Infectious Pneumonia and Lung Ultrasound: A Review
Abstract
1. Introduction
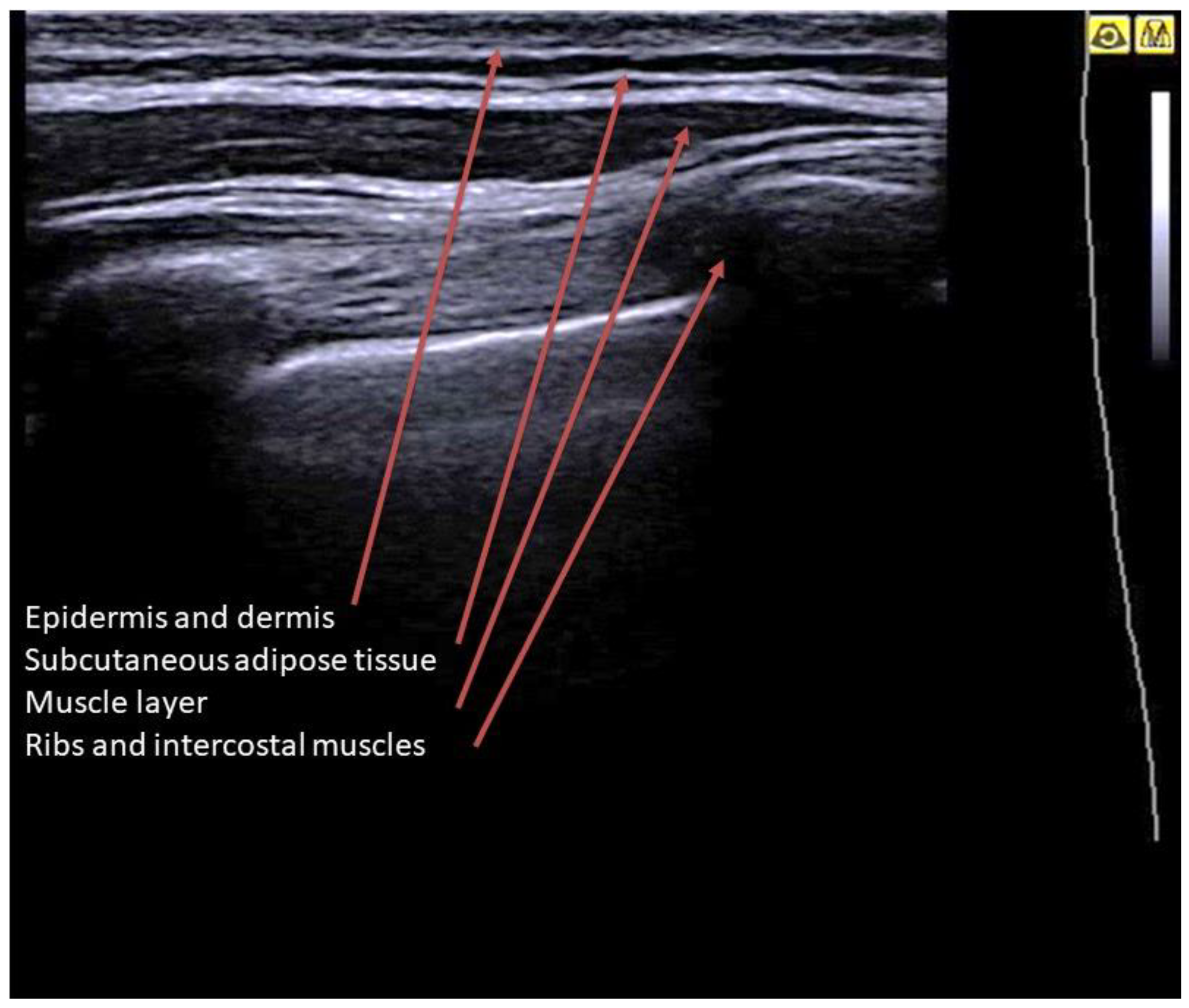
2. Basic Lung Ultrasound
3. Lung Ultrasound Evaluation
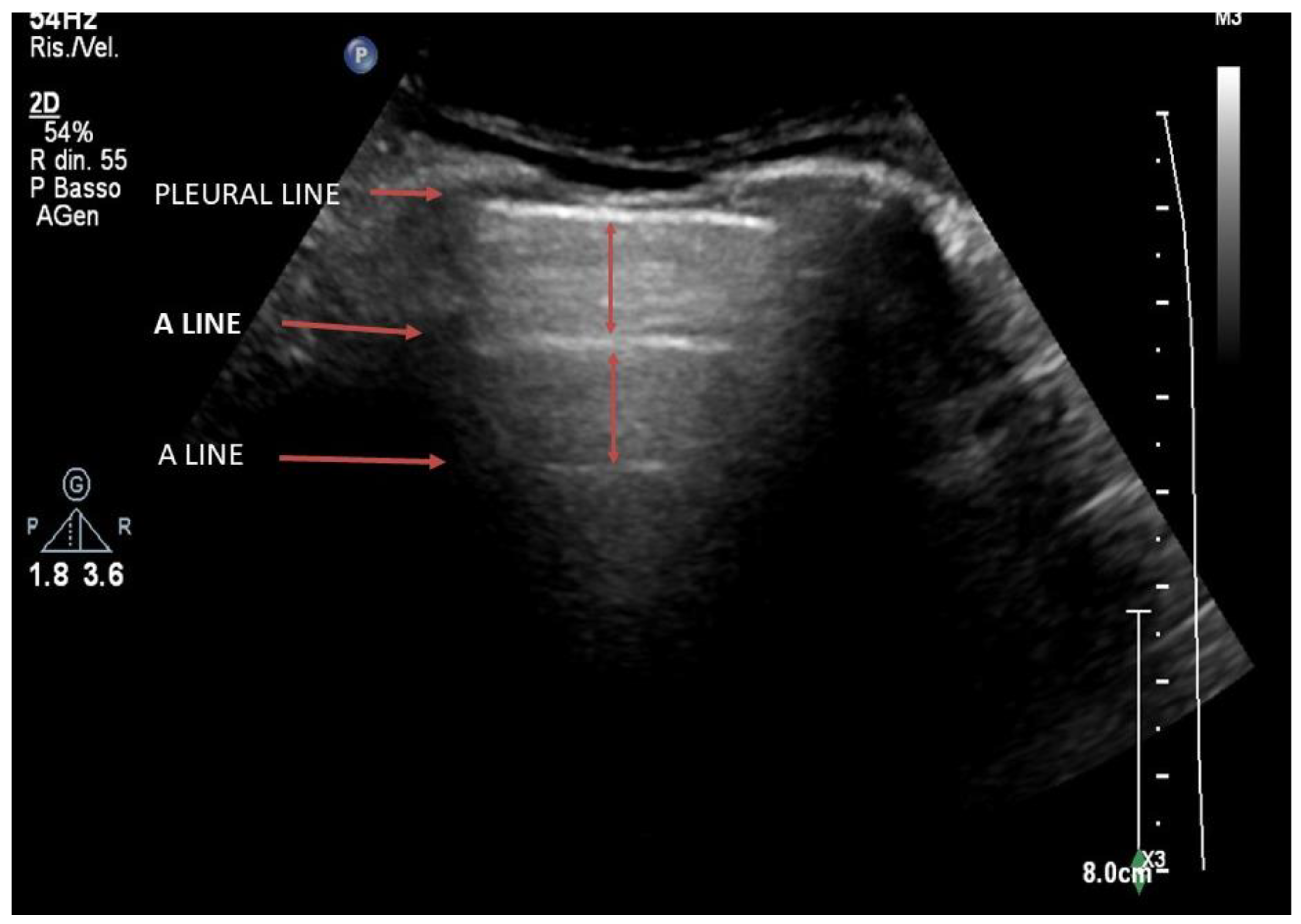
4. Lung Ultrasound on Physiological Lung
5. From the B-Lines to the Pathological Lung
5.1. Interstitial Syndrome
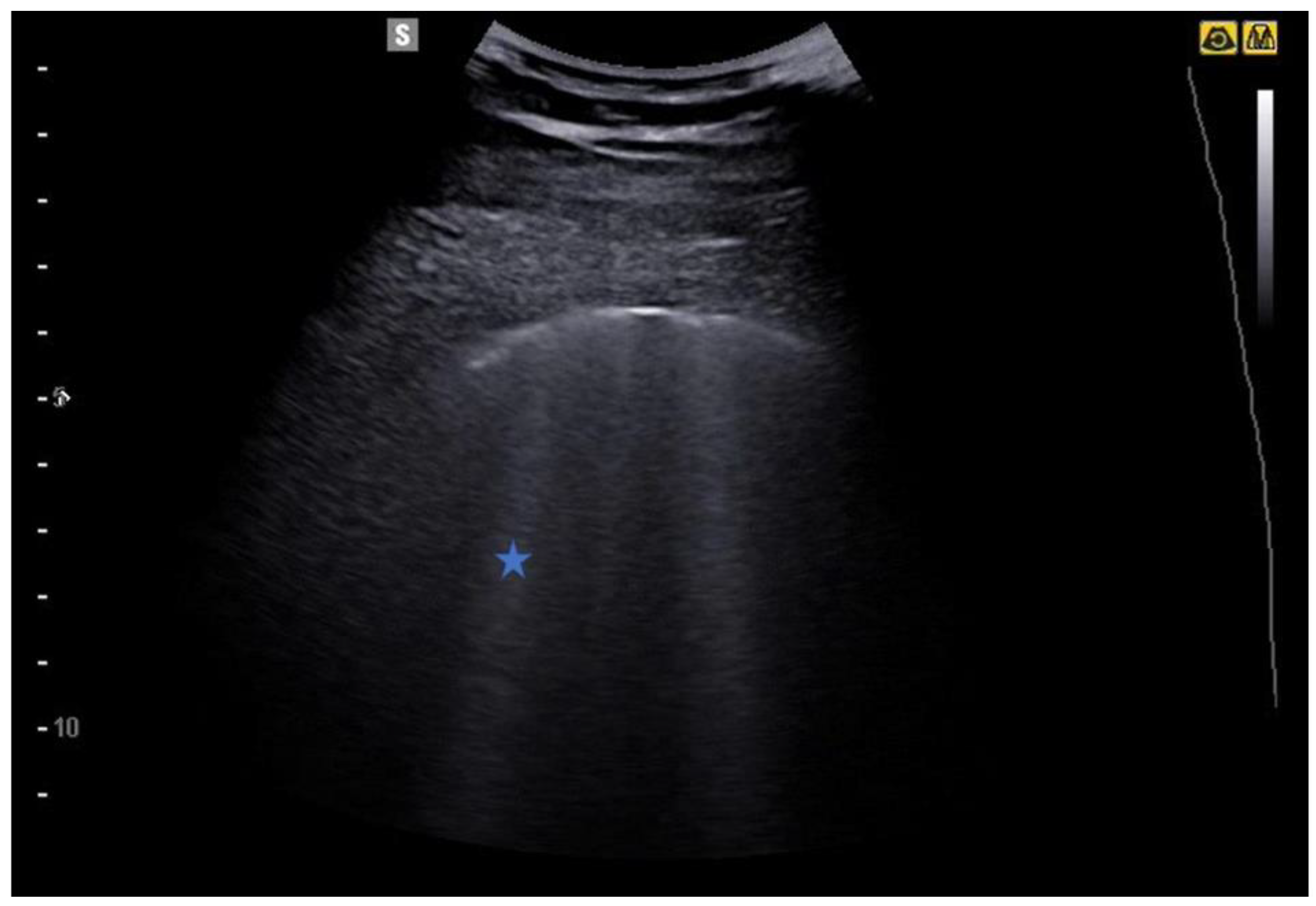
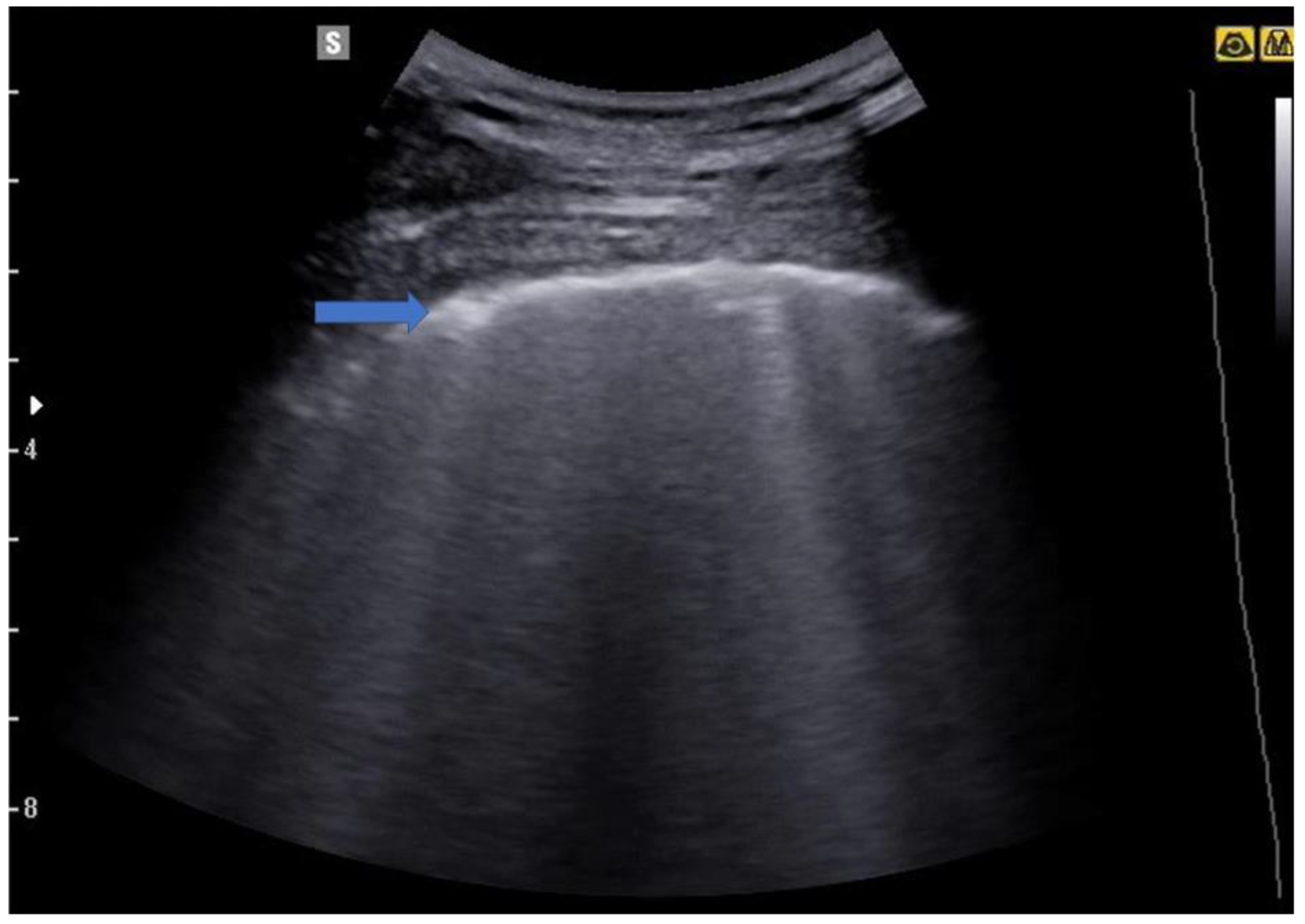
5.2. Lung Consolidations
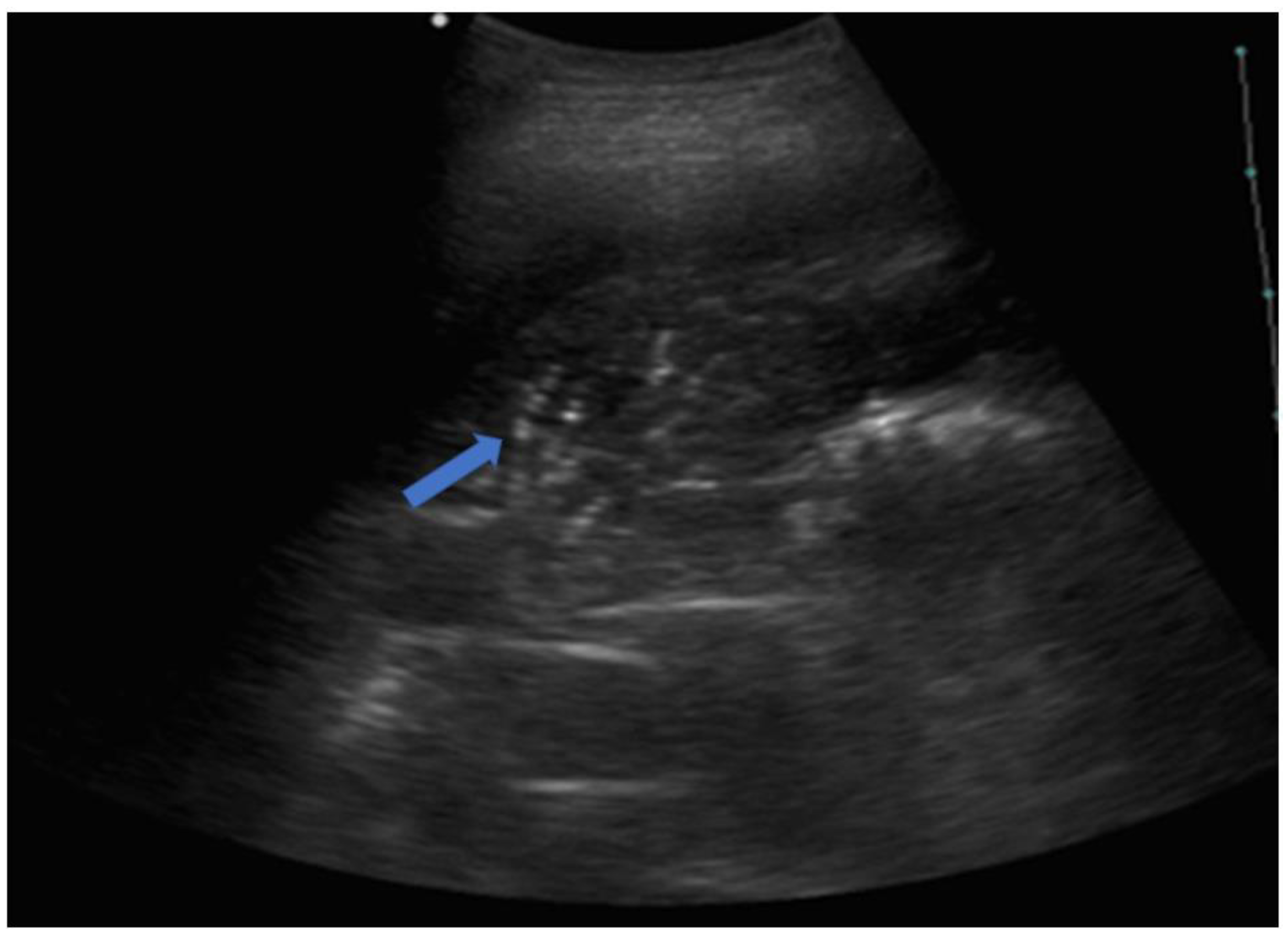
6. Pneumonia
7. Lung Abscess
8. Pulmonary Tuberculosis
9. Lung Ultrasound in COVID-19 Pneumonia
10. Fields of Application of Lung Ultrasound
11. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
Abbreviations
| ICU | Intensive care unit |
| PLAPS | Posterolateral and/or pleural alveolar syndrome |
| COPD | Chronic obstructive pulmonary disease |
| ARDS | Acute respiratory distress syndrome |
| CAP | Community-acquired pneumonia |
| HCAP | Healthcare-associated pneumonia |
| AIDS | Acquired Immuno Deficiency Syndrome |
| CEUS | Contrast-enhanced ultrasound |
| HRCT | High-resolution computed tomography |
References
- Gargani, L.; Volpicelli, G. How I do it: Lung ultrasound. Cardiovasc. Ultrasound 2014, 12, 25. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, D.A.; Meziere, G.A. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: The BLUE protocol. Chest 2008, 134, 117–125. [Google Scholar] [CrossRef]
- Lichtenstein, D.A. Lung ultrasound in the critically ill. Ann. Intensive Care 2014, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, D.; Meziere, G. A lung ultrasound sign allowing bedside distinction between pulmonary edema and COPD: The comet-tail artifact. Intensive Care Med. 1998, 24, 1331–1334. [Google Scholar] [CrossRef]
- Lichtenstein, D.A.; Menu, Y. A bedside ultrasound sign ruling out pneumothorax in the critically ill. Lung sliding. Chest 1995, 108, 1345–1348. [Google Scholar] [CrossRef] [PubMed]
- Parlamento, S.; Copetti, R.; Di Bartolomeo, S. Evaluation of lung ultrasound for the diagnosis of pneumonia in the ED. Am. J. Emerg. Med. 2009, 27, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Boccatonda, A. Emergency Ultrasound: Is It Time for Artificial Intelligence? J. Clin. Med. 2022, 11, 3823. [Google Scholar] [CrossRef] [PubMed]
