Weight-Based Bisphosphonate Administration for Multiple Myeloma Patients and the Risks of Skeletal Complications
Abstract
1. Introduction
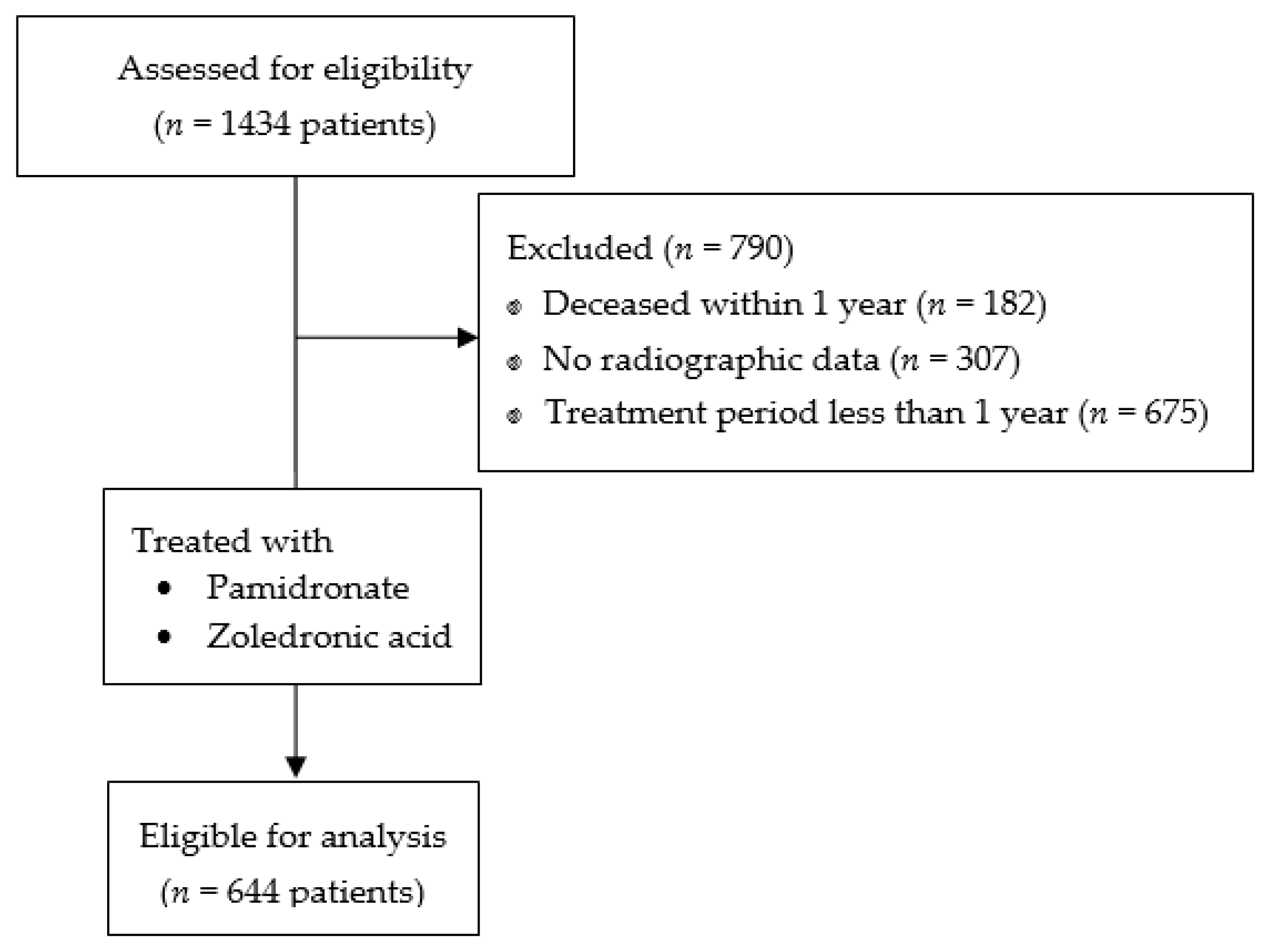
2. Materials and Methods
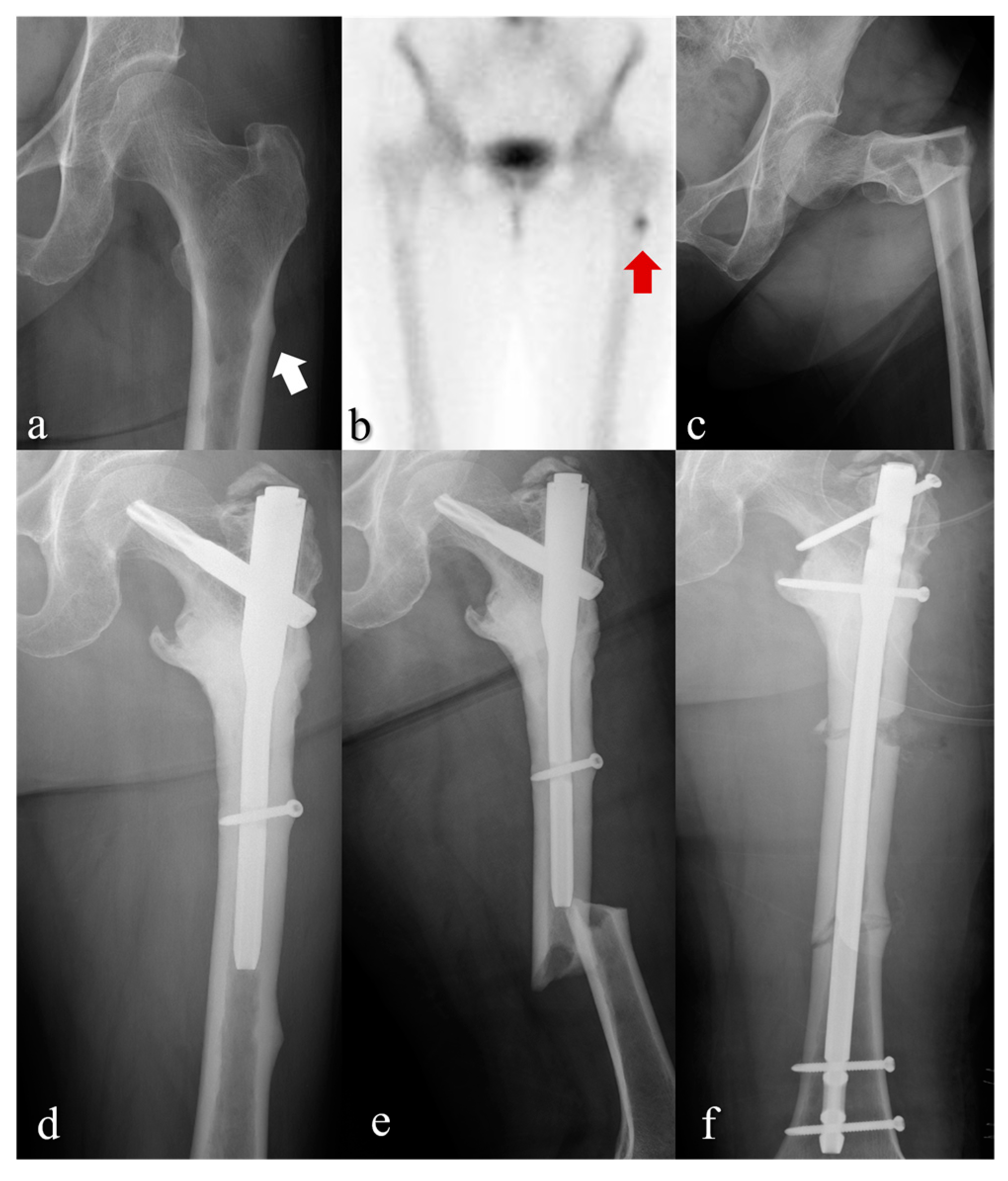
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kyle, R.A.; Gertz, M.A.; Witzig, T.E.; Lust, J.A.; Lacy, M.Q.; Dispenzieri, A.; Fonseca, R.; Rajkumar, S.V.; Offord, J.R.; Larson, D.R. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin. Proc. 2003, 78, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Dispenzieri, A.; Kyle, R.; Merlini, G.; Miguel, J.; Ludwig, H.; Hájek, R.; Palumbo, A.; Jagannath, S.; Bladé, J.; Lonial, S. International Myeloma Working Group guidelines for serum-free light chain analysis in multiple myeloma and related disorders. Leukemia 2009, 23, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Marino, S.; Roodman, G.D. Multiple myeloma and bone: The fatal interaction. Cold Spring Harb. Perspect. Med. 2018, 8, a031286. [Google Scholar] [CrossRef]
- Giuliani, N.; Rizzoli, V.; Roodman, G.D. Multiple myeloma bone disease: Pathophysiology of osteoblast inhibition. Blood 2006, 108, 3992–3996. [Google Scholar] [CrossRef] [PubMed]
- Cowan, A.J.; Green, D.J.; Kwok, M.; Lee, S.; Coffey, D.G.; Holmberg, L.A.; Tuazon, S.; Gopal, A.K.; Libby, E.N. Diagnosis and Management of Multiple Myeloma: A Review. JAMA 2022, 327, 464–477. [Google Scholar] [CrossRef] [PubMed]
- Guenther, A.; Gordon, S.; Tiemann, M.; Burger, R.; Bakker, F.; Green, J.R.; Baum, W.; Roelofs, A.J.; Rogers, M.J.; Gramatzki, M. The bisphosphonate zoledronic acid has antimyeloma activity in vivo by inhibition of protein prenylation. Int. J. Cancer 2010, 126, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Kimachi, K.; Kajiya, H.; Nakayama, S.; Ikebe, T.; Okabe, K. Zoledronic acid inhibits rank expression and migration of osteoclast precursors during osteoclastogenesis. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2011, 383, 297–308. [Google Scholar] [CrossRef]
- Terpos, E.; Zamagni, E.; Lentzsch, S.; Drake, M.T.; García-Sanz, R.; Abildgaard, N.; Ntanasis-Stathopoulos, I.; Schjesvold, F.; de la Rubia, J.; Kyriakou, C. Treatment of multiple myeloma-related bone disease: Recommendations from the Bone Working Group of the International Myeloma Working Group. Lancet Oncol. 2021, 22, e119–e130. [Google Scholar] [CrossRef]
