Osteoma of the Jaw as First Clinical Sign of Gardner’s Syndrome: The Experience of Two Italian Centers and Review
Abstract
:1. Introduction
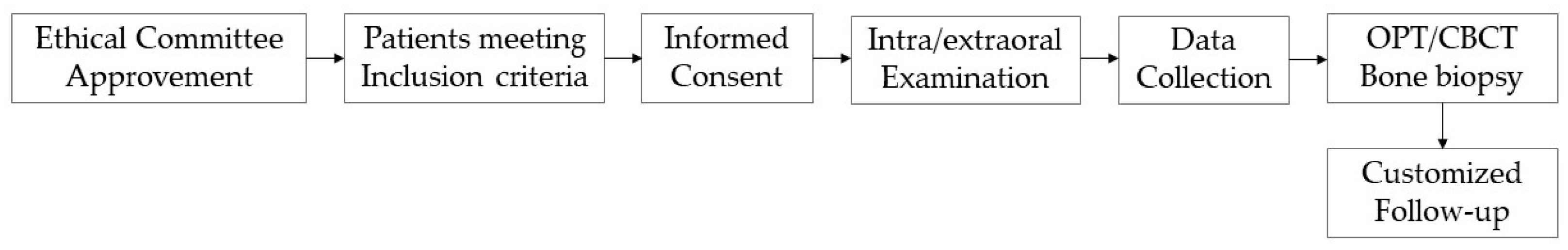
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Study Design
2.3. Literature Review
- Population: Adults, adolescents, and children.
- Intervention: Osteomas of the jaw in GS patients.
- Comparator: Systemically healthy subjects.
- Outcome: The earlier presence of oral osteomas.
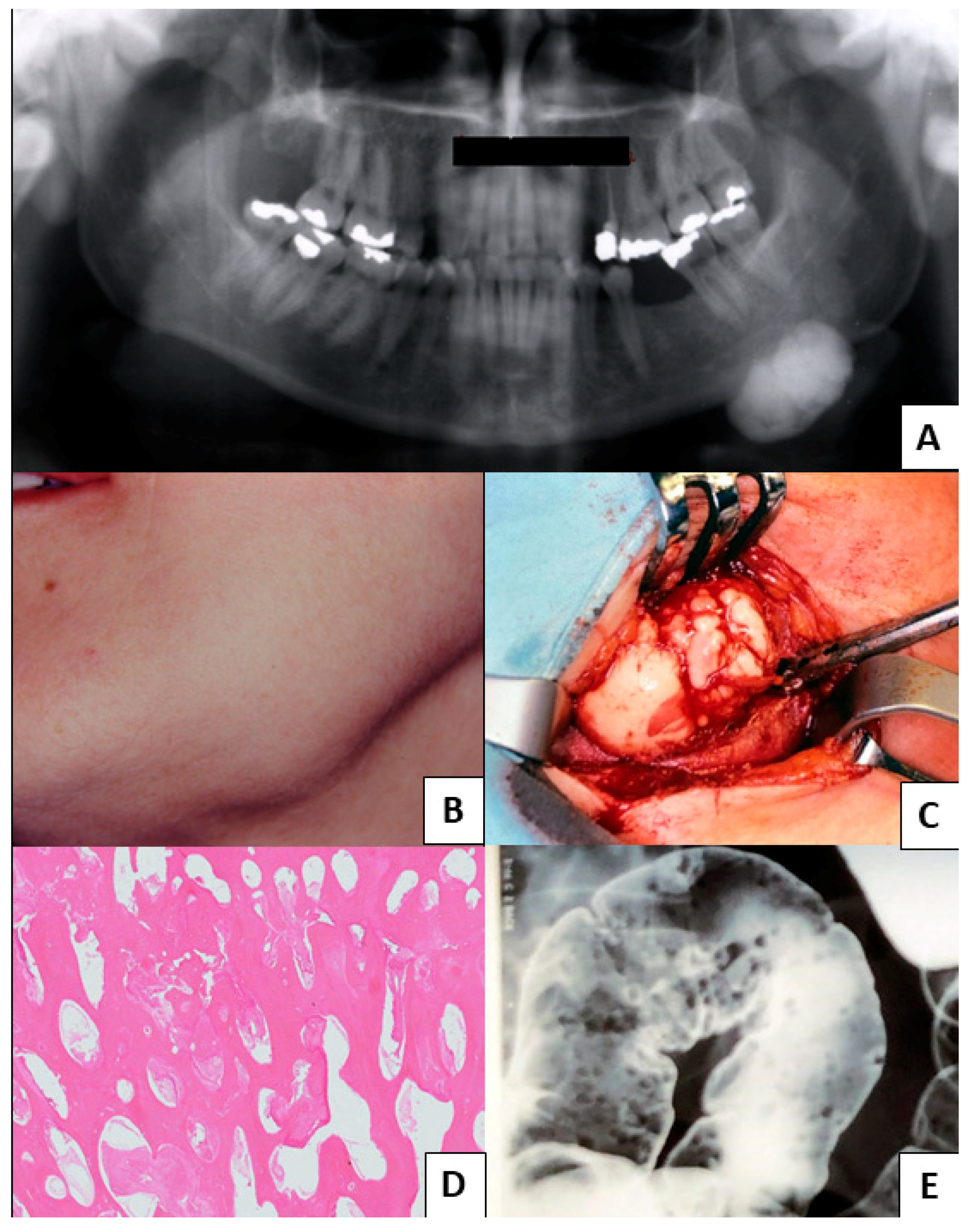
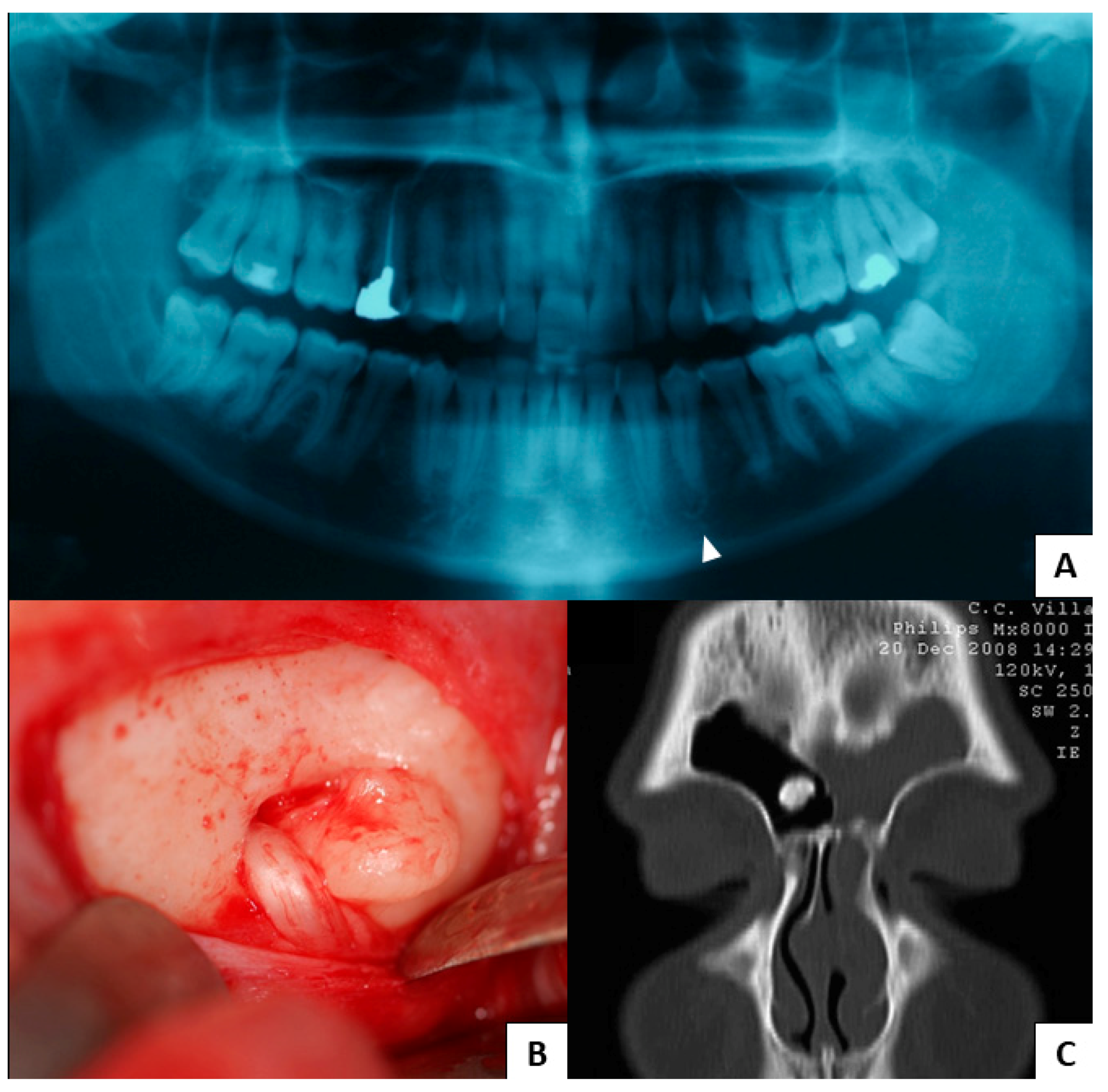
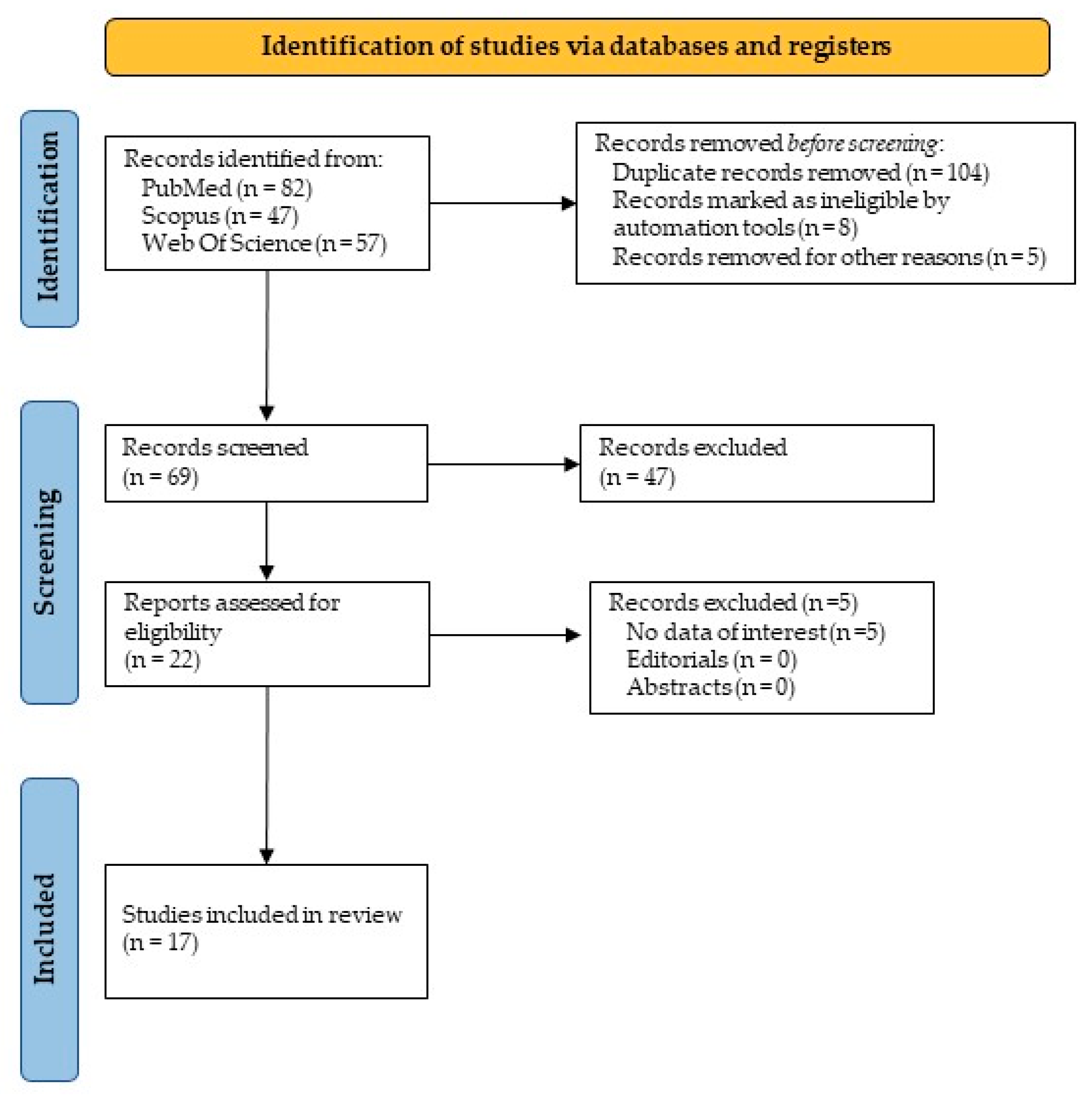
3. Results
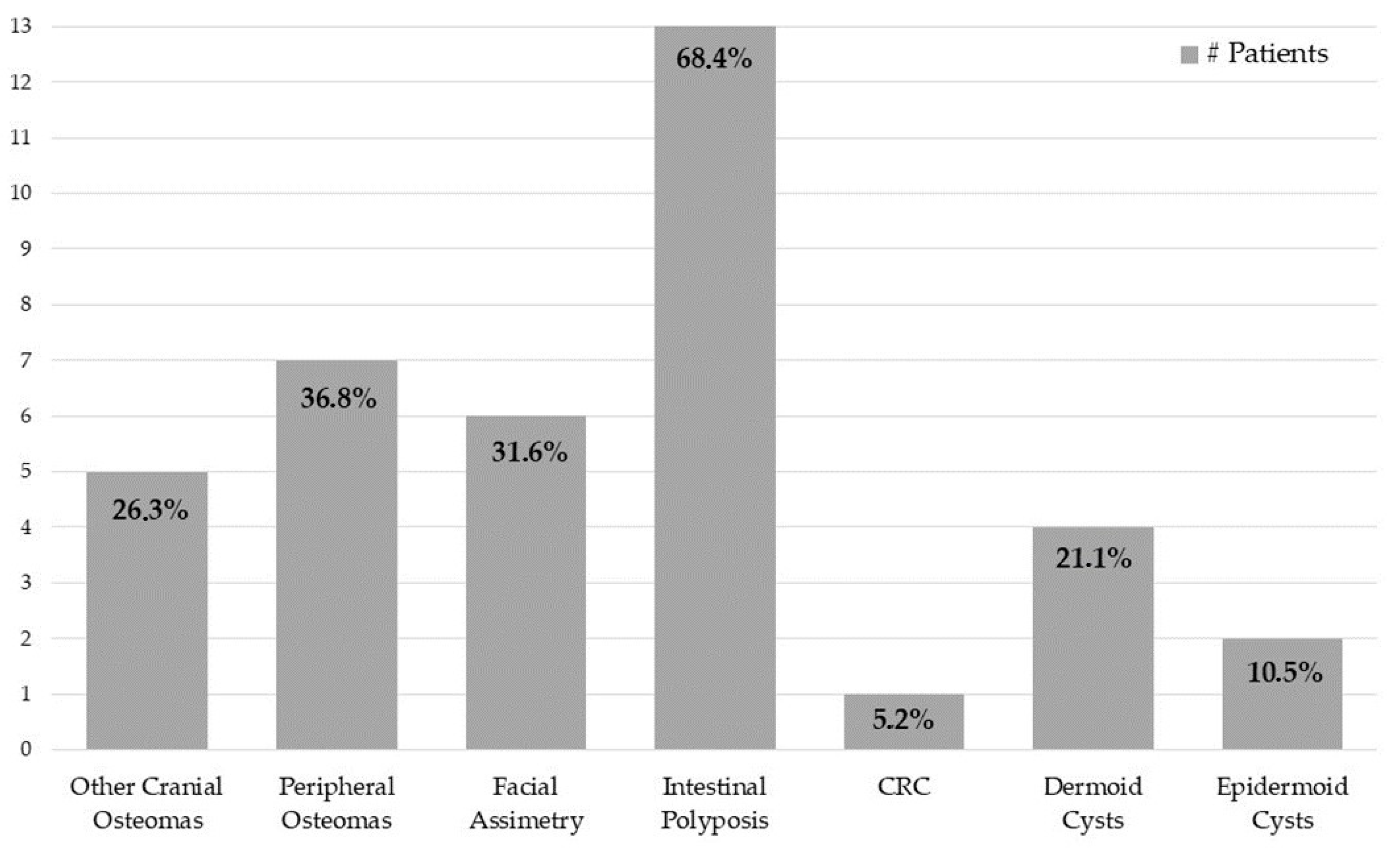
3.1. Study Results
3.2. Literature Review Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Authors | Year | Early Oral Symptoms |
|---|---|---|
| Adisen et al. [4] | 2018 | Multiple osteomas of the jaw |
| Antohi et al. [13] | 2021 | Multiple osteomas and dental abnormalities |
| Baldino et al. [6] | 2019 | Craniomaxillofacial osteomas |
| Baykul et al. [15] | 2003 | Multiple mandibular osteomas |
| Capodiferro et al. [14] | 2005 | Multiple mandibular osteomas |
| de Oliveira Ribas et al. [21] | 2009 | Multiple osteomas |
| Groen et al. [22] | 2008 | Multiple osteomas |
| Katou et al. [23] | 1989 | Multiple osteomas |
| Kubo et al. [24] | 1989 | Multiple osteomas |
| Payne et al. [25] | 2002 | Multiple osteomas |