- Sperandeo, M.; Carnevale, V.; Muscarella, S.; Sperandeo, G.; Varriale, A.; Filabozzi, P.; Piattelli, M.L.; D’Alessandro, V.; Copetti, M.; Pellegrini, F.; et al. Clinical application of transthoracic ultrasonography in inpatients with pneumonia. Eur. J. Clin. Investig. 2011, 41, 1–7. [Google Scholar] [CrossRef]
- Reissig, A.; Kroegel, C. Sonographic diagnosis and follow-up of pneumonia: A prospective study. Respir. Int. Rev. Thorac. Dis. 2007, 74, 537–547. [Google Scholar] [CrossRef]
- Reissig, A.; Gramegna, A.; Aliberti, S. The role of lung ultrasound in the diagnosis and follow-up of community-acquired pneumonia. Eur. J. Intern. Med. 2012, 23, 391–397. [Google Scholar] [CrossRef]
- Reissig, A.; Copetti, R. Lung ultrasound in community-acquired pneumonia and in interstitial lung diseases. Respiration 2014, 87, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Volpicelli, G.; Lamorte, A.; Villén, T. What’s new in lung ultrasound during the COVID-19 pandemic. Intensive Care Med. 2020, 46, 1445–1448. [Google Scholar] [CrossRef] [PubMed]
- Volpicelli, G.; Gargani, L. Sonographic signs and patterns of COVID-19 pneumonia. Ultrasound J. 2020, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Soldati, G.; Smargiassi, A. On Lung Ultrasound Patterns Specificity in the Management of COVID-19 Patients. J. Ultrasound Med. 2020, 39, 2283–2284. [Google Scholar] [CrossRef] [PubMed]
- Sofia, S.; Boccatonda, A.; Montanari, M.; Spampinato, M.; D’Ardes, D.; Cocco, G.; Accogli, E.; Cipollone, F.; Schiavone, C. Thoracic ultrasound and SARS-COVID-19: A pictorial essay. J. Ultrasound 2020, 23, 217–221. [Google Scholar] [CrossRef]
- Sofia, S. Bedside US imaging in multiple trauma patients. Part 1: US findings and techniques. J. Ultrasound 2013, 16, 147–159. [Google Scholar] [CrossRef]
- Mongodi, S.; Via, G.; Girard, M.; Rouquette, I.; Misset, B.; Braschi, A.; Mojoli, F.; Bouhemad, B. Lung Ultrasound for Early Diagnosis of Ventilator-Associated Pneumonia. Chest 2016, 149, 969–980. [Google Scholar] [CrossRef]
- Volpicelli, G.; Caramello, V.; Cardinale, L.; Mussa, A.; Bar, F.; Frascisco, M.F. Bedside ultrasound of the lung for the monitoring of acute decompensated heart failure. Am. J. Emerg. Med. 2008, 26, 585–591. [Google Scholar] [CrossRef]