- Anderson, K.; Ismaila, N.; Flynn, P.J.; Halabi, S.; Jagannath, S.; Ogaily, M.S.; Omel, J.; Raje, N.; Roodman, G.D.; Yee, G.C. Role of bone-modifying agents in multiple myeloma: American Society of Clinical Oncology clinical practice guideline update. J. Clin. Oncol. 2018, 36, 812–818. [Google Scholar] [CrossRef]
- Terpos, E.; Kleber, M.; Engelhardt, M.; Zweegman, S.; Gay, F.; Kastritis, E.; van de Donk, N.W.; Bruno, B.; Sezer, O.; Broijl, A. European Myeloma Network guidelines for the management of multiple myeloma-related complications. Haematologica 2015, 100, 1254. [Google Scholar] [CrossRef]
- Kyle, R.A.; Yee, G.C.; Somerfield, M.R.; Flynn, P.J.; Halabi, S.; Jagannath, S.; Orlowski, R.Z.; Roodman, D.G.; Twilde, P.; Anderson, K. American Society of Clinical Oncology 2007 clinical practice guideline update on the role of bisphosphonates in multiple myeloma. J. Clin. Oncol. 2007, 25, 2464–2472. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Moreau, P.; Terpos, E.; Mateos, M.-V.; Zweegman, S.; Cook, G.; Delforge, M.; Hájek, R.; Schjesvold, F.; Cavo, M. Multiple myeloma: EHA-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.; Callander, N.S.; Hillengass, J.; Liedtke, M.; Baljevic, M.; Campagnaro, E.; Castillo, J.J.; Chandler, J.C.; Cornell, R.F.; Costello, C. NCCN guidelines insights: Multiple myeloma, version 1.2020: Featured updates to the NCCN guidelines. J. Natl. Compr. Cancer Netw. 2019, 17, 1154–1165. [Google Scholar] [CrossRef] [PubMed]
- Camacho, P.M.; Petak, S.M.; Binkley, N.; Diab, D.L.; Eldeiry, L.S.; Farooki, A.; Harris, S.T.; Hurley, D.L.; Kelly, J.; Lewiecki, E.M. American Association of Clinical Endocrinologists/American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis—2020 update. Endocr. Pract. 2020, 26, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.G.; Chretien, K.C.; Meoni, L.A.; Liu, Y.-P.; Klag, M.J.; Levine, M.A. Comparison of intravenous pamidronate to standard therapy for osteoporosis: Use in patients unable to take oral bisphosphonates. J. Clin. Rheumatol. 2005, 11, 2–7. [Google Scholar] [CrossRef]
