Development of a Reinforcement Learning Algorithm to Optimize Corticosteroid Therapy in Critically Ill Patients with Sepsis
Abstract
:1. Introduction
2. Materials and Methods
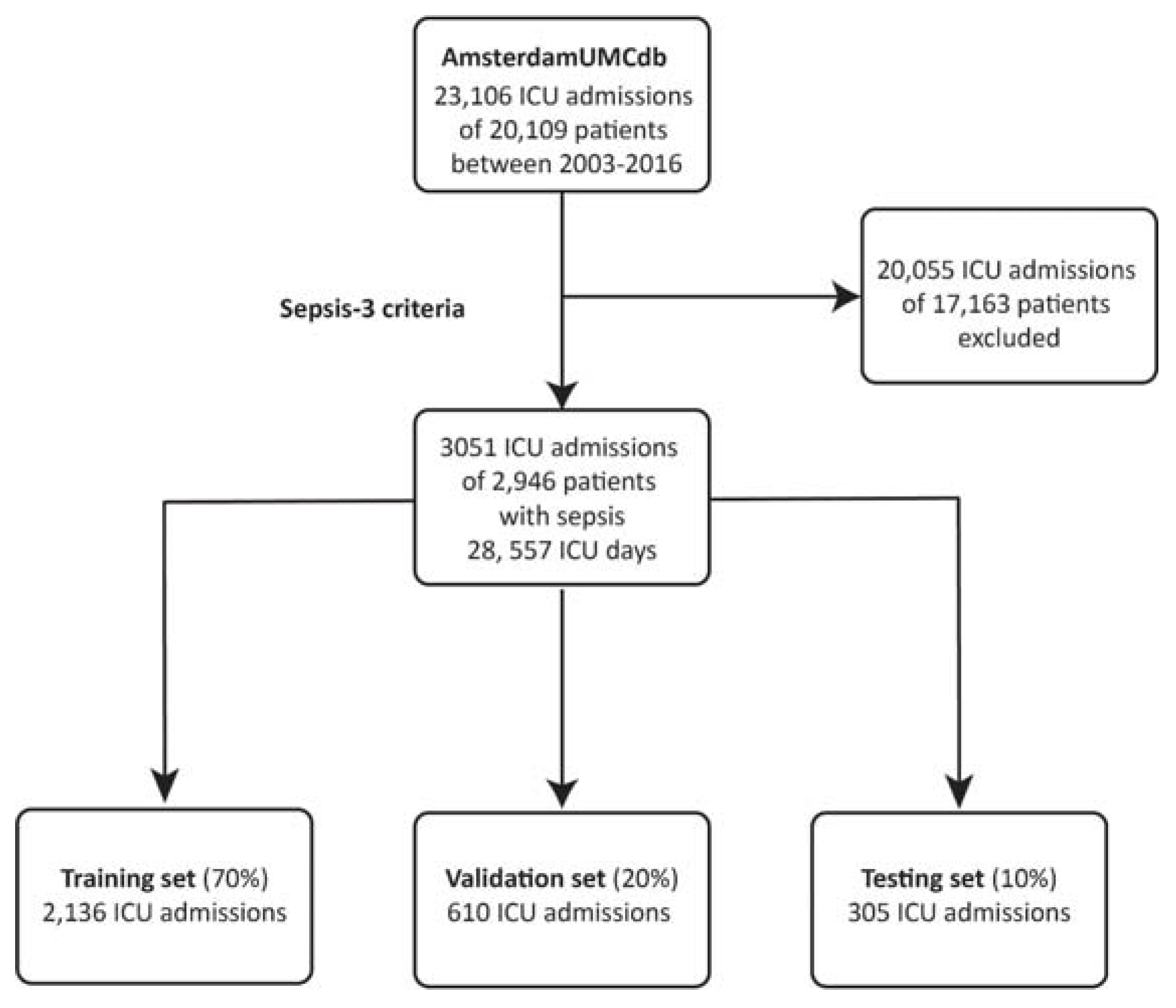
2.1. Data Sources and Data Processing
2.2. Algorithm Development
2.3. Evaluation of the Algorithm
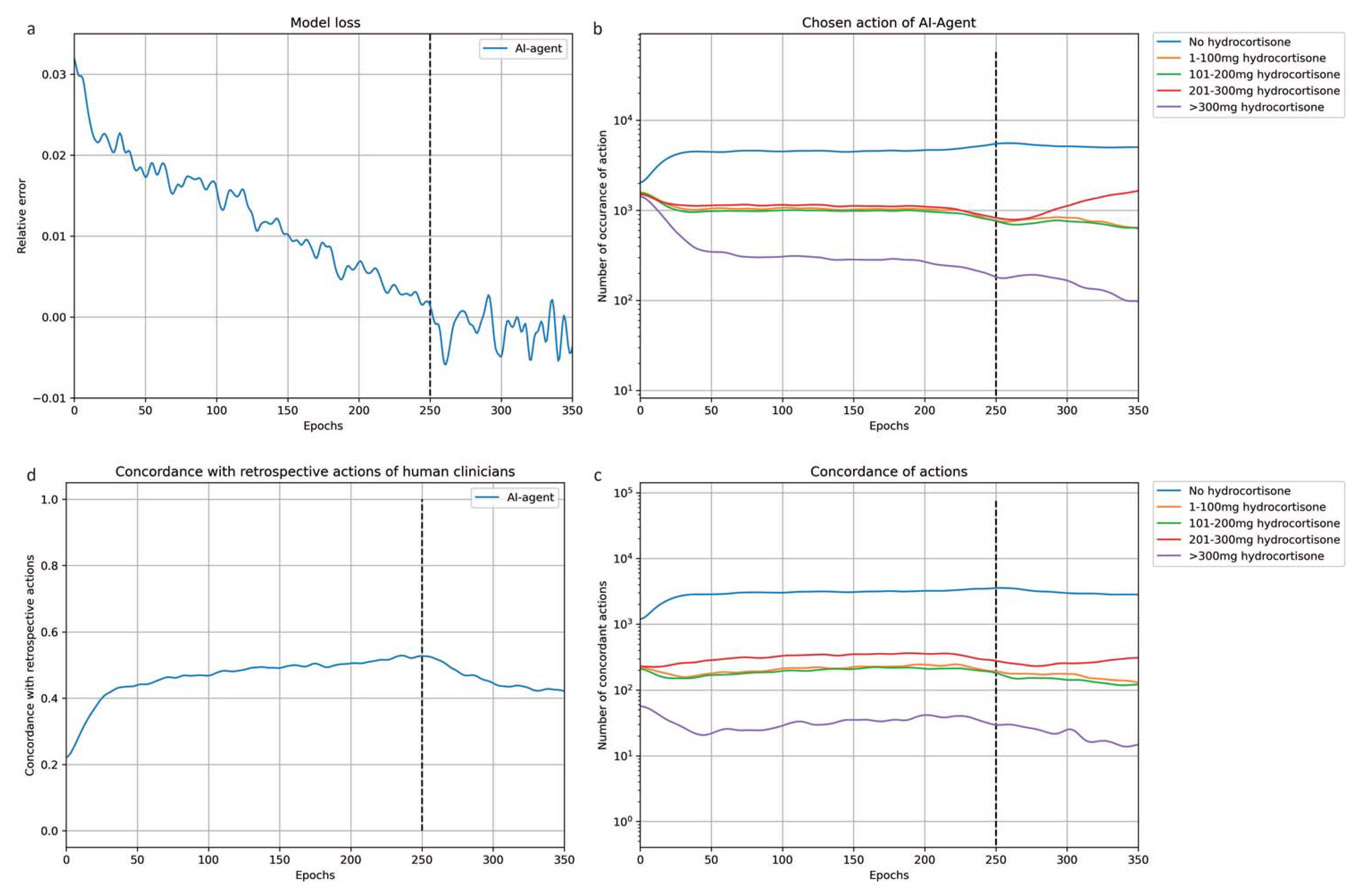
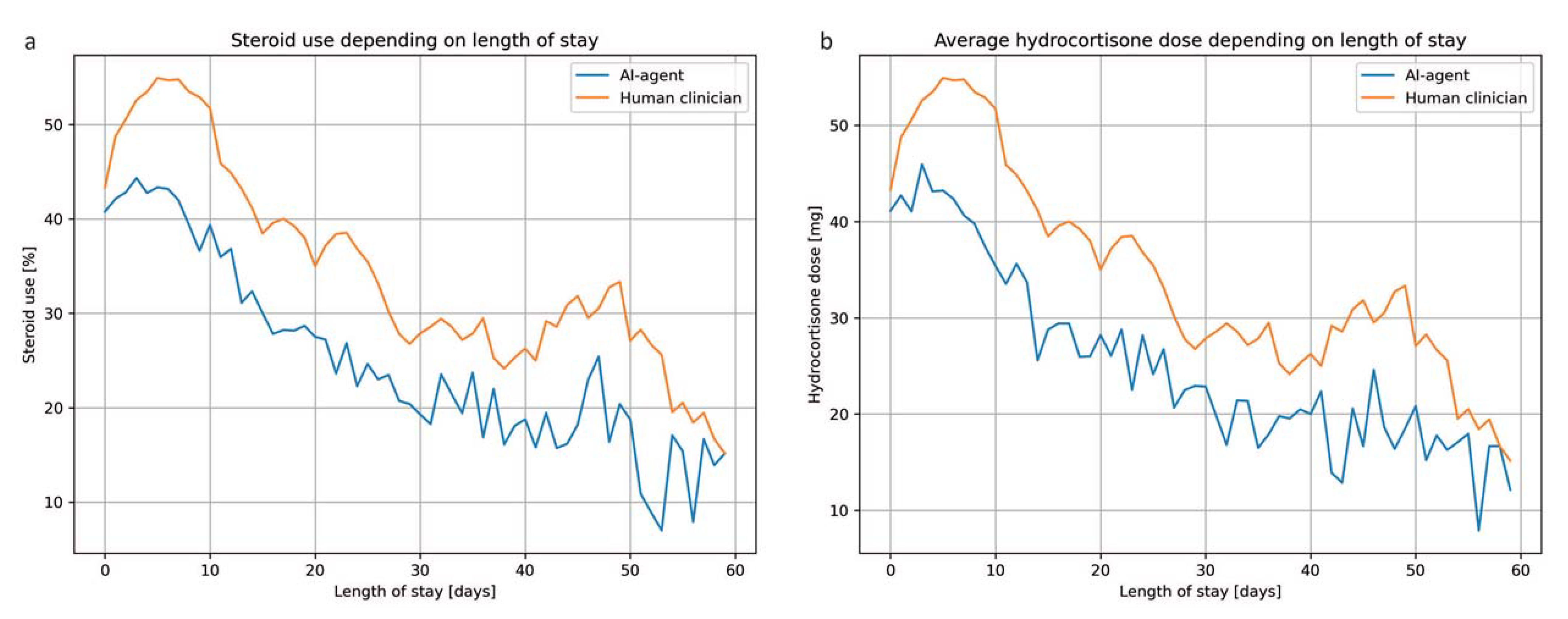
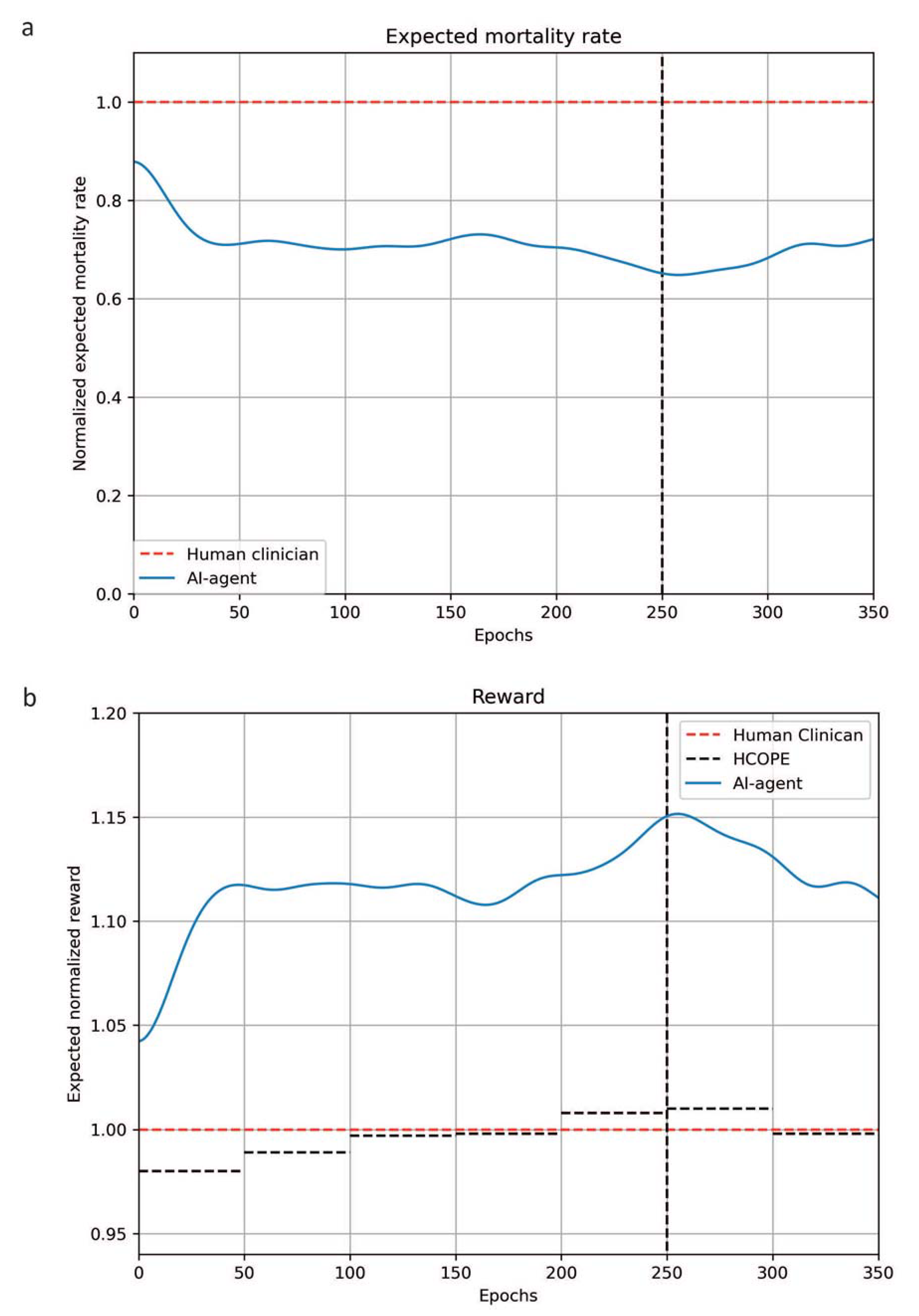
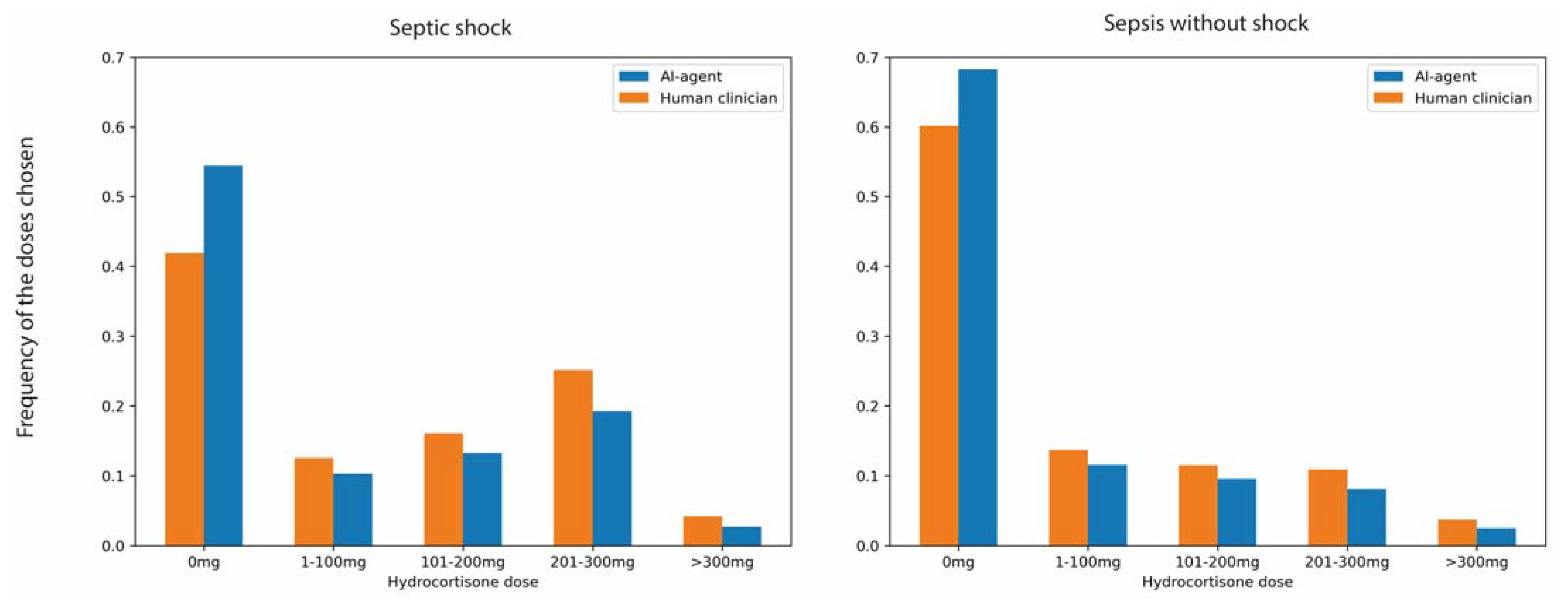
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Seymour, C.W.; Kennedy, J.N.; Wang, S.; Chang, C.-C.H.; Elliott, C.; Xu, Z.; Berry, S.; Clermont, G.; Cooper, G.; Gomez, H.; et al. Derivation, Validation, and Potential Treatment Implications of Novel Clinical Phenotypes for Sepsis. JAMA 2019, 321, 2003–2017. [Google Scholar] [CrossRef]
- Iwashyna, T.J.; Burke, J.F.; Sussman, J.B.; Prescott, H.C.; Hayward, R.A.; Angus, D.C. Implications of Heterogeneity of Treatment Effect for Reporting and Analysis of Randomized Trials in Critical Care. Am. J. Respir. Crit. Care Med. 2015, 192, 1045–1051. [Google Scholar] [CrossRef]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef]
- Cook, C.; Smith, C. Sepsis and cortisone. Nature 1952, 170, 980. [Google Scholar] [CrossRef]
- Annane, D.; Pastores, S.M.; Arlt, W.; Balk, R.A.; Beishuizen, A.; Briegel, J.; Carcillo, J.; Christ-Crain, M.; Cooper, M.S.; Marik, P.E.; et al. Critical illness-related corticosteroid insufficiency (CIRCI): A narrative review from a Multispecialty Task Force of the Society of Critical Care Medicine (SCCM) and the European Society of Intensive Care Medicine (ESICM). Intensiv. Care Med. 2017, 43, 1781–1792. [Google Scholar] [CrossRef] [PubMed]