- Mongodi, S.; Bouhemad, B.; Orlando, A.; Stella, A.; Tavazzi, G.; Via, G.; Iotti, G.A.; Braschi, A.; Mojoli, F. Modified Lung Ultrasound Score for Assessing and Monitoring Pulmonary Aeration. Ultraschall Der Med. 2017, 38, 530–537. [Google Scholar] [CrossRef]
- Boussuges, A.; Gole, Y.; Blanc, P. Diaphragmatic motion studied by m-mode ultrasonography: Methods, reproducibility, and normal values. Chest 2009, 135, 391–400. [Google Scholar] [CrossRef]
- Koh, D.M.; Burke, S.; Davies, N.; Padley, S.P. Transthoracic US of the Chest: Clinical Uses and Applications; Radiographics: A review publication of the Radiological Society of North America, Inc.; RSNA: Oak Brook, IL, USA, 2002; Volume 22, p. e1. [Google Scholar]
- Volpicelli, G.; Elbarbary, M.; Blaivas, M.; Lichtenstein, D.A.; Mathis, G.; Kirkpatrick, A.W.; Melniker, L.; Gargani, L.; Noble, V.E.; Via, G.; et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012, 38, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Mayo, P.H.; Copetti, R.; Feller-Kopman, D.; Mathis, G.; Maury, E.; Mongodi, S.; Mojoli, F.; Volpicelli, G.; Zanobetti, M. Thoracic ultrasonography: A narrative review. Intensive Care Med. 2019, 45, 1200–1211. [Google Scholar] [CrossRef] [PubMed]
- Volpicelli, G.; Melniker, L.A.; Cardinale, L.; Lamorte, A.; Frascisco, M.F. Lung ultrasound in diagnosing and monitoring pulmonary interstitial fluid. La Radiol. Med. 2013, 118, 196–205. [Google Scholar] [CrossRef]
- Soldati, G.; Demi, M. The use of lung ultrasound images for the differential diagnosis of pulmonary and cardiac interstitial pathology. J. Ultrasound 2017, 20, 91–96. [Google Scholar] [CrossRef]
- Lichtenstein, D.; Meziere, G.; Biderman, P.; Gepner, A.; Barre, O. The comet-tail artifact. An ultrasound sign of alveolar-interstitial syndrome. Am. J. Respir. Crit. Care Med. 1997, 156, 1640–1646. [Google Scholar] [CrossRef]
- Wang, Y.; Gargani, L.; Barskova, T.; Furst, D.E.; Cerinic, M.M. Usefulness of lung ultrasound B-lines in connective tissue disease-associated interstitial lung disease: A literature review. Arthritis Res. Ther. 2017, 19, 206. [Google Scholar] [CrossRef] [PubMed]
- Tardella, M.; Di Carlo, M.; Carotti, M.; Filippucci, E.; Grassi, W.; Salaffi, F. Ultrasound B-lines in the evaluation of interstitial lung disease in patients with systemic sclerosis: Cut-off point definition for the presence of significant pulmonary fibrosis. Medicine 2018, 97, e0566. [Google Scholar] [CrossRef]
