Effect of a Contrast Agent on Bone Mineral Density Measurement in the Spine and Hip Using QCT-Conversion Factor Recommendation
Abstract
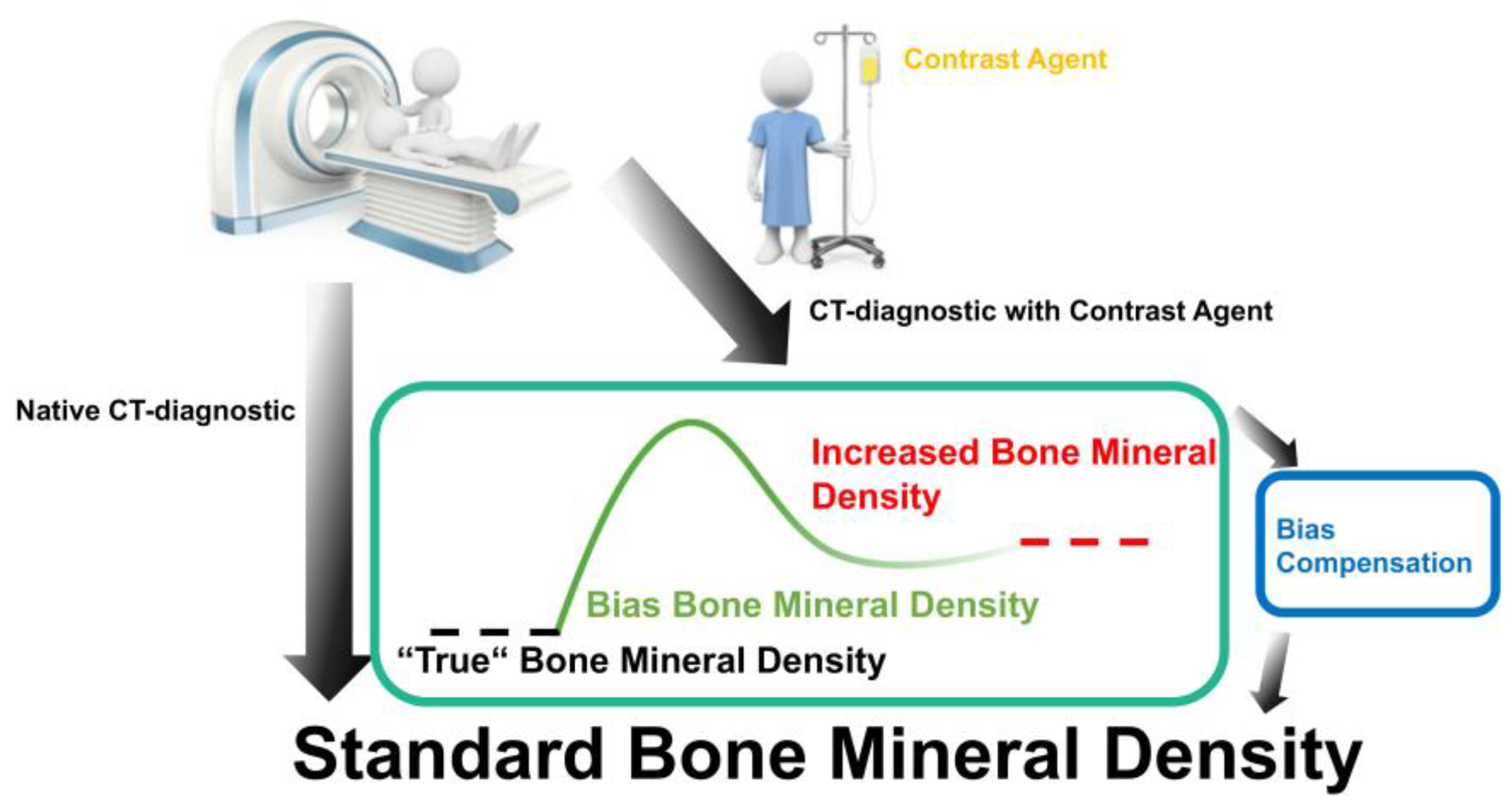
:1. Introduction
2. Materials and Methods
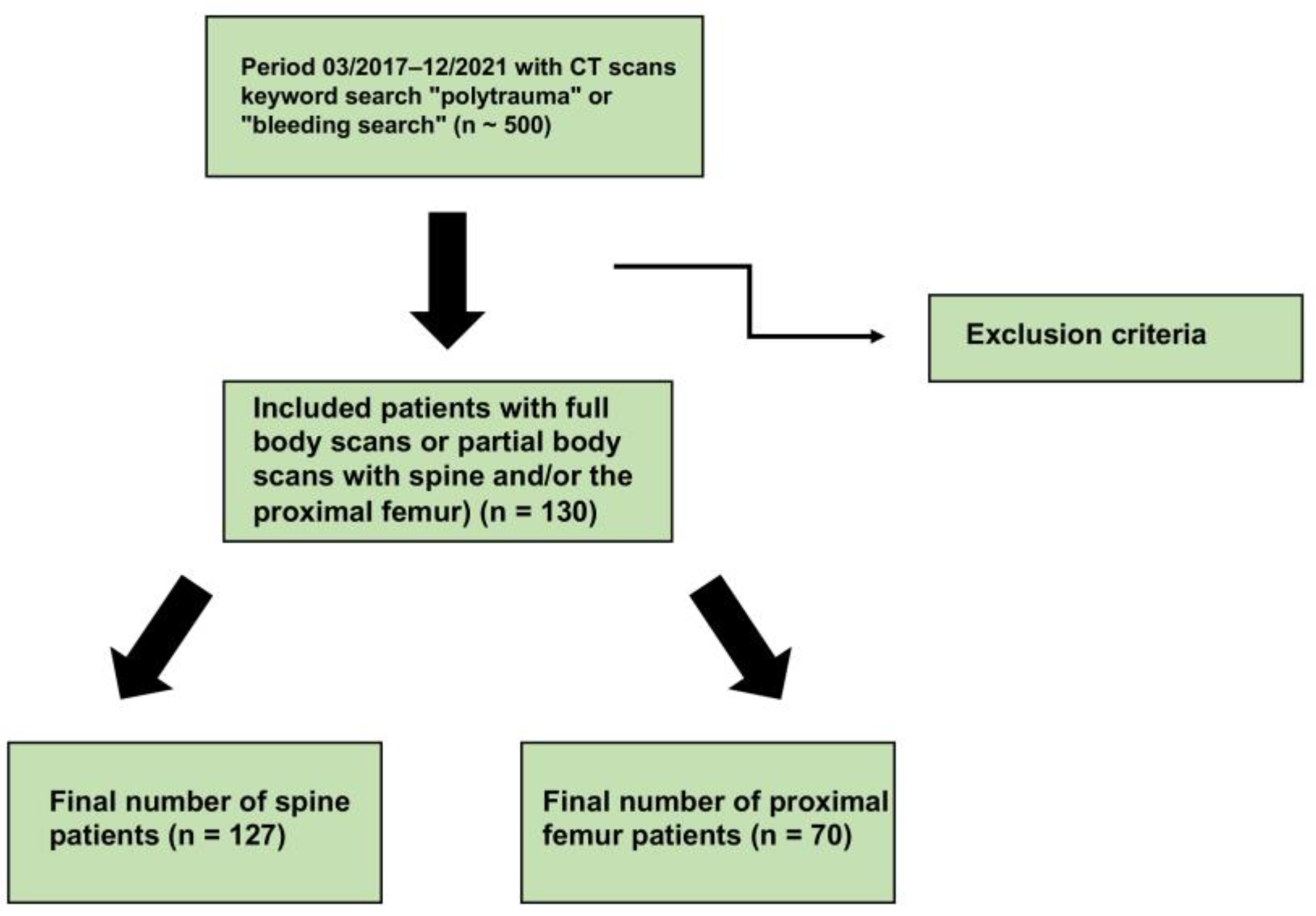
2.1. Study Design and Subjects
2.2. Contrast Agent Administration
2.3. Statistics
3. Results
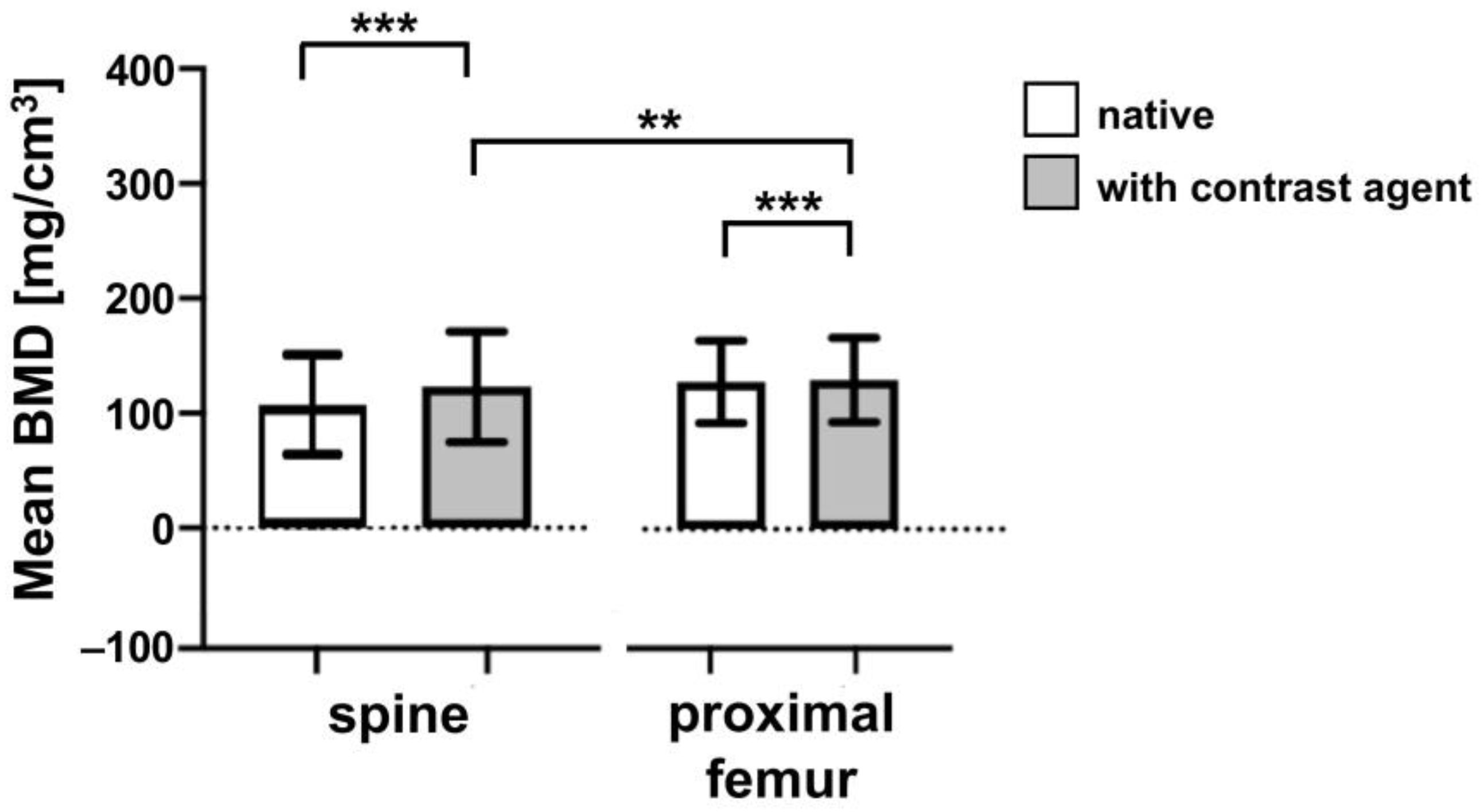
3.1. Comparison of the BMD Values of Patients with and without Contrast Agent Administration
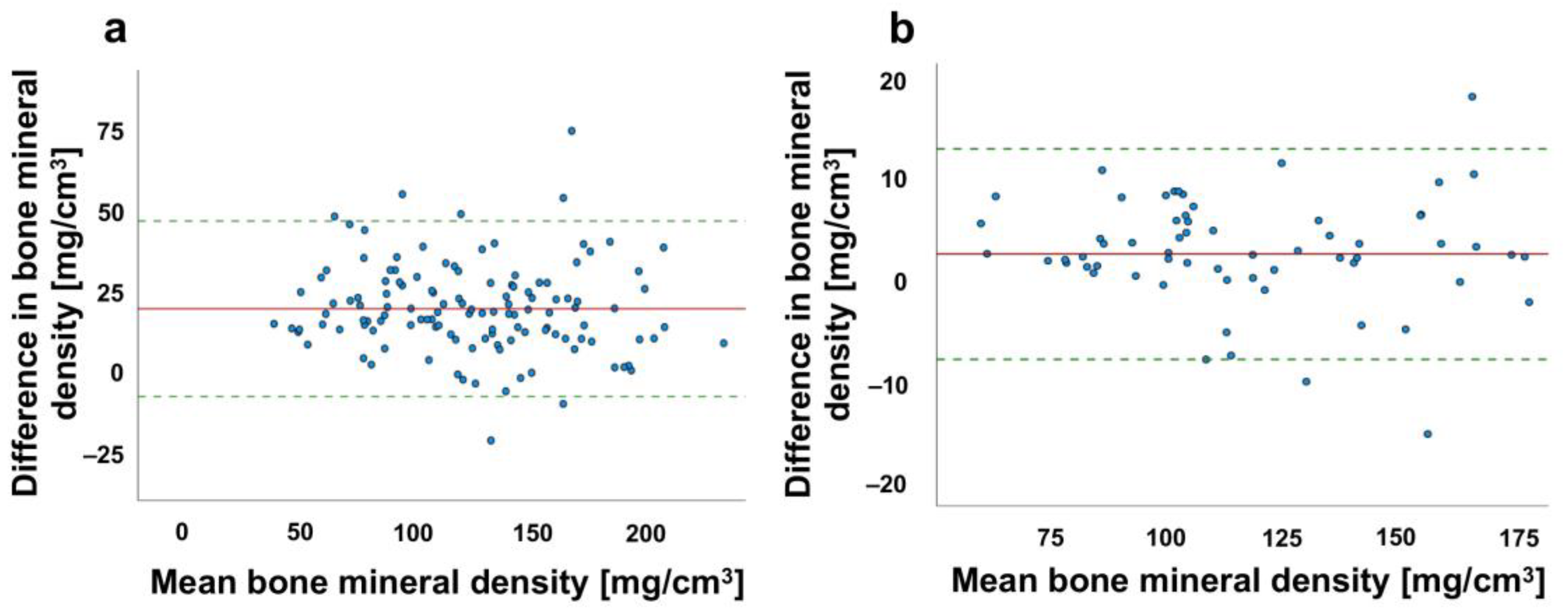
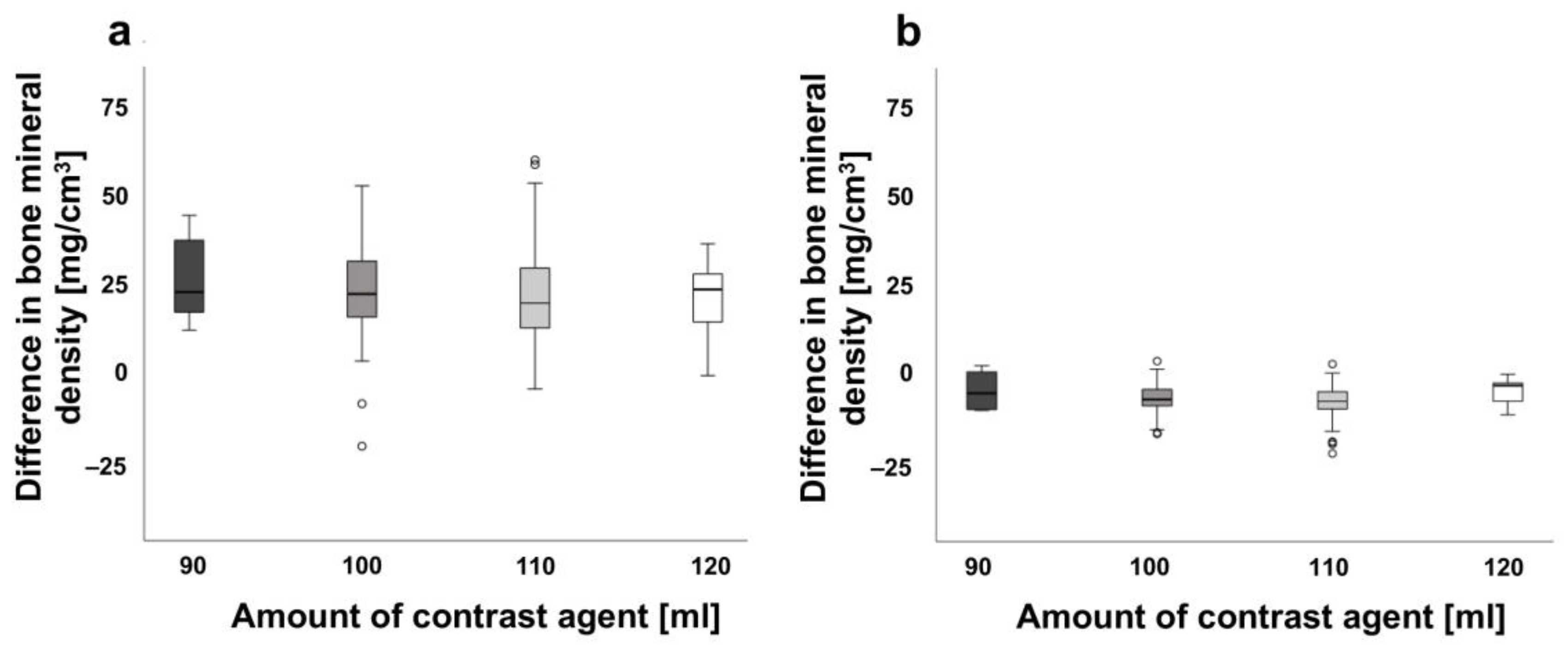