- Annane, D.; Bellissant, E.; Bollaert, P.E.; Briegel, J.; Confalonieri, M.; De Gaudio, R.; Keh, D.; Kupfer, Y.; Oppert, M.; Meduri, G.U. Corticosteroids in the treatment of severe sepsis and septic shock in adults: A systematic review. JAMA 2009, 301, 2362–2375. [Google Scholar] [CrossRef]
- Annane, D.; Bellissant, E.; Bollaert, P.E.; Briegel, J.; Keh, D.; Kupfer, Y. Corticosteroids for treating sepsis. Cochrane Database Syst. Rev. 2015, 12, CD002243. [Google Scholar] [CrossRef]
- Rygård, S.L.; Butler, E.; Granholm, A.; Møller, M.H.; Cohen, J.; Finfer, S.; Perner, A.; Myburgh, J.; Venkatesh, B.; Delaney, A. Low-dose corticosteroids for adult patients with septic shock: A systematic review with meta-analysis and trial sequential analysis. Intensiv. Care Med. 2018, 44, 1003–1016. [Google Scholar] [CrossRef] [PubMed]
- Pirracchio, R.; Hubbard, A.; Sprung, C.L.; Chevret, S.; Annane, D.; for the Rapid Recognition of Corticosteroid Resistant or Sensitive Sepsis (RECORDS) Collaborators. Assessment of Machine Learning to Estimate the Individual Treatment Effect of Corticosteroids in Septic Shock. JAMA Netw. Open 2020, 3, e2029050. [Google Scholar] [CrossRef]
- Antcliffe, D.B.; Burnham, K.L.; Al-Beidh, F.; Santhakumaran, S.; Brett, S.J.; Hinds, C.J.; Ashby, D.; Knight, J.C.; Gordon, A.C. Transcriptomic Signatures in Sepsis and a Differential Response to Steroids. From the VANISH Randomized Trial. Am. J. Respir. Crit. Care Med. 2019, 199, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Doya, K. Reinforcement learning: Computational theory and biological mechanisms. HFSP J. 2007, 1, 30–40. [Google Scholar] [CrossRef]