| Seehra et al. [2] | 2016 | Multiple osteomas |
| Ragupathy et al. [26] | 2015 | Mandibular osteoma |
| Oliveira et al. [7] | 2016 | Multiple osteomas |
| Verma et al. [27] | 2016 | Palatal osteoma |
| Thomaidis et al. [5] | 2019 | Multiple osteomas and soft tissue lesions |
| Wijn et al. [28] | 2007 | Multiple osteomas |
| Yu et al. [3] | 2018 | Multiple osteomas and dental abnormalities |
References
- Agrawal, D.; Newaskar, V.; Shrivastava, S.; Nayak, P.A. External manifestations of Gardner’s syndrome as the presenting clinical entity. BMJ Case Rep. 2014, 2014, bcr2013200293. [Google Scholar] [CrossRef] [PubMed]
- Seehra, J.; Patel, S.; Bryant, C. Gardner’s Syndrome revisited: A clinical case and overview of the literature. J. Orthod. 2016, 43, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Cw, B.N.; Zhu, H.; Liu, J.; Lin, Y. Bone and dental abnormalities as first signs of familial Gardner’s syndrome in a Chinese family: A literature review and a case report. Med. Sci. 2018, 34, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Adisen, M.Z.; Okkesim, A.; Misirlioglu, M. The importance of early diagnosis of gardner’s syndrome in dental examination. Niger. J. Clin. Pract. 2018, 21, 114–116. [Google Scholar] [CrossRef] [PubMed]