3.2. Effects of the Contrast Agent Amount on Bone Density Measurements in Spine and Proximal Femur
3.3. Correlation of Body Mass Index and the Administration of Contrast Agents
3.4. Comparison between Contrast Agent Amount and Bone Mineral Quantity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klibanski, A.; Adams-Campbell, L.; Bassford, T.; Blair, S.N.; Boden, S.D.; Dickersin, K.; Gifford, D.R.; Glasse, L.; Goldring, S.R.; Hruska, K.; et al. National Institutes of Health: Osteoporosis prevention, diagnosis, and therapy. JAMA 2001, 285, 785–795. [Google Scholar]
- Osterkamp, R. Population developments in Germany until 2050: Demographic and economic consequences for geriatric medicine and surgery. Der Chir. 2005, 76, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Duquet, N. Osteoporosis: Treatment and pharmaceutical care. J. Pharm. Belg. 2014, 2, 14–24. [Google Scholar]
- Löffler, M.T.; Sollmann, N.; Mei, K.; Valentinitsch, A.; Noël, P.B.; Kirschke, J.S.; Baum, T. X-ray-based quantitative osteoporosis imaging at the spine. Osteoporos. Int. 2019, 31, 233–250. [Google Scholar] [CrossRef] [PubMed]
- Melton, L.J., III; Atkinson, E.J.; Cooper, C.; O’Fallon, W.M.; Riggs, B.L. Vertebral fractures predict subsequent fractures. Osteoporos. Int. 1999, 10, 214–221. [Google Scholar] [CrossRef]
- Haghighat Khah, H.R.; Moradi, N.; Taheri, T.; Sanei Taheri, M.; Rayegani, S.M. Investigating comparability of quantitative computed tomography with dual energy x-ray absorptiometry in assessing bone mineral density of patients with chronic spinal cord injury. Spinal Cord 2018, 56, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.E. Quantitative computed tomography. Eur. J. Radiol. 2009, 71, 415–424. [Google Scholar] [CrossRef] [PubMed]
- QCT PROTM. Mineral Densitometry Software Report Content and Interpretation Module Version 5.1—Revision 20130102, Copyright 2013; Mindways Software, Inc.: Austin, TX, USA, 2013. [Google Scholar]
- American College of Radiology. ACR–SPR–SSR Practice Parameter for the Performance of Quantitative Computed Tomography (QCT) Bone Densitometry. 2014. Available online: https://www.acr.org/-/media/ACR/Files/Practice-Parameters/QCT.pdf?la=en (accessed on 11 November 2022).