- Komorowski, M.; Celi, L.A.; Badawi, O.; Gordon, A.C.; Faisal, A.A. The Artificial Intelligence Clinician learns optimal treatment strategies for sepsis in intensive care. Nat. Med. 2018, 24, 1716–1720. [Google Scholar] [CrossRef]
- Liu, S.; See, K.C.; Ngiam, K.Y.; Celi, L.A.; Sun, X.; Feng, M. Reinforcement Learning for Clinical Decision Support in Critical Care: Comprehensive Review. J. Med. Internet Res. 2020, 22, e18477. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Ngiam, K.Y.; Feng, M. Deep Reinforcement Learning for Clinical Decision Support: A Brief Survey. arXiv 2019, arXiv:1907.09475. [Google Scholar]
- Thoral, P.J.; Peppink, J.M.; Driessen, R.H.; Sijbrands, E.J.; Kompanje, E.J.; Kaplan, L.; Bailey, H.; Kesecioglu, J.; Cecconi, M.; Churpek, M.; et al. Sharing ICU Patient Data Responsibly Under the Society of Critical Care Medicine/European Society of Intensive Care Medicine Joint Data Science Collaboration: The Amsterdam University Medical Centers Database (AmsterdamUMCdb) Example. Crit. Care Med. 2021, 49, e563–e577. [Google Scholar] [CrossRef] [PubMed]
- Lambden, S.; Laterre, P.F.; Levy, M.M.; Francois, B. The SOFA score—Development, utility and challenges of accurate assessment in clinical trials. Crit. Care 2019, 23, 374. [Google Scholar] [CrossRef]
- Thoral, P.J.; Driessen, R.H.; Peppink, J.M. AmsterdamUMCdb Github Repository. 2020. Available online: https://github.com/AmsterdamUMC/AmsterdamUMCdb (accessed on 15 September 2021).
- Shin, J.; Badgwell, T.A.; Liu, K.-H.; Lee, J.H. Reinforcement Learning—Overview of recent progress and implications for process control. Comput. Chem. Eng. 2019, 127, 282–294. [Google Scholar] [CrossRef]