- Thomaidis, V.; Seretis, K.; Tsoucalas, G.; Razos, K.; Vasilopoulos, A.; Efenti, G.M.; Fiska, A. A Case of Early FAP Diagnosis with Extraintestinal Manifestations on the Face. Acta Med. Acad. 2019, 48, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Baldino, M.E.; Koth, V.S.; Silva, D.N.; Figueiredo, M.A.; Salum, F.G.; Cherubini, K. Gardner syndrome with maxillofacial manifestation: A case report. Spec. Care Dentist. 2019, 39, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.R.; Rodrigues, W.C.; Gabrielli, M.F.R.; Gabrielli, M.; Onofre, M.A.; Filho, V.O. Gardner Syndrome with Unusual Maxillofacial Manifestation. J. Craniofac. Surg. 2016, 27, 1253–1255. [Google Scholar] [CrossRef] [PubMed]
- Guignard, N.; Cartier, C.; Crampette, L.; Akkari, M. Gardner’s syndrome presenting with a fibromatous tumour of the parotid. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2016, 133, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Halawi, A.M.; Maley, J.; Robinson, R.; Swenson, C.; Graham, S. Craniofacial osteoma: Clinical presentation and patterns of growth. Am. J. Rhinol. Allergy 2013, 27, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Herford, A.S.; Stoffella, E.; Tandon, R. Osteomas involving the facial skeleton: A report of 2 cases and review of the literature. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 115, e1–e6. [Google Scholar] [CrossRef] [PubMed]