- Janssen, I.; Ryang, Y.M.; Gempt, J.; Bette, S.; Gerhardt, J.; Kirschke, J.S.; Meyer, B. Risk of cement leakage and pulmonary embolism by bone cement-augmented pedicle screw fixation of the thoracolumbar spine. Spine J. 2017, 17, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ran, L.; Zha, X.; Zhao, K.; Yang, Y.; Shuang, Q.; Liu, Y.; Hind, K.; Cheng, X.; Blake, G.M. Adjustment of DXA BMD measurements for anthropometric factors and its impact on the diagnosis of osteoporosis. Arch. Osteoporos. 2020, 15, 155. [Google Scholar] [CrossRef] [PubMed]
- Link, T.M. Radiology of Osteoporosis. Can. Assoc. Radiol. J. 2016, 67, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.S.; Henning, T.D.; Müeller, D.; Lu, Y.; Majumdar, S.; Link, T.M. Volumetric Quantitative CT of the Spine and Hip Derived from Contrast-Enhanced MDCT: Conversion Factors. AJR 2007, 188, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Ziemlewicz, T.J.; Maciejewski, A.; Binkley, N.; Brett, A.D.; Brown, J.K.; Pickhardt, P.J. Direct Comparison of Unenhanced and Contrast-Enhanced CT for Opportunistic Proximal Femur Bone Mineral Density Measurement: Implications for Osteoporosis Screening. AJR Am. J. Roentgenol. 2016, 206, 694–698. [Google Scholar] [CrossRef] [PubMed]
- Kutleša, Z.; Jerković, K.; Ordulj, I.; Budimir Mršić, D. The effect of contrast media on CT measures of bone mineral density: A systematic review. Skelet. Radiol. 2022, 52, 687–694. [Google Scholar] [CrossRef] [PubMed]
| Description | Spine (n = 127) | Proximal Femur (n = 70) | p |
|---|---|---|---|
| Age mean [years] ± SD | 51.23 ± 21.89 | 57.86 ± 18.63 | |
| Gender | |||
| Female [n] | 50 | 31 | |
| Male [n] | 77 | 39 | |
| Mean BMD native [mg/cm3] Mean BMD contrast agent [mg/cm3] | 122.92 ± 48.16 143.80 ± 46.40 | 123.18 ± 37.48 126.03 ± 36.66 | <0.001 (***) <0.001 (***) |
| BMD [mg/cm3] | |||
| Amount contrast agent 90 mL | 23.56 ± 12.42 | 4.86 ± 5.22 | |
| Amount contrast agent 100 mL | 20.55. ± 16.31 | 3.06 ± 4.24 | |