- Li, L.; Komorowski, M.; Faisal, A.A. The Actor Search Tree Critic (ASTC) for Off-Policy POMDP Learning in Medical Decision Making. arXiv 2018, arXiv:1805.11548. [Google Scholar]
- Haarnoja, T.; Zhou, A.; Hartikainen, K.; Tucker, G.; Ha, S.; Tan, J.; Kumar, V.; Zhu, H.; Gupta, A.; Abbeel, P.; et al. Soft Actor-Critic Algorithms and Applications. arXiv 2018, arXiv:181205905. [Google Scholar]
- Liu, D.; Ahmet, A.; Ward, L.; Krishnamoorthy, P.; Mandelcorn, E.D.; Leigh, R.; Brown, J.P.; Cohen, A.; Kim, H. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin. Immunol. 2013, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Abadi, M.; Barham, P.; Chen, J.; Chen, Z.; Davis, A.; Dean, J.; Devin, M.; Ghemawat, S.; Irving, G.; Isard, M.; et al. TensorFlow: Large-scale machine learning on heterogeneous systems. In Proceedings of the 12th USENIX Symposium on Operating Systems Design and Implementation, Savannah, GA, USA, 2–4 November 2015. [Google Scholar]
- Thomas, P.; Theocharous, G.; Ghavamzadeh, M. High-Confidence Off-Policy Evaluation. In Proceedings of the AAAI Conference on Artificial Intelligence, Hollywood, FL, USA, 18–20 May 2015. [Google Scholar] [CrossRef]
- Thomas, P.; Theocharous, G.; Ghavamzadeh, M. High Confidence Policy Improvement. In Proceedings of the 32nd International Conference on Machine Learning, Lille, France, 7–9 July 2015; Proceedings of Machine Learning Research, PMLR. Francis, B., David, B., Eds.; MLResearch Press: San Francisco, CA, USA, 2015; Volume 37, pp. 2380–2388. [Google Scholar]