- Song, G.; Bae, S.C.; Lee, Y.H. Diagnostic accuracy of lung ultrasound for interstitial lung disease in patients with connective tissue diseases: A meta-analysis. Clin. Exp. Rheumatol. 2016, 34, 11–16. [Google Scholar]
- Olson, A.L.; Swigris, J.J.; Sprunger, D.B.; Fischer, A.; Fernandez-Perez, E.R.; Solomon, J.; Murphy, J.; Cohen, M.; Raghu, G.; Brown, K.K. Rheumatoid arthritis-interstitial lung disease-associated mortality. Am. J. Respir. Crit. Care Med. 2011, 183, 372–378. [Google Scholar] [CrossRef]
- Moazedi-Fuerst, F.C.; Kielhauser, S.M.; Scheidl, S.; Tripolt, N.; Lutfi, A.; Yazdani-Biuki, B.; Dejaco, C.; Graninger, W. Ultrasound screening for interstitial lung disease in rheumatoid arthritis. Clin. Exp. Rheumatol. 2014, 32, 199–203. [Google Scholar]
- Moazedi-Fuerst, F.C.; Kielhauser, S.; Brickmann, K.; Tripolt, N.; Meilinger, M.; Lufti, A.; Graninger, W. Sonographic assessment of interstitial lung disease in patients with rheumatoid arthritis, systemic sclerosis and systemic lupus erythematosus. Clin. Exp. Rheumatol. 2015, 33 (Suppl. S91), S87–S91. [Google Scholar] [PubMed]
- Martinez, F.J.; Flaherty, K. Pulmonary function testing in idiopathic interstitial pneumonias. Proc. Am. Thorac. Soc. 2006, 3, 315–321. [Google Scholar] [CrossRef]
- Ma, H.; Yan, W.; Liu, J. Diagnostic value of lung ultrasound for neonatal respiratory distress syndrome: A meta-analysis and systematic review. Med. Ultrason. 2020, 22, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Levitus, M.; Eisen, L.; Shiloh, A.L.; Fein, D. Lung Ultrasound for the Diagnosis and Management of Acute Respiratory Failure. Lung 2020, 198, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bello, G.; Blanco, P. Lung Ultrasonography for Assessing Lung Aeration in Acute Respiratory Distress Syndrome: A Narrative Review. J. Ultrasound Med. 2019, 38, 27–37. [Google Scholar] [CrossRef]
- Mathis, G. Thoraxsonography—Part II: Peripheral pulmonary consolidation. Ultrasound Med. Biol. 1997, 23, 1141–1153. [Google Scholar] [CrossRef]
- Reissig, A.; Kroegel, C. Relevance of subpleural consolidations in chest ultrasound. Chest 2009, 136, 1706. [Google Scholar] [CrossRef]