| Amount contrast agent 110 mL | 18.83 ± 13.31 | 2.01 ± 6.38 | |
| Amount contrast agent 120 mL | 18.01 ± 10.43 | 4.76 ± 3.44 | |
| Mean BMD [mg/cm3] difference | 20.88 | 2.85 | <0.003 (**) |
| Mean Body mass index (BMI) [kg/m2] | 26.00 | 26.03 | 0.020 (*)//0.806 (n.s.) |
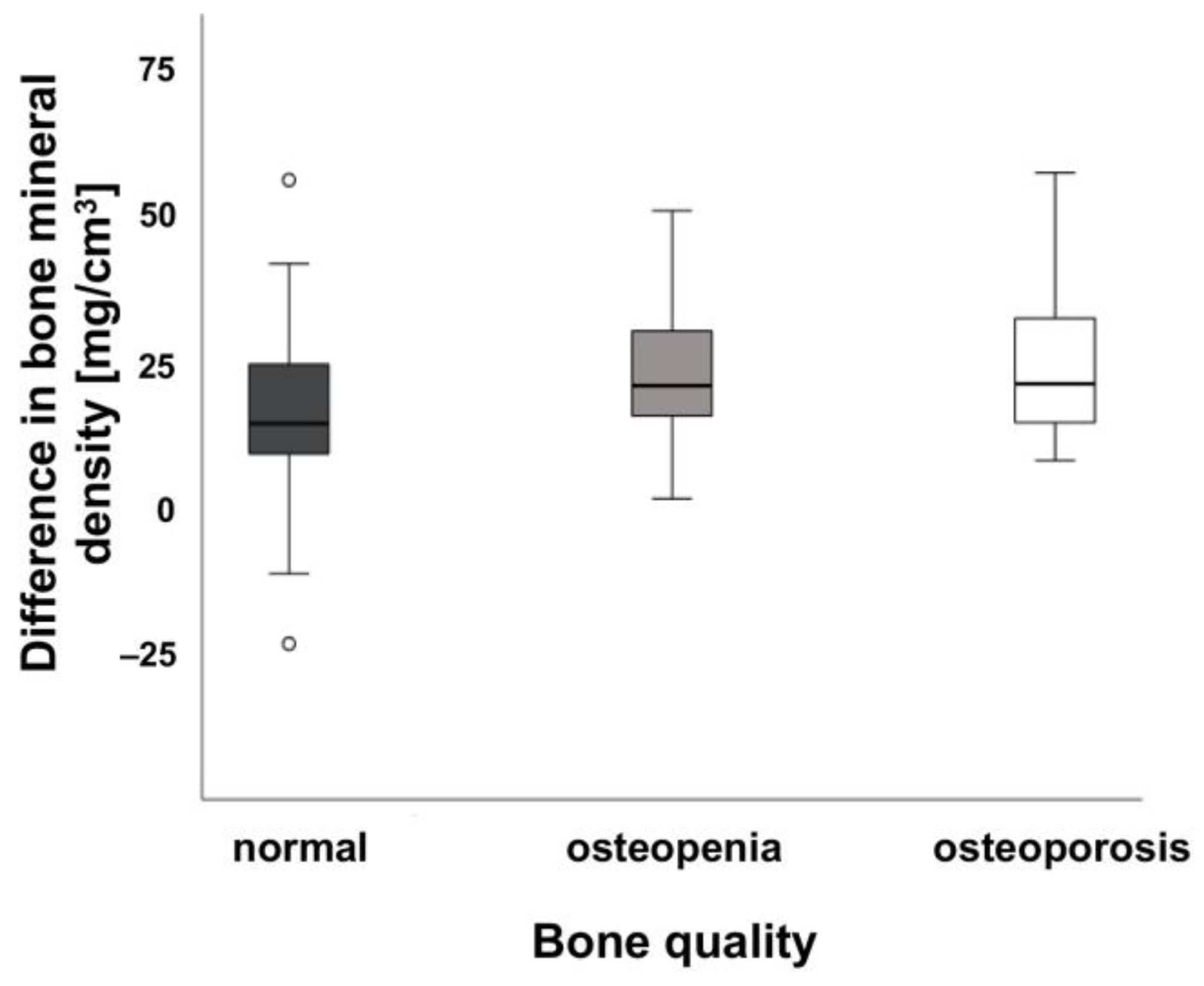
| Bone quality and BMD [mg/cm3] | |||
| Normal (>120 mg/cm3) | 23.14 ± 8.23 | 0.036 (n.s.) | |
| Osteopenia (80–120 mg/cm3) | 24.94 ± 8.96 | 0.048 (n.s.) | |
| Osteoporosis (<80 mg/cm3) | 24.12 ± 10.46 | 0.067 (n.s.) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jäckle, K.; Lüken, S.; Roch, P.J.; Klockner, F.S.; Reinhold, M.; Meier, M.-P.; Hawellek, T.; Lehmann, W.; Weiser, L. Effect of a Contrast Agent on Bone Mineral Density Measurement in the Spine and Hip Using QCT-Conversion Factor Recommendation. J. Clin. Med. 2023, 12, 1456. https://doi.org/10.3390/jcm12041456
Jäckle K, Lüken S, Roch PJ, Klockner FS, Reinhold M, Meier M-P, Hawellek T, Lehmann W, Weiser L. Effect of a Contrast Agent on Bone Mineral Density Measurement in the Spine and Hip Using QCT-Conversion Factor Recommendation. Journal of Clinical Medicine. 2023; 12(4):1456. https://doi.org/10.3390/jcm12041456
Chicago/Turabian StyleJäckle, Katharina, Sophia Lüken, Paul Jonathan Roch, Friederike Sophie Klockner, Max Reinhold, Marc-Pascal Meier, Thelonius Hawellek, Wolfgang Lehmann, and Lukas Weiser. 2023. "Effect of a Contrast Agent on Bone Mineral Density Measurement in the Spine and Hip Using QCT-Conversion Factor Recommendation" Journal of Clinical Medicine 12, no. 4: 1456. https://doi.org/10.3390/jcm12041456
APA StyleJäckle, K., Lüken, S., Roch, P. J., Klockner, F. S., Reinhold, M., Meier, M.-P., Hawellek, T., Lehmann, W., & Weiser, L. (2023). Effect of a Contrast Agent on Bone Mineral Density Measurement in the Spine and Hip Using QCT-Conversion Factor Recommendation. Journal of Clinical Medicine, 12(4), 1456. https://doi.org/10.3390/jcm12041456