- Argyris, P.P.; Koutlas, I.G. Orthokeratinized Odontogenic Cyst with an Associated Keratocystic Odontogenic Tumor Component and Ghost Cell Keratinization and Calcifications in a Patient with Gardner Syndrome. Head Neck Pathol. 2017, 11, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Antohi, C.; Haba, D.; Caba, L.; Ciofu, M.L.; Drug, V.L.; Bărboi, O.B.; Dobrovăț, B.I.; Pânzaru, M.C.; Gorduza, N.C.; Lupu, V.V.; et al. Novel Mutation in APC Gene Associated with Multiple Osteomas in a Family and Review of Genotype-Phenotype Correlations of Extracolonic Manifestations in Gardner Syndrome. Diagnostics 2021, 11, 1560. [Google Scholar] [CrossRef] [PubMed]
- Capodiferro, S.; Lacaita, M.G.; Scivetti, M.; Manco, R.; Testa, N.F. Multiple and giant mandibular osteomas in a Gardner’s syndrome. Case report. Minerva Stomatol. 2005, 54, 165–169. [Google Scholar]
- Baykul, T.; Heybeli, N.; Oyar, O.; Doğru, H. Multiple huge osteomas of the mandible causing disfigurement related with Gardner’s syndrome: Case report. Auris Nasus Larynx 2003, 30, 447–451. [Google Scholar] [CrossRef]
- Larsson Wexell, C.; Bergenblock, S.; Kovács, A. A Case Report on Gardner Syndrome with Dental Implant Treatment and a Long-Term Follow-Up. J. Oral Maxillofac. Surg. 2019, 77, 1617–1627. [Google Scholar] [CrossRef]