- Musher, D.M.; Thorner, A.R. Community-acquired pneumonia. N. Engl. J. Med. 2014, 371, 1619–1628. [Google Scholar] [CrossRef]
- American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 2005, 171, 388–416. [CrossRef]
- Jain, S.; Self, W.H.; Wunderink, R.G.; Fakhran, S.; Balk, R.; Bramley, A.M.; Chappell, J.D. Community-Acquired Pneumonia Requiring Hospitalization among U.S. Adults. N. Engl. J. Med. 2015, 373, 415–427. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Herold, C.J.; Sailer, J.G. Community-acquired and nosocomial pneumonia. Eur. Radiol. 2004, 14 (Suppl. S3), E2–E20. [Google Scholar] [CrossRef] [PubMed]
- Copetti, R.; Cattarossi, L. Ultrasound diagnosis of pneumonia in children. Radiol. Med. 2008, 113, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Gehmacher, O.; Mathis, G.; Kopf, A.; Scheier, M. Ultrasound imaging of pneumonia. Ultrasound Med. Biol. 1995, 21, 1119–1122. [Google Scholar] [CrossRef]
- Lichtenstein, D. Should lung ultrasonography be more widely used in the assessment of acute respiratory disease? Expert Rev. Respir. Med. 2010, 4, 533–538. [Google Scholar] [CrossRef]
- Weinberg, B.; Diakoumakis, E.E.; Kass, E.G.; Seife, B.; Zvi, Z.B. The air bronchogram: Sonographic demonstration. AJR Am. J. Roentgenol. 1986, 147, 593–595. [Google Scholar] [CrossRef]
- Lichtenstein, D.; Mezière, G.; Seitz, J. The dynamic air bronchogram. A lung ultrasound sign of alveolar consolidation ruling out atelectasis. Chest 2009, 135, 1421–1425. [Google Scholar] [CrossRef]
- Lichtenstein, D.A.; Lascols, N.; Mezière, G.; Gepner, A. Ultrasound diagnosis of alveolar consolidation in the critically ill. Intensive Care Med. 2004, 30, 276–281. [Google Scholar] [CrossRef]
- Targhetta, R.; Chavagneux, R.; Bourgeois, J.M.; Dauzat, M.; Balmes, P.; Pourcelot, L. Sonographic approach to diagnosing pulmonary consolidation. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 1992, 11, 667–672. [Google Scholar] [CrossRef]
- Reissig, A.; Copetti, R.; Mathis, G.; Mempel, C.; Schuler, A.; Zechner, P.; Aliberti, S.; Neumann, R.; Kroegel, C.; Hoyer, H. Lung ultrasound in the diagnosis and follow-up of community-acquired pneumonia: A prospective, multicenter, diagnostic accuracy study. Chest 2012, 142, 965–972. [Google Scholar] [CrossRef]