- Reyes, C.; Hitz, M.; Prieto-Alhambra, D.; Abrahamsen, B. Risks and benefits of bisphosphonate therapies. J. Cell. Biochem. 2016, 117, 20–28. [Google Scholar] [CrossRef]
- Schilcher, J.; Michaëlsson, K.; Aspenberg, P. Bisphosphonate use and atypical fractures of the femoral shaft. N. Engl. J. Med. 2011, 364, 1728–1737. [Google Scholar] [CrossRef]
- Black, D.M.; Geiger, E.J.; Eastell, R.; Vittinghoff, E.; Li, B.H.; Ryan, D.S.; Dell, R.M.; Adams, A.L. Atypical femur fracture risk versus fragility fracture prevention with bisphosphonates. N. Engl. J. Med. 2020, 383, 743–753. [Google Scholar] [CrossRef]
- Ruggiero. American Association of Oral and Maxillofacial Surgeons Position Paper on Medication-Related Osteonecrosis of the Jaw-2014 Update (vol 72, pg 1938, 2014). J. Oral Maxillofac. Surg. 2015, 73, 1879. [Google Scholar]
- Lee, Y.-K.; Ahn, S.; Kim, K.M.; Suh, C.S.; Koo, K.-H. Incidence rate of atypical femoral fracture after bisphosphonates treatment in Korea. J. Korean Med. Sci. 2018, 33, e38. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, Y.-K.; Kim, T.-Y.; Ha, Y.-C.; Jang, S.; Kim, H.Y. Incidence of and risk for osteonecrosis of the jaw in Korean osteoporosis patients treated with bisphosphonates: A nationwide cohort-study. Bone 2021, 143, 115650. [Google Scholar] [CrossRef] [PubMed]
- Shane, E.; Burr, D.; Abrahamsen, B.; Adler, R.A.; Brown, T.D.; Cheung, A.M.; Cosman, F.; Curtis, J.R.; Dell, R.; Dempster, D.W. Atypical subtrochanteric and diaphyseal femoral fractures: Second report of a task force of the American Society for Bone and Mineral Research. J. Bone Miner. Res. 2014, 29, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, S.L.; Dodson, T.B.; Assael, L.A.; Landesberg, R.; Marx, R.E.; Mehrotra, B. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws—2009 update. J. Oral Maxillofac. Surg. 2009, 67, 2–12. [Google Scholar]
- Zacharis, C.K.; Tzanavaras, P.D. Determination of bisphosphonate active pharmaceutical ingredients in pharmaceuticals and biological material: A review of analytical methods. J. Pharm. Biomed. Anal. 2008, 48, 483–496. [Google Scholar] [CrossRef]