- Montavon, G.; Binder, A.; Lapuschkin, S.; Samek, W.; Müller, K.-R. Layer-Wise Relevance Propagation: An Overview. In Explainable AI: Interpreting, Explaining and Visualizing Deep Learning; Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 2019; pp. 193–209. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Sligl, W.I.; Milner, J.D.A.; Sundar, S.; Mphatswe, W.; Majumdar, S.R. Safety and Efficacy of Corticosteroids for the Treatment of Septic Shock: A Systematic Review and Meta-Analysis. Clin. Infect. Dis. 2009, 49, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.-L. Steroids in sepsis: Another swing of the pendulum in our clinical trials. Crit. Care 2008, 12, 141. [Google Scholar] [CrossRef]
- Sprung, C.L.; Annane, D.; Keh, D.; Moreno, R.; Singer, M.; Freivogel, K.; Weiss, Y.G.; Benbenishty, J.; Kalenka, A.; Forst, H.; et al. Hydrocortisone Therapy for Patients with Septic Shock. New Engl. J. Med. 2008, 358, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, B.; Finfer, S.; Cohen, J.; Rajbhandari, D.; Arabi, Y.; Bellomo, R.; Billot, L.; Correa, M.; Glass, P.; Harward, M.; et al. Adjunctive Glucocorticoid Therapy in Patients with Septic Shock. New Engl. J. Med. 2018, 378, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.D.; MacCallum, N.S.; Harris, S.; Brealey, D.A.; Palmer, E.; Hetherington, J.; Shi, S.; Perez-Suarez, D.; Ercole, A.; Watkinson, P.J.; et al. Descriptors of Sepsis Using the Sepsis-3 Criteria: A Cohort Study in Critical Care Units Within the U.K. National Institute for Health Research Critical Care Health Informatics Collaborative. Crit. Care Med. 2021, 49, 1883. [Google Scholar] [CrossRef]