- Chavez, M.A.; Shams, N.; Ellington, L.E.; Naithani, N.; Gilman, R.H.; Steinhoff, M.C.; Santosham, M.; Black, R.E.; Price, C.; Gross, M.; et al. Lung ultrasound for the diagnosis of pneumonia in adults: A systematic review and meta-analysis. Respir. Res. 2014, 15, 50. [Google Scholar] [CrossRef] [PubMed]
- Cortellaro, F.; Colombo, S.; Coen, D.; Duca, P.G. Lung ultrasound is an accurate diagnostic tool for the diagnosis of pneumonia in the emergency department. Emerg. Med. J. EMJ 2012, 29, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Tsung, J.W.; Kessler, D.O.; Shah, V.P. Prospective application of clinician-performed lung ultrasonography during the 2009 H1N1 influenza A pandemic: Distinguishing viral from bacterial pneumonia. Crit. Ultrasound J. 2012, 4, 16. [Google Scholar] [CrossRef]
- Testa, A.; Soldati, G.; Copetti, R.; Giannuzzi, R.; Portale, G.; Gentiloni-Silveri, N. Early recognition of the 2009 pandemic influenza A (H1N1) pneumonia by chest ultrasound. Crit. Care 2012, 16, R30. [Google Scholar] [CrossRef] [PubMed]
- Deganello, A.; Rafailidis, V.; Sellars, M.E.; Ntoulia, A.; Kalogerakou, K.; Ruiz, G.; Cosgrove, D.O.; Sidhu, P.S. Intravenous and Intracavitary Use of Contrast-Enhanced Ultrasound in the Evaluation and Management of Complicated Pediatric Pneumonia. J. Ultrasound Med. 2017, 36, 1943–1954. [Google Scholar] [CrossRef]
- Linde, H.N.; Holland, A.; Greene, B.H.; Görg, C. Contrast-enhancend sonography (CEUS) in pneumonia: Typical patterns and clinical value—A retrospective study on n = 50 patients. Ultraschall Der Med. 2012, 33, 146–151. [Google Scholar] [CrossRef]
- Tang, M.; Xie, Q.; Wang, J.; Zhai, X.; Lin, H.; Zheng, X.; Wei, G.; Tang, Y.; Zeng, F.; Chu, Y.; et al. Time Difference of Arrival on Contrast-Enhanced Ultrasound in Distinguishing Benign Inflammation From Malignant Peripheral Pulmonary Lesions. Front. Oncol. 2020, 10, 578884. [Google Scholar] [CrossRef]
- Boccatonda, A.; Susca, V.; Primomo, G.L.; Cocco, G.; Cinalli, S.; Di Resta, V.; Martino, L.; Mucilli, F.; Marinari, S.; Cipollone, F.; et al. Role of shear-wave and strain elastography to differentiate malignant vs benign subpleural lung lesions. Medicine 2021, 100, e24123. [Google Scholar] [CrossRef]