- Green, J.R.; Müller, K.; Jaeggi, K.A. Preclinical pharmacology of CGP 42′ 446, a new, potent, heterocyclic bisphosphonate compound. J. Bone Miner. Res. 1994, 9, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Berenson, J.R.; Hillner, B.E.; Kyle, R.A.; Anderson, K.; Lipton, A.; Yee, G.C.; Biermann, J.S. American Society of Clinical Oncology clinical practice guidelines: The role of bisphosphonates in multiple myeloma. J. Clin. Oncol. 2002, 20, 3719–3736. [Google Scholar] [CrossRef]
- Zeng, C.; Wen, W.; Morgans, A.K.; Pao, W.; Shu, X.-O.; Zheng, W. Disparities by race, age, and sex in the improvement of survival for major cancers: Results from the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program in the United States, 1990 to 2010. JAMA Oncol. 2015, 1, 88–96. [Google Scholar] [CrossRef]
- Sneyd, M.J.; Gray, A.R.; Morison, I.M. Trends in survival from myeloma, 1990–2015: A competing risks analysis. BMC Cancer 2021, 21, 821. [Google Scholar] [CrossRef]
- Dickinson, M.; Prince, H.; Kirsa, S.; Zannettino, A.; Gibbs, S.; Mileshkin, L.; O’Grady, J.; Seymour, J.; Szer, J.; Horvath, N. Osteonecrosis of the jaw complicating bisphosphonate treatment for bone disease in multiple myeloma: An overview with recommendations for prevention and treatment. Intern. Med. J. 2009, 39, 304–316. [Google Scholar] [CrossRef]
- Bamias, A.; Kastritis, E.; Bamia, C.; Moulopoulos, L.A.; Melakopoulos, I.; Bozas, G.; Koutsoukou, V.; Gika, D.; Anagnostopoulos, A.; Papadimitriou, C. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: Incidence and risk factors. J. Clin. Oncol. 2005, 23, 8580–8587. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Kastritis, E.; Anagnostopoulos, A.; Melakopoulos, I.; Gika, D.; Moulopoulos, L.A.; Bamia, C.; Terpos, E.; Tsionos, K.; Bamias, A. Osteonecrosis of the jaw in patients with multiple myeloma treated with bisphosphonates: Evidence of increased risk after treatment with zoledronic acid. Haematologica 2006, 91, 968–971. [Google Scholar] [PubMed]
- Chang, S.T.; Tenforde, A.S.; Grimsrud, C.D.; O’Ryan, F.S.; Gonzalez, J.R.; Baer, D.M.; Chandra, M.; Lo, J.C. Atypical femur fractures among breast cancer and multiple myeloma patients receiving intravenous bisphosphonate therapy. Bone 2012, 51, 524–527. [Google Scholar] [CrossRef]
- Wernecke, G.; Namduri, S.; DiCarlo, E.F.; Schneider, R.; Lane, J. Case report of spontaneous, nonspinal fractures in a multiple myeloma patient on long-term pamidronate and zoledronic acid. HSS J. 2008, 4, 123–127. [Google Scholar] [CrossRef]