- Rhee, C.; Murphy, M.V.; Li, L.; Platt, R.; Klompas, M.; for the Centers for Disease Control and Prevention Epicenters Program. Comparison of Trends in Sepsis Incidence and Coding Using Administrative Claims Versus Objective Clinical Data. Clin. Infect. Dis. 2015, 60, 88–95. [Google Scholar] [CrossRef]
- Valik, J.K.; Ward, L.; Tanushi, H.; Müllersdorf, K.; Ternhag, A.; Aufwerber, E.; Färnert, A.; Johansson, A.F.; Mogensen, M.L.; Pickering, B.; et al. Validation of automated sepsis surveillance based on the Sepsis-3 clinical criteria against physician record review in a general hospital population: Observational study using electronic health records data. BMJ Qual. Saf. 2020, 29, 735–745. [Google Scholar] [CrossRef]
- Wang, F.; Kaushal, R.; Khullar, D. Should Health Care Demand Interpretable Artificial Intelligence or Accept “Black Box” Medicine? Ann. Intern. Med. 2020, 172, 59–60. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, M.C.; McIntyre, P.; Prasad, K.; van de Beek, D. Corticosteroids for acute bacterial meningitis. Cochrane Database Syst. Rev. 2015, 2015, CD004405. [Google Scholar] [CrossRef] [PubMed]
- Cunha, B.A.; Lortholary, O.; Cunha, C.B. Fever of unknown origin: A clinical approach. Am. J. Med. 2015, 128, 1138.e1–1138.e15. [Google Scholar] [CrossRef] [PubMed]
- Teblick, A.; Peeters, B.; Langouche, L.; Van den Berghe, G. Adrenal function and dysfunction in critically ill patients. Nat. Rev. Endocrinol. 2019, 15, 417–427. [Google Scholar] [CrossRef]
- Walker, B.R.; Yau, J.L.; Brett, L.P.; Seckl, J.R.; Monder, C.; Williams, B.C.; Edwards, C.R. 11 beta-hydroxysteroid dehydrogenase in vascular smooth muscle and heart: Implications for cardiovascular responses to glucocorticoids. Endocrinology 1991, 129, 3305–3312. [Google Scholar] [CrossRef]