- Lewis, A.D.; Bridwell, M.R.; Hambuchen, M.D.; Clay, T.B.; Orwig, K.W. Correlation of MRSA polymerase chain reaction (PCR) nasal swab in ventilator-associated pneumonia, lung abscess, and empyema. Diagn. Microbiol. Infect. Dis. 2023, 105, 115836. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Hirche, T.O.; Schreiber, D.; Wagner, T.O. Sonographie von pleura und lunge. Ultraschall Der Med. 2003, 24, 303–311. [Google Scholar] [CrossRef]
- Gorg, C.; Bert, T. Transcutaneous colour Doppler sonography of lung consolidations: Review and pictorial essay. Part 2: Colour Doppler sonographic patterns of pulmonary consolidations. Ultraschall Der Med. 2004, 25, 285–291. [Google Scholar] [CrossRef]
- Piscaglia, F.; Nolsøe, C.; Dietrich, C.A.; Cosgrove, D.O.; Gilja, O.H.; Nielsen, M.B.; Albrecht, T.; Barozzi, L.; Bertolotto, M.; Catalano, O.; et al. The EFSUMB Guidelines and Recommendations on the Clinical Practice of Contrast Enhanced Ultrasound (CEUS): Update 2011 on non-hepatic applications. Ultraschall Der Med. 2012, 33, 33–59. [Google Scholar] [CrossRef] [PubMed]
- Cocco, G.; Boccatonda, A.; Rossi, I.; D’Ardes, D.; Corvino, A.; Delli Pizzi, A.; Ucciferri, C.; Katia, F.; Jacopo, V. Early detection of pleuro-pulmonary tuberculosis by bedside lung ultrasound: A case report and review of literature. Clin. Case Rep. 2022, 10, e05739. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, S.A.; Perera, P. Tuberculous pleural effusion. West. J. Emerg. Med. 2012, 13, 313–314. [Google Scholar] [CrossRef] [PubMed]
- Boccatonda, A.; Grignaschi, A.; Lanotte, A.M.G.; Cocco, G.; Vidili, G.; Giostra, F.; Schiavone, C. Role of Lung Ultrasound in the Management of Patients with Suspected SARS-CoV-2 Infection in the Emergency Department. J. Clin. Med. 2022, 11, 2067. [Google Scholar] [CrossRef]
- Xing, C.; Li, Q.; Du, H.; Kang, W.; Lian, J.; Yuan, L. Lung ultrasound findings in patients with COVID-19 pneumonia. Crit. Care 2020, 24, 174. [Google Scholar] [CrossRef]
- Guarracino, F.; Vetrugno, L.; Forfori, F.; Corradi, F.; Orso, D.; Bertini, P.; Ortalda, A.; Federici, N.; Copetti, R.; Bove, T. Lung, Heart, Vascular, and Diaphragm Ultrasound Examination of COVID-19 Patients: A Comprehensive Approach. J. Cardiothorac. Vasc. Anesth. 2020, 35, 1866–1874. [Google Scholar] [CrossRef]