- Ota, S.; Inoue, R.; Shiozaki, T.; Yamamoto, Y.; Hashimoto, N.; Takeda, O.; Yoshikawa, K.; Ito, J.; Ishibashi, Y. Atypical femoral fracture after receiving antiresorptive drugs in breast cancer patients with bone metastasis. Breast Cancer 2017, 24, 601–607. [Google Scholar] [CrossRef]
- Raje, N.; Terpos, E.; Willenbacher, W.; Shimizu, K.; García-Sanz, R.; Durie, B.; Legieć, W.; Krejčí, M.; Laribi, K.; Zhu, L. Denosumab versus zoledronic acid in bone disease treatment of newly diagnosed multiple myeloma: An international, double-blind, double-dummy, randomised, controlled, phase 3 study. Lancet Oncol. 2018, 19, 370–381. [Google Scholar] [CrossRef]
- Hageman, K.; Patel, K.C.; Mace, K.; Cooper, M.R. The role of denosumab for prevention of skeletal-related complications in multiple myeloma. Ann. Pharmacother. 2013, 47, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Morgan, G.; Dimopoulos, M.A.; Drake, M.T.; Lentzsch, S.; Raje, N.; Sezer, O.; García-Sanz, R.; Shimizu, K.; Turesson, I. International Myeloma Working Group recommendations for the treatment of multiple myeloma–related bone disease. J. Clin. Oncol. 2013, 31, 2347. [Google Scholar] [CrossRef] [PubMed]
- Morgan, G.J.; Davies, F.E.; Gregory, W.M.; Cocks, K.; Bell, S.E.; Szubert, A.J.; Navarro-Coy, N.; Drayson, M.T.; Owen, R.G.; Feyler, S. First-line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): A randomised controlled trial. Lancet 2010, 376, 1989–1999. [Google Scholar] [CrossRef] [PubMed]
- Body, J.-J. Clinical research update: Zoledronate. Cancer Interdiscip. Int. J. Am. Cancer Soc. 1997, 80, 1699–1701. [Google Scholar] [CrossRef]
- Lipton, A.; Small, E.; Saad, F.; Gleason, D.; Gordon, D.; Smith, M.; Rosen, L.; Ortu Kowalski, M.; Reitsma, D.; Seaman, J. The new bisphosphonate, Zometa®(zoledronic acid), decreases skeletal complications in both osteolytic and osteoblastic lesions: A comparison to pamidronate. Cancer Investig. 2002, 20, 45–54. [Google Scholar] [CrossRef]
- Rosen, L.S.; Gordon, D.; Kaminski, M.; Howell, A.; Belch, A.; Mackey, J.; Apffelstaedt, J.; Hussein, M.; Coleman, R.E.; Reitsma, D.J. Zoledronic acid versus pamidronate in the treatment of skeletal metastases in patients with breast cancer or osteolytic lesions of multiple myeloma: A phase III, double-blind, comparative trial. Cancer J. 2001, 7, 377–387. [Google Scholar] [PubMed]
