Sustained Myocarditis following Messenger RNA Vaccination against Coronavirus Disease 2019: Relation to Neutralizing Antibody and Amelioration by Low-Dose Booster Vaccination
Abstract
1. Introduction
2. Methods
2.1. Patient Selection
2.2. Definitions
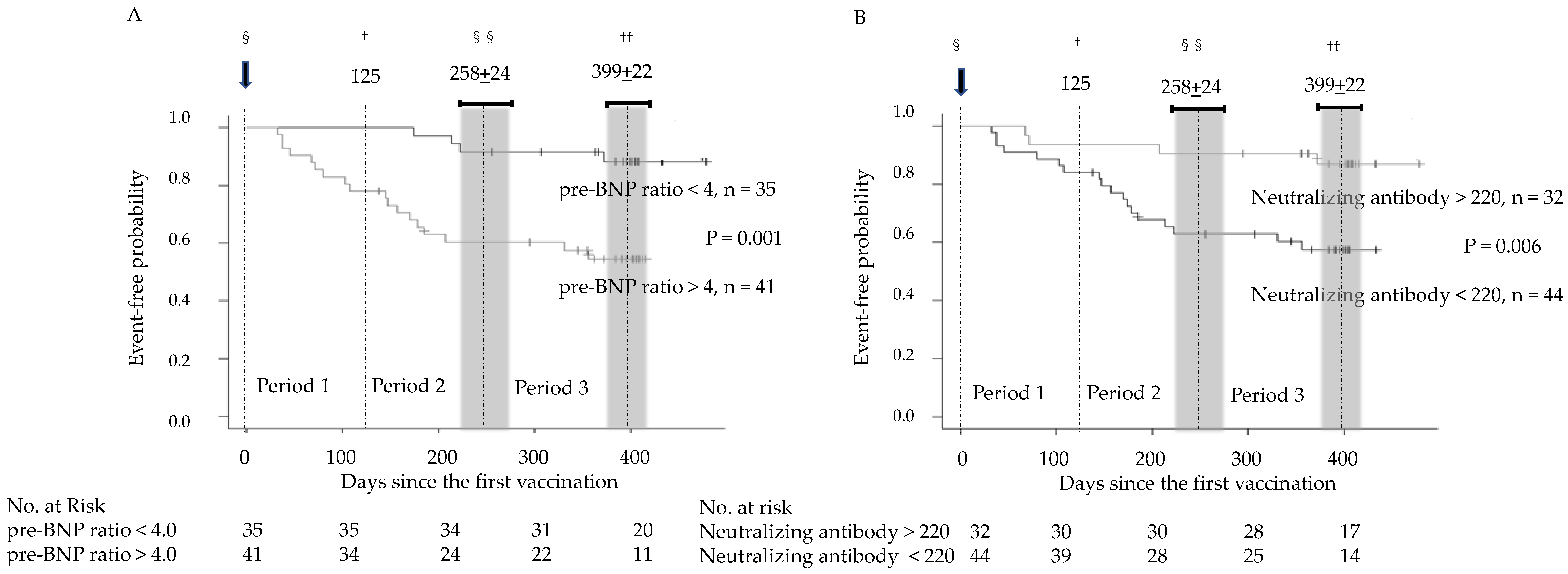
2.3. Study Protocol
2.4. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Abu-Raddad, L.J.; Chemaitelly, H.; Ayoub, H.H.; AlMukdad, S.; Yassine, H.M.; Al-Khatib, H.A.; Smatti, M.K.; Tang, P.; Hasan, M.R.; Coyle, P.; et al. Effect of mRNA vaccine boosters against SARS-CoV-2 omicron infection in Qatar. N. Engl. J. Med. 2022, 386, 1804–1816. [Google Scholar] [CrossRef] [PubMed]
- Magen, O.; Waxman, J.G.; Makov-Assif, M.; Vered, R.; Dicker, D.; Hernán, M.A.; Lipsitch, M.; Reis, B.Y.; Balicer, R.D.; Dagan, N. Fourth dose of BNT162b2 mRNA COVID-19 vaccine in a nationwide setting. N. Engl. J. Med. 2022, 386, 1603–1614. [Google Scholar] [CrossRef]
- Bhatt, A.S.; Jering, K.S.; Vaduganathan, M.; Claggett, B.L.; Cunningham, J.W.; Rosenthal, N.; Signorovitch, J.; Thune, J.J.; Vardeny, O.; Solomon, S.D. Clinical outcomes in patients with heart failure hospitalized with COVID-19. JACC Heart Fail. 2021, 9, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Rosano, G.; Jankowska, E.A.; Ray, R.; Metra, M.; Abdelhamid, M.; Adamopoulos, S.; Anker, S.D.; Bayes-Genis, A.; Belenkov, Y.; Gal, T.B.; et al. COVID-19 vaccination in patients with heart failure: A position paper of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2021, 23, 1806–1818. [Google Scholar] [CrossRef]
- Truong, D.T.; Dionne, A.; Muniz, J.C.; McHugh, K.E.; Portman, M.A.; Lambert, L.M.; Thacker, D.; Elias, M.D.; Li, J.S.; Toro-Salazar, O.H.; et al. Clinically suspected myocarditis temporally related to COVID-19 vaccination in adolescents and young adults. Circulation 2021, 145, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Mevorach, D.; Anis, E.; Cedar, N.; Bromberg, M.; Haas, E.J.; Nadir, E.; Olsha-Castell, S.; Arad, D.; Hasin, T.; Levi, N.; et al. Myocarditis after BNT162b2 mRNA vaccine against COVID-19 in Israel. N. Engl. J. Med. 2021, 385, 2140–2149. [Google Scholar] [CrossRef] [PubMed]