- Shoenfeld, Y.; Gurewich, Y.; Gallant, L.A.; Pinkhas, J. Prednisone-induced leukocytosis. Am. J. Med. 1981, 71, 773–778. [Google Scholar] [CrossRef]
- Van de Sande, D.; van Genderen, M.E.; Huiskens, J.; Gommers, D.; van Bommel, J. Moving from bytes to bedside: A systematic review on the use of artificial intelligence in the intensive care unit. Intensive Care Med. 2021, 47, 750–760. [Google Scholar] [CrossRef]
- Richard, S. Sutton and Andrew G. Barto, Reinforcement Learning: An Introduction. IEEE Trans. Neural Netw 2015, 9, 1054. [Google Scholar]
- Brockman, G.; Cheung, V.; Pettersson, L.; Schneider, J.; Schulman, J.; Tang, J.; Zaremba, W. Openai gym. arXiv 2016, arXiv:1606.01540. [Google Scholar]
- Haarnoja, T.; Zhou, A.; Abbeel, P.; Levine, S. Soft Actor-Critic: Off-Policy Maximum Entropy Deep Reinforcement Learning with a Stochastic Actor. In Proceedings of the International Conference on Machine Learning, PMLR 2022, Virtual Event, 7–8 April 2022. [Google Scholar] [CrossRef]
| Characteristics | Summary (Total) | Summary (Survivors) | Summary (Non-Survivors) |
|---|---|---|---|
| Total number of ICU admissions | 3051 | 2336 | 715 |
| Male sex, No. (%) | 1758 (57.6%) | 1353 (57.9%) | 405 (56.6%) |
| Age group (years), No. (%) | -- | -- | -- |
| 18–39 | 342 (11.2%) | 303 (12.9%) | 39 (5.4%) |
| 40–49 | 322 (10.5%) | 265 (11.3%) | 57 (7.9%) |
| 50–59 | 518 (17.0%) | 414 (17.7%) | 104 (14.5%) |
| 60–69 | 757 (24.8%) | 591 (25.2%) | 166 (23.2%) |
| 70–79 | 709 (23.2%) | 506 (21.6%) | 203 (28.3%) |
| >80 | 403 (13.2%) | 257 (11%) | 146 (20.4%) |
| Highest SOFA score during the ICU stay, Median (IQR) | 10 (6) | 9 (6) | 13 (6) |