- Álvarez Salgado, J.A.; González-Llanos Fernández de Mesa, F.; Villaseñor Ledezma, J.J.; Cañizares Méndez, M.L.; Paredes Sansinenea, I.; Rodríguez de Lope-Llorca, A.; Mollejo Villanueva, M. Craneofaringioma ectópico y síndrome de Gardner: A propósito de un caso y revisión de la literatura [Ectopic craniopharyngioma and Gardner’s syndrome: Case report and literature review]. Neurocirugia 2017, 28, 97–101. (In Spanish) [Google Scholar] [CrossRef]
- Koppány, F.; Joób-Fancsaly, A.; Pataky, L.; Martonffy, K.; Ujpál, M.; Németh, Z.; Hrabák, K. Gardner-szindróma. Esetismertetés [Gardner syndrome. Case reports]. Fogorv. Sz. 2002, 95, 253–256. (In Hungarian) [Google Scholar]
- Fotiadis, C.; Tsekouras, D.K.; Antonakis, P.; Sfiniadakis, J.; Genetzakis, M.; Zografos, G.C. Gardner’s syndrome: A case report and review of the literature. World J. Gastroenterol. 2005, 11, 5408–5411. [Google Scholar] [CrossRef]
- Nandakumar, G.; Morgan, J.A.; Silverberg, D.; Steinhagen, R.M. Familial polyposis coli: Clinical manifestations, evaluation, management and treatment. Mt. Sinai J. Med. 2004, 71, 384–391. [Google Scholar]
- de Oliveira Ribas, M.; Martins, W.D.; de Sousa, M.H.; de Aguiar Koubik, A.C.; Avila, L.F.; Zanferrari, F.L.; Martins, G. Oral and maxillofacial manifestations of familial adenomatous polyposis (Gardner’s syndrome): A report of two cases. J. Contemp. Dent Pract. 2009, 10, 82–90. [Google Scholar] [PubMed]