- Patone, M.; Mei, X.W.; Handunnetthi, L.; Dixon, S.; Zaccardi, F.; Shankar-Hari, M.; Watkinson, P.; Khunti, K.; Harnden, A.; Coupland, C.A.C.; et al. Risk of myocarditis after sequential doses of COVID-19 vaccine and SARS-CoV-2 infection by age and sex. Circulation 2022, 146, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Oster, M.E.; Shay, D.K.; Su, J.R.; Gee, J.; Creech, C.B.; Broder, K.R.; Edwards, K.; Soslow, J.H.; Dendy, J.M.; Schlaudecker, E.; et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. J. Am. Med. Assoc. 2022, 327, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Kracalik, I.; Oster, M.E.; Broder, K.R.; Cortese, M.M.; Glover, M.; Shields, K.; Creech, C.B.; Romanson, B.; Novosad, S.; Soslow, J.; et al. Outcomes at least 90 days since onset of myocarditis after mRNA COVID-19 vaccination in adolescents and young adults in the USA: A follow-up surveillance study. Lancet Child. Adolesc. Health 2022, 6, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, J.; Ryan, M.; Engler, R.; Hoffman, D.; McClenathan, B.; Collins, L.; Loran, D.; Hrncir, D.; Herring, K.; Platzer, M.; et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol. 2021, 6, 1202–1206. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B.; Kamat, I.; Hotez, P.J. Myocarditis with COVID-19 mRNA vaccines. Circulation 2021, 144, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Pillay, J.; Gaudet, L.; Wingert, A.; Bial, L.; Mackie, A.S.; Paterson, D.I.; Hartling, L. Incidence, risk factors, natural history, and hypothesised mechanisms of myocarditis and pericarditis following COVID-19 vaccination: Living evidence syntheses and review. Br. Med. J. 2022, 378, e069445. [Google Scholar] [CrossRef] [PubMed]
- Tamimoto, R.; Fujii, T.; Nagamatsu, H.; Murakami, T.; Miyazaki, K.; Goto, S.; Cho, Y.; Mori, H. Sub-acute effects of vaccination with a messenger RNA-based vaccine against coronavirus disease 2019 on elderly Japanese patients with cardiac disorder. Sys. Rev. Pharm. 2021, 13, 631–635. [Google Scholar]
- Rus, K.R.; Korva, M.; Knap, N.; Županc, T.A.; Poljak, M. Performance of the rapid high-throughput automated electrochemiluminescence immunoassay targeting total antibodies to the SARS-CoV-2 spike protein receptor binding domain in comparison to the neutralization assay. J. Clin. Virol. 2021, 139, 104820. [Google Scholar]
- Murphy, W.J.; Longo, D.L. A possible role for anti-idiotype antibodies in SARS-CoV-2 infection and vaccination. N. Engl. J. Med. 2022, 386, 394–396. [Google Scholar] [CrossRef] [PubMed]
- Paque, R.E.; Miller, R.U. Autoanti-idiotypes exhibit mimicry of myocyte antigens in virus-induced myocarditis. J. Virol. 1991, 6, 16–22. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Baseline | Period 1 † | Period 2 †† | Period 3 ††† |
