGF and Stem Cells in BPH. Differentiation 2011, 82, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Kramer, G.; Steiner, G.E.; Handisurya, A.; Stix, U.; Haitel, A.; Knerer, B.; Gessl, A.; Lee, C.; Marberger, M. Increased Expression of Lymphocyte-Derived Cytokines in Benign Hyperplastic Prostate Tissue, Identification of the Producing Cell Types, and Effect of Differentially Expressed Cytokines on Stromal Cell Proliferation. Prostate 2002, 52, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Kahokehr, A.; Vather, R.; Nixon, A.; Hill, A.G. Non-Steroidal Anti-Inflammatory Drugs for Lower Urinary Tract Symptoms in Benign Prostatic Hyperplasia: Systematic Review and Meta-Analysis of Randomized Controlled Trials. BJU Int. 2013, 111, 304–311. [Google Scholar] [CrossRef]
- Qiao, J.; Gan, Y.; Gong, Y.; Song, Q.; Zhang, B.; Li, B.; Ru, F.; Li, Y.; He, Y. Combination Therapy with Curcumin plus Tamsulosin and Finasteride in the Treatment of Men with Benign Prostatic Hyperplasia: A Single Center, Randomized Control Study. Transl. Androl. Urol. 2021, 10, 3432–3439. [Google Scholar] [CrossRef] [PubMed]
- Roehrborn, C.G. BPH Progression: Concept and Key Learning from MTOPS, ALTESS, COMBAT, and ALF-ONE. BJU Int. 2008, 101, 17–21. [Google Scholar] [CrossRef]
| Group 1 (α1-Adrenoceptor Antagonists Only) | Group 2 (α1-Adrenoceptor Antagonists Plus Qurmin) | p Value | |
|---|---|---|---|
| Age, years, median (IQR) | 66.5 (4) | 68.0 (4) | 0.357 |
| PV, mL, median (IQR) | 52.0 (13) | 56.00 (18) | 0.379 |
| IPSS, median (IQR) | 15.5 (8) | 15.0 (5) | 0.958 |
| PVR, mL, median (IQR) | 62.5 (18) | 55.0 (15) | 0.08 |
| Qmax, mL/s, median (IQR) | 16.60 (3.3) | 15.75 (2.9) | 0.465 |
| PSA, ng/mL, median (IQR) | 2.60 (2.6) | 3.25 (1.8) | 0.828 |
| Type of α1-adrenoceptor antagonist n (%) | 0.81 | ||
| Tamsulosin | 9 (45.0) | 10 (50.0) | |
| Alfuzosin | 5 (25.0) | 4 (20.0) | |
| Sylodosin | 6 (30.0) | 6 (30.0) |
| Group 1 (α1-Adrenoceptor Antagonists Only) | Group 2 (α1-Adrenoceptor Antagonists Plus Qurmin) | p Value | |
|---|---|---|---|