- Groen, E.J.; Roos, A.; Muntinghe, F.L.; Enting, R.H.; de Vries, J.; Kleibeuker, J.H.; Witjes, M.J.; Links, T.P.; van Beek, A.P. Extra-intestinal manifestations of familial adenomatous polyposis. Ann Surg Oncol. 2008, 15, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Katou, F.; Motegi, K.; Baba, S. Mandibular lesions in patients with adenomatosis coli. J. Craniomaxillofac. Surg. 1989, 17, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Kubo, K.; Miyatani, H.; Takenoshita, Y.; Abe, K.; Oka, M.; Iida, M.; Itoh, H. Widespread radiopacity of jaw bones in familial adenomatosis coli. J. Craniomaxillofac. Surg. 1989, 17, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Payne, M.; Anderson, J.A.; Cook, J. Gardner’s syndrome—A case report. Br. Dent. J. 2002, 193, 383–384. [Google Scholar] [CrossRef]
- Ragupathy, K.; Priyadharsini, I.; Sanjay, P.; Yuvaraj, V.; Balaji, T.S. Peripheral Osteoma of the Body of Mandible: A Case Report. J. Maxillofac. Oral Surg. 2015, 14, 1004–1008. [Google Scholar] [CrossRef]
- Verma, P.; Surya, V.; Kadam, S.; Umarji, H.R. Classical presentation of Gardner’s syndrome in an Indian patient: A case report. Contemp. Clin. Dent. 2016, 7, 277–280. [Google Scholar] [CrossRef]
- Wijn, M.A.; Keller, J.J.; Giardiello, F.M.; Brand, H.S. Oral and maxillofacial manifestations of familial adenomatous polyposis. Oral Dis. 2007, 13, 360–365. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Agostino, S.; Dell’Olio, F.; Tempesta, A.; Cervinara, F.; D’Amati, A.; Dolci, M.; Favia, G.; Capodiferro, S.; Limongelli, L. Osteoma of the Jaw as First Clinical Sign of Gardner’s Syndrome: The Experience of Two Italian Centers and Review. J. Clin. Med. 2023, 12, 1496. https://doi.org/10.3390/jcm12041496
D’Agostino S, Dell’Olio F, Tempesta A, Cervinara F, D’Amati A, Dolci M, Favia G, Capodiferro S, Limongelli L. Osteoma of the Jaw as First Clinical Sign of Gardner’s Syndrome: The Experience of Two Italian Centers and Review. Journal of Clinical Medicine. 2023; 12(4):1496. https://doi.org/10.3390/jcm12041496
Chicago/Turabian StyleD’Agostino, Silvia, Fabio Dell’Olio, Angela Tempesta, Francesca Cervinara, Antonio D’Amati, Marco Dolci, Gianfranco Favia, Saverio Capodiferro, and Luisa Limongelli. 2023. "Osteoma of the Jaw as First Clinical Sign of Gardner’s Syndrome: The Experience of Two Italian Centers and Review" Journal of Clinical Medicine 12, no. 4: 1496. https://doi.org/10.3390/jcm12041496
APA StyleD’Agostino, S., Dell’Olio, F., Tempesta, A., Cervinara, F., D’Amati, A., Dolci, M., Favia, G., Capodiferro, S., & Limongelli, L. (2023). Osteoma of the Jaw as First Clinical Sign of Gardner’s Syndrome: The Experience of Two Italian Centers and Review. Journal of Clinical Medicine, 12(4), 1496. https://doi.org/10.3390/jcm12041496