|---|---|---|---|---|
| Age, years (range) | 83.5 (79.8–88.5) | |||
| Males, n (%) | 32 (42.1%) | |||
| Height, cm (range) | 155.0 (145.0–162.3) | |||
| Weight, kg (range) | 48.5 (42–59) | |||
| Body mass index (range) | 21.1 (18.6–23.3) | |||
| Hypertension, n (%) | 43 (57%) | |||
| Dyslipidemia, n (%) | 41 (54%) | |||
| Diabetes mellitus, n (%) | 25 (33%) | |||
| Old myocardial infarction, n (%) | 11 (14%) | |||
| Valvular heart disease, n (%) | 24 (32%) | |||
| Hypertensive heart disease, n (%) | 56 (74%) | |||
| Anticoagulation, n (%) | 26 (34%) | |||
| Warfarin, n (%) | 14 (18%) | |||
| Direct oral anticoagulants, n (%) | 12 (16%) | |||
| Serum creatinine, mg/dL (range), missing n | 0.9 (0.7–1.1), 0 | 0.9 (0.7–1.1), 12 NS | 0.9 (0.7–1.1), 5 NS | 0.9 (0.7–1.1), 6 NS |
| Urea nitrogen, mg/mL (range), missing n | 19 (16–24), 2 | 21 (16–25), 13 NS | 22 (17–27), 5 NS | 20 (17–26), 6 NS |
| Aspartate aminotransferase, IU/L (range), missing n | 21 (17–26), 4 | 22 (17–27), 14 NS | 22 (18–26), 7 NS | 21 (17–26), 7 NS |
| Alanine aminotransferase, IU/L (range), missing n | 14 (11–20), 4 | 15 (11–22), 14 NS | 16 (11–22), 7 NS | 16 (12–19), 7 NS |
| Haemoglobin A1c, % (range), missing n | 5.7 (5.4–6.2), 11 | 5.8 (5.3–6.1), 22 **, NS | 5.6 (5.3–5.9), 14 NS | 5.7 (5.3–6.0), 13 NS |
| Troponin T, ng/mL (range), missing n | 0.019 (0.010–0.033), 26 | 0.025 (0.010–0.037), 28 | 0.020 (0.012–0.035), 19 | 0.023 (0.013–0.037), 22 ¶¶1 |
| C-reactive protein, mg/dL (range), missing n | 0.071 (0.028–0.407), 24 | 0.126 (0.041–0.377), 18 | 0.150 (0.040–0.455), 13 | 0.129 (0.041–0.695), 11 ¶1, ¶2 |
| BNP ratio (range), n (missing n) | 4.5 (1.1–15.3), 76 (0) | 9.8 (2.7–19.1), 76 (16) **, NS | 6.4 (1.6–18.0), 76 (7) NS | 6.1 (1.4–17.1), 68 (2) NS |
| pre-BNP § < 4 (range), n (missing n) | 1.1 (0.8–2.4), 35 (0) | 1.8 (1.2–2.9), 35 (14) **, NS | 1.6 (1.0–2.8), 35 (3) NS | 1.5 (0.9–3.1), 33 (0) NS |
| pre-BNP > 4 (range), n (missing n) | 9.5 (6.3–19.4), 41 (0) | 14.8 (9.5–29.5), 41 (2) **, NS | 16.2 (8.6–32.1), 41 (4) NS | 16.0 (9.0–21.0), 35 (2) NS |
| Death, n/patients, n (%) | 0/76 (0%) | 5/76 (7%) | 5/68 (7%) | |
| Changes in BNP ratio > 10 compared with baseline (%) | 9/76 (12%) | 13/76 (17%) | 8/68 (12%) |
| Crude Hazard Ratio | pValue | |
| pre-BNP | 1.021 (1.006–1.037) | 0.007 |
| Neutralizing antibodies | 0.245 (0.083–0.726) | 0.011 |
| AdjustedHazard Ratio | pValue | |
| pre-BNP | 1.032 (1.010–1.055) | 0.005 |