| Sofa score at sepsis onset, Median (IQR) | 9 (6) | 8 (5) | 11 (7) |
| Septic shock, No. (%) | 1395 (45.7%) | 845 (36.1%) | 550 (76.9%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bologheanu, R.; Kapral, L.; Laxar, D.; Maleczek, M.; Dibiasi, C.; Zeiner, S.; Agibetov, A.; Ercole, A.; Thoral, P.; Elbers, P.; et al. Development of a Reinforcement Learning Algorithm to Optimize Corticosteroid Therapy in Critically Ill Patients with Sepsis. J. Clin. Med. 2023, 12, 1513. https://doi.org/10.3390/jcm12041513
Bologheanu R, Kapral L, Laxar D, Maleczek M, Dibiasi C, Zeiner S, Agibetov A, Ercole A, Thoral P, Elbers P, et al. Development of a Reinforcement Learning Algorithm to Optimize Corticosteroid Therapy in Critically Ill Patients with Sepsis. Journal of Clinical Medicine. 2023; 12(4):1513. https://doi.org/10.3390/jcm12041513
Chicago/Turabian StyleBologheanu, Razvan, Lorenz Kapral, Daniel Laxar, Mathias Maleczek, Christoph Dibiasi, Sebastian Zeiner, Asan Agibetov, Ari Ercole, Patrick Thoral, Paul Elbers, and et al. 2023. "Development of a Reinforcement Learning Algorithm to Optimize Corticosteroid Therapy in Critically Ill Patients with Sepsis" Journal of Clinical Medicine 12, no. 4: 1513. https://doi.org/10.3390/jcm12041513
APA StyleBologheanu, R., Kapral, L., Laxar, D., Maleczek, M., Dibiasi, C., Zeiner, S., Agibetov, A., Ercole, A., Thoral, P., Elbers, P., Heitzinger, C., & Kimberger, O. (2023). Development of a Reinforcement Learning Algorithm to Optimize Corticosteroid Therapy in Critically Ill Patients with Sepsis. Journal of Clinical Medicine, 12(4), 1513. https://doi.org/10.3390/jcm12041513