- Piper, P.K., Jr.; Gruntmanis, U. Management of osteoporosis in the aging male: Focus on zoledronic acid. Clin. Interv. Aging 2009, 4, 289. [Google Scholar] [CrossRef]
- Dempster, D.W.; Bolognese, M.A. Ibandronate: The evolution of a once-a-month oral therapy for postmenopausal osteoporosis. J. Clin. Densitom. 2006, 9, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Shaw, N.; Bishop, N. Bisphosphonate treatment of bone disease. Arch. Dis. Child. 2005, 90, 494–499. [Google Scholar] [CrossRef]
- Morgan, G.J.; Child, J.A.; Gregory, W.M.; Szubert, A.J.; Cocks, K.; Bell, S.E.; Navarro-Coy, N.; Drayson, M.T.; Owen, R.G.; Feyler, S. Effects of zoledronic acid versus clodronic acid on skeletal morbidity in patients with newly diagnosed multiple myeloma (MRC Myeloma IX): Secondary outcomes from a randomised controlled trial. Lancet Oncol. 2011, 12, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, S.L.; Dodson, T.B.; Aghaloo, T.; Carlson, E.R.; Ward, B.B.; Kademani, D. American Association of Oral and Maxillofacial Surgeons’ Position Paper on medication-related osteonecrosis of the jaw–2022 update. J. Oral Maxillofac. Surg. 2022, 80, 920–943. [Google Scholar] [CrossRef] [PubMed]
| Items | |
|---|---|
| Patients for assessment | 644 |
| Sex | |
| Male | 325 (50.5%) |
| Female | 319 (49.5%) |
| Mean follow-up ± SD (range), months | 69.8 ± 33.1 (12.1–128.3) |
| Mean age ± SD (range), years | 67.2 ± 9.5 (18–89) |
| Mean body weight ± SD (range), kg | 60.8 ± 13.1 (31.2–108) |
| Mean BMI ± SD (range), kg/m2 | 23.7 ± 3.6 (13.5–62.2) |
| Bisphosphonate treatment options | |
| Pamidronate only | 381 (59.2%) |
| Zoledronic acid only | 18 (2.8%) |
| Pamidronate + zoledronic acid | 245 (38.0%) |
| Atypical Femoral Fracture (AFF) | Medication-Related Osteonecrosis of the Jaw (MRONJ) | |||||
|---|---|---|---|---|---|---|
| Item | OR | 95% C.I. | p | OR | 95% C.I. | p |
| Age | 1.024 | 0.937–1.120 | 0.594 | 1.012 | 0.981–1.045 | 0.444 |
| Sex (women: men) | 2.063 | 0.375–11.349 | 0.405 | 0.511 | 0.245–1.064 | 0.073 |
| Body weight (kg) | 0.990 | 0.919–1.066 | 0.792 | 0.982 | 0.922–1.046 | 0.580 |