| Neutralizing antibodies | 0.155 (0.041–0.587) | 0.006 |
| Age | 1.022 (0.957–1.091) | 0.51 |
| Male sex | 0.597 (0.195–1.824) | 0.37 |
| Body mass index | 1.069 (0.926–1.234) | 0.36 |
| Serum creatinine | 0.344 (0.066–1.802) | 0.21 |
| Anticoagulation therapy | 3.246 (1.106–9.529) | 0.032 |
| A | |||||||||
| (BNP ratio in Period 1–2 minus pre-BNP ratio) < 10 | (BNP ratio in Period 1–2 minus pre-BNP ratio) > 10 | p value | |||||||
| Neutralizing antibodies < 220 U/mL | 30 | 14 | 0.03 | ||||||
| Neutralizing antibodies > 220 U/mL | 29 | 3 | |||||||
| B | C | ||||||||
| pre-BNP ratio < 4.0 | pre-BNP ratio > 4.0 | p value | BNP ratio in Period 1–2 † < 10 | BNP ratio in Period 1–2 >10 | p value | ||||
| Neutralizing antibodies < 220 U/mL | 18 | 26 | 0.35 | Neutralizing antibodies < 220 U/mL | 23 | 21 | 0.17 | ||
| Neutralizing antibodies > 220 U/mL | 17 | 15 | Neutralizing antibodies > 220 U/mL | 22 | 10 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyazaki, K.; Fujii, T.; Mori, K.; Tamimoto, R.; Nagamatsu, H.; Murakami, T.; Cho, Y.; Goto, S.; Mori, H. Sustained Myocarditis following Messenger RNA Vaccination against Coronavirus Disease 2019: Relation to Neutralizing Antibody and Amelioration by Low-Dose Booster Vaccination. J. Clin. Med. 2023, 12, 1421. https://doi.org/10.3390/jcm12041421
Miyazaki K, Fujii T, Mori K, Tamimoto R, Nagamatsu H, Murakami T, Cho Y, Goto S, Mori H. Sustained Myocarditis following Messenger RNA Vaccination against Coronavirus Disease 2019: Relation to Neutralizing Antibody and Amelioration by Low-Dose Booster Vaccination. Journal of Clinical Medicine. 2023; 12(4):1421. https://doi.org/10.3390/jcm12041421
Chicago/Turabian StyleMiyazaki, Koji, Toshiharu Fujii, Kikue Mori, Ryuichi Tamimoto, Hirofumi Nagamatsu, Tsutomu Murakami, Yasunori Cho, Shinya Goto, and Hidezo Mori. 2023. "Sustained Myocarditis following Messenger RNA Vaccination against Coronavirus Disease 2019: Relation to Neutralizing Antibody and Amelioration by Low-Dose Booster Vaccination" Journal of Clinical Medicine 12, no. 4: 1421. https://doi.org/10.3390/jcm12041421
APA StyleMiyazaki, K., Fujii, T., Mori, K., Tamimoto, R., Nagamatsu, H., Murakami, T., Cho, Y., Goto, S., & Mori, H. (2023). Sustained Myocarditis following Messenger RNA Vaccination against Coronavirus Disease 2019: Relation to Neutralizing Antibody and Amelioration by Low-Dose Booster Vaccination. Journal of Clinical Medicine, 12(4), 1421. https://doi.org/10.3390/jcm12041421