| PV, mL, median (IQR) | 62.5 (18.0) | 55.0 (15.0) | 0.04 |
| IPSS, median (IQR) | 18 (9.25) | 13.5 (3.75) | 0.009 |
| PVR, mL, median (IQR) | 60.5 (17) | 56.5 (15) | 0.33 |
| Qmax, mL/s, median (IQR) | 14.5 (4.2) | 15.85 (2.9) | 0.022 |
| PSA, ng/mL, median (IQR) | 3.05 (2.7) | 2.5 (1.5) | 0.009 |
| Group 1 (α1-Adrenoceptor Antagonists Only) | Group 2 (α1-Adrenoceptor Antagonists Plus Qurmin) | p Value | |
|---|---|---|---|
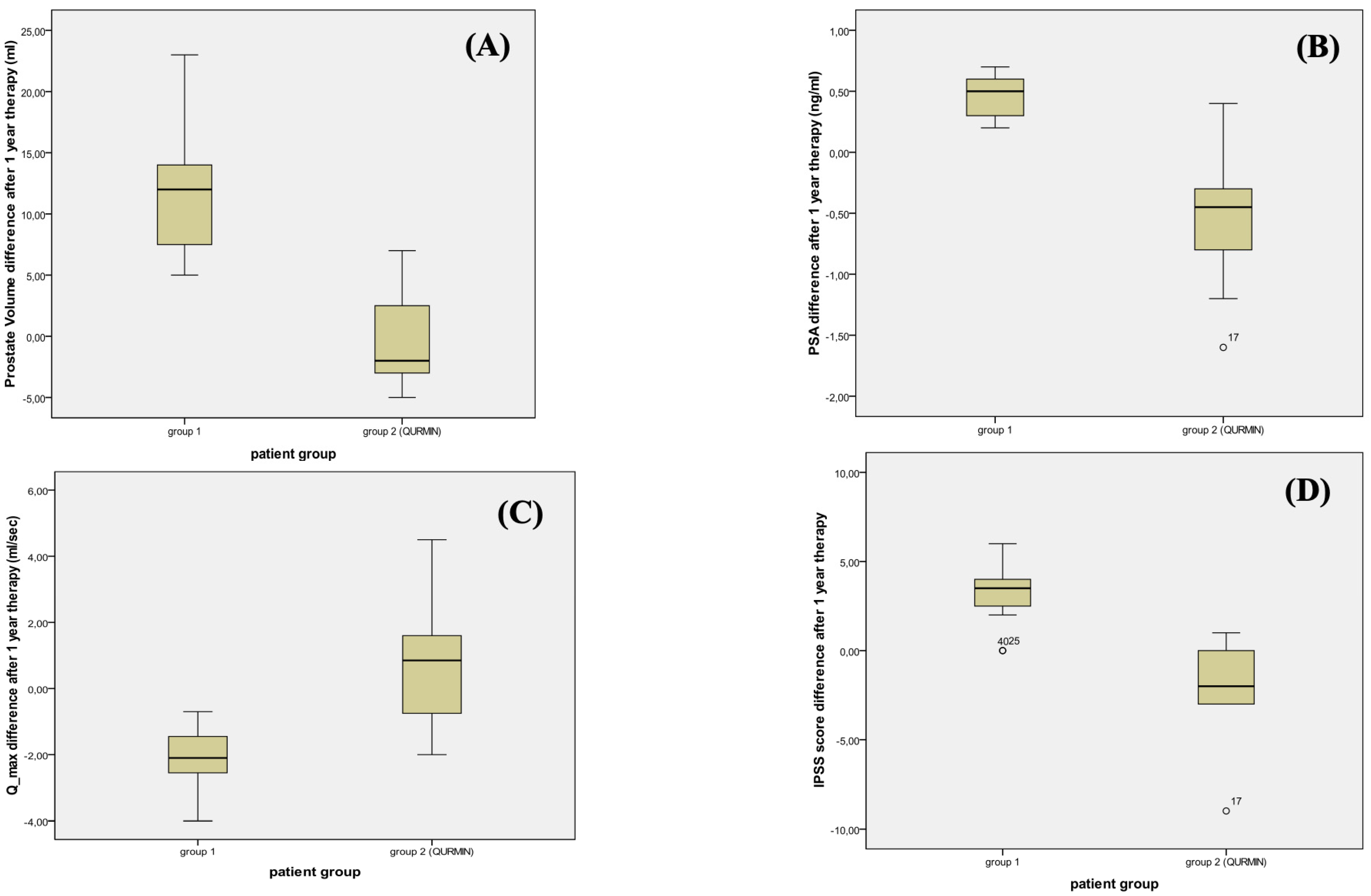
| PV, mL, median (IQR) | 12 (6.75) | −2 (5.75) | <0.001 |
| IPSS, median (IQR) | 3.5 (1.75) | −2 (3) | <0.001 |
| PVR, mL, median (IQR) | 2.3 (6.9) | 2.1 (7.8) | 0.33 |
| Qmax, mL/s, median (IQR) | −2.1 (1.15) | 0.85 (2.52) | 0.001 |
| PSA, ng/mL, median (IQR) | 0.50 (0.30) | −0.45 (0.55) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milanese, G.; Agostini, E.; De Angelis, M.V.; Pretore, E.; Galosi, A.B.; Castellani, D. Efficacy of 1-Year Cavacurmin® Therapy in Reducing Prostate Growth in Men Suffering from Lower Urinary Tract Symptoms. J. Clin. Med. 2023, 12, 1689. https://doi.org/10.3390/jcm12041689
Milanese G, Agostini E, De Angelis MV, Pretore E, Galosi AB, Castellani D. Efficacy of 1-Year Cavacurmin® Therapy in Reducing Prostate Growth in Men Suffering from Lower Urinary Tract Symptoms. Journal of Clinical Medicine. 2023; 12(4):1689. https://doi.org/10.3390/jcm12041689
Chicago/Turabian StyleMilanese, Giulio, Edoardo Agostini, Maria Vittoria De Angelis, Eugenio Pretore, Andrea Benedetto Galosi, and Daniele Castellani. 2023. "Efficacy of 1-Year Cavacurmin® Therapy in Reducing Prostate Growth in Men Suffering from Lower Urinary Tract Symptoms" Journal of Clinical Medicine 12, no. 4: 1689. https://doi.org/10.3390/jcm12041689
APA StyleMilanese, G., Agostini, E., De Angelis, M. V., Pretore, E., Galosi, A. B., & Castellani, D. (2023). Efficacy of 1-Year Cavacurmin® Therapy in Reducing Prostate Growth in Men Suffering from Lower Urinary Tract Symptoms. Journal of Clinical Medicine, 12(4), 1689. https://doi.org/10.3390/jcm12041689