| BMI (kg/m2) | 0.983 | 0.778–1.241 | 0.885 | 1.014 | 0.865–1.189 | 0.863 |
| Accumulative treatment dose (mg) | ||||||
| Pamidronate | 1.000 | 1.000–1.001 | 0.755 | 1.000 | 0.999–1.001 | 0.786 |
| Zoledronate | 1.017 | 1.004–1.029 | 0.009 † | 1.008 | 0.972–1.044 | 0.675 |
| Accumulative dose(mg)/BMI (kg/m2) | ||||||
| Pamidronate | 1.013 | 0.947–1.083 | 0.716 | 1.002 | 0.998–1.006 | 0.351 |
| Zoledronate | 1.441 | 1.085–1.914 | 0.012 † | 1.262 | 1.110–1.435 | <0.001 † |
| Accumulative dose(mg)/Bwt (kg) | ||||||
| Pamidronate | 1.005 | 0.976–1.034 | 0.762 | 1.004 | 0.994–1.014 | 0.401 |
| Zoledronate | 2.641 | 1.277–5.462 | 0.009 † | 1.816 | 1.313–2.513 | <0.001 † |
| Potency-weighted accumulative dose (mg)/Bwt (kg) | ||||||
| Pamidronate | 1.010 | 0.976–1.034 | 0.762 | 1.004 | 0.994–1.014 | 0.401 |
| Zoledronate | 1.010 | 1.002–1.017 | 0.009 † | 1.006 | 1.003–1.009 | <0.001 † |
| Pamidronate + zoledronate total dose | 1.010 | 1.003–1.017 | 0.005 † | 1.007 | 1.003–1.010 | <0.001 † |
| ⸫ for AFF: pamidronate ∗ 90 mg + zoledronate ∗ 4 mg ∗ 100 ≥ 77 ∗ Bwt (kg) |
| ⸫ for MRONJ: pamidronate ∗ 90 mg + zoledronate ∗ 4 mg ∗ 100 ≥ 57.70 ∗ Bwt (kg) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bahk, J.H.; Jo, W.-L.; Kwon, S.-Y.; Park, H.C.; Lim, Y.W. Weight-Based Bisphosphonate Administration for Multiple Myeloma Patients and the Risks of Skeletal Complications. J. Clin. Med. 2023, 12, 1637. https://doi.org/10.3390/jcm12041637
Bahk JH, Jo W-L, Kwon S-Y, Park HC, Lim YW. Weight-Based Bisphosphonate Administration for Multiple Myeloma Patients and the Risks of Skeletal Complications. Journal of Clinical Medicine. 2023; 12(4):1637. https://doi.org/10.3390/jcm12041637
Chicago/Turabian StyleBahk, Ji Hoon, Woo-Lam Jo, Soon-Yong Kwon, Hyung Chul Park, and Young Wook Lim. 2023. "Weight-Based Bisphosphonate Administration for Multiple Myeloma Patients and the Risks of Skeletal Complications" Journal of Clinical Medicine 12, no. 4: 1637. https://doi.org/10.3390/jcm12041637
APA StyleBahk, J. H., Jo, W.-L., Kwon, S.-Y., Park, H. C., & Lim, Y. W. (2023). Weight-Based Bisphosphonate Administration for Multiple Myeloma Patients and the Risks of Skeletal Complications. Journal of Clinical Medicine, 12(4), 1637. https://doi.org/10.3390/jcm12041637