- Volpicelli, G.; Gargani, L.; Perlini, S.; Spinelli, S.; Barbieri, G.; Lanotte, A.; Casasola, G.G.; Nogué-Bou, R.; Lamorte, A.; Agricola, E.; et al. Lung ultrasound for the early diagnosis of COVID-19 pneumonia: An international multicenter study. Intensive Care Med. 2021, 47, 444–454. [Google Scholar] [CrossRef]
- Pan, F.; Ye, T. Time Course of Lung Changes at Chest CT during Recovery from Coronavirus Disease 2019 (COVID-19). Radiology 2020, 295, 715–721. [Google Scholar] [CrossRef]
- Lomoro, P.; Verde, F.; Zerboni, F.; Simonetti, I.; Borghi, C.; Fachinetti, C.; Natalizi, A.; Martegani, A. COVID-19 pneumonia manifestations at the admission on chest ultrasound, radiographs, and CT: Single-center study and comprehensive radiologic literature review. Eur. J. Radiol. Open 2020, 7, 100231. [Google Scholar] [CrossRef]
- Boccatonda, A.; Primomo, G.; Cocco, G.; D’Ardes, D.; Marinari, S.; Montanari, M.; Giostra, F.; Schiavone, C. Not all abolished lung sliding are pneumothorax: The case of a particular lung atelectasis. J. Ultrasound 2020, 24, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Huang, Y.; Gao, F.; Yuan, L.; Wang, Z. Lung ultrasonography versus chest CT in COVID-19 pneumonia: A two-centered retrospective comparison study from China. Intensiv. Care Med. 2020, 46, 1761–1763. [Google Scholar] [CrossRef] [PubMed]
- Boccatonda, A.; Cocco, G.; Ianniello, E.; Montanari, M.; D’Ardes, D.; Borghi, C.; Giostra, F.; Copetti, R.; Schiavone, C. One year of SARS-CoV-2 and lung ultrasound: What has been learned and future perspectives. J. Ultrasound 2021, 24, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, D.A.; Mezière, G.; Lascols, N.; Biderman, P.; Courret, J.-P.; Gepner, A.; Goldstein, I.; Tenoudji-Cohen, M. Ultrasound diagnosis of occult pneumothorax. Crit. Care Med. 2005, 33, 1231–1238. [Google Scholar] [CrossRef]
- Boccatonda, A.; Cocco, G.; Sofia, S.; Accogli, E.; Vicari, S.; Schiavone, C. A New Type of Outpatient: Lung Ultrasound After COVID-19 Infection. J. Ultrasound Med. 2022, 41, 2113–2114. [Google Scholar] [CrossRef]
- Inchingolo, R.; Copetti, R.; Smargiassi, A.; Gerardi, R.E.; Conte, E.G.; Corbo, G.M.; Gatto, A.; Pierandrei, C.; Capossela, L.; Lazzareschi, I.; et al. Air bronchogram integrated lung ultrasound score to monitor community-acquired pneumonia in a pilot pediatric population. J. Ultrasound 2021, 24, 191–200. [Google Scholar] [CrossRef]
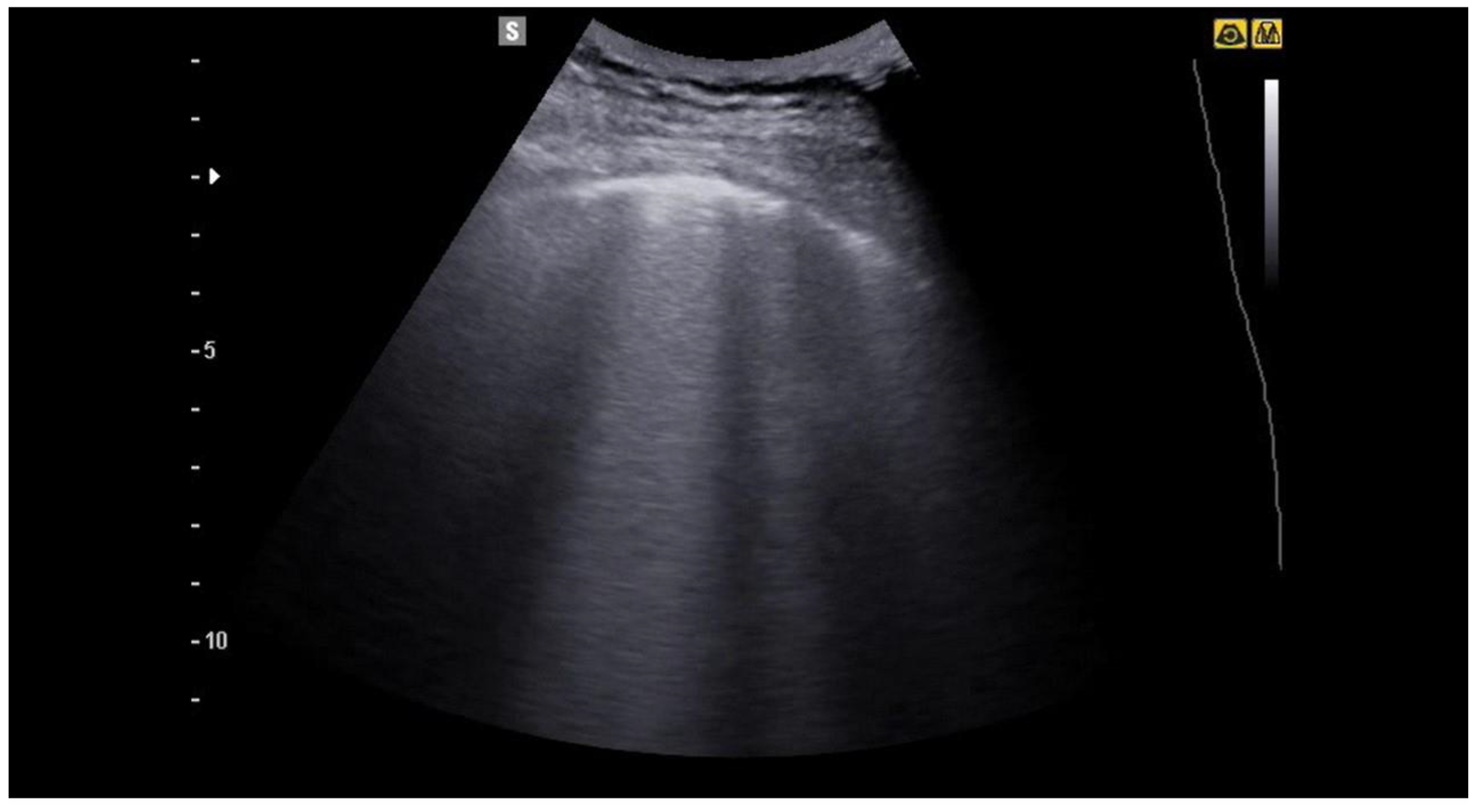
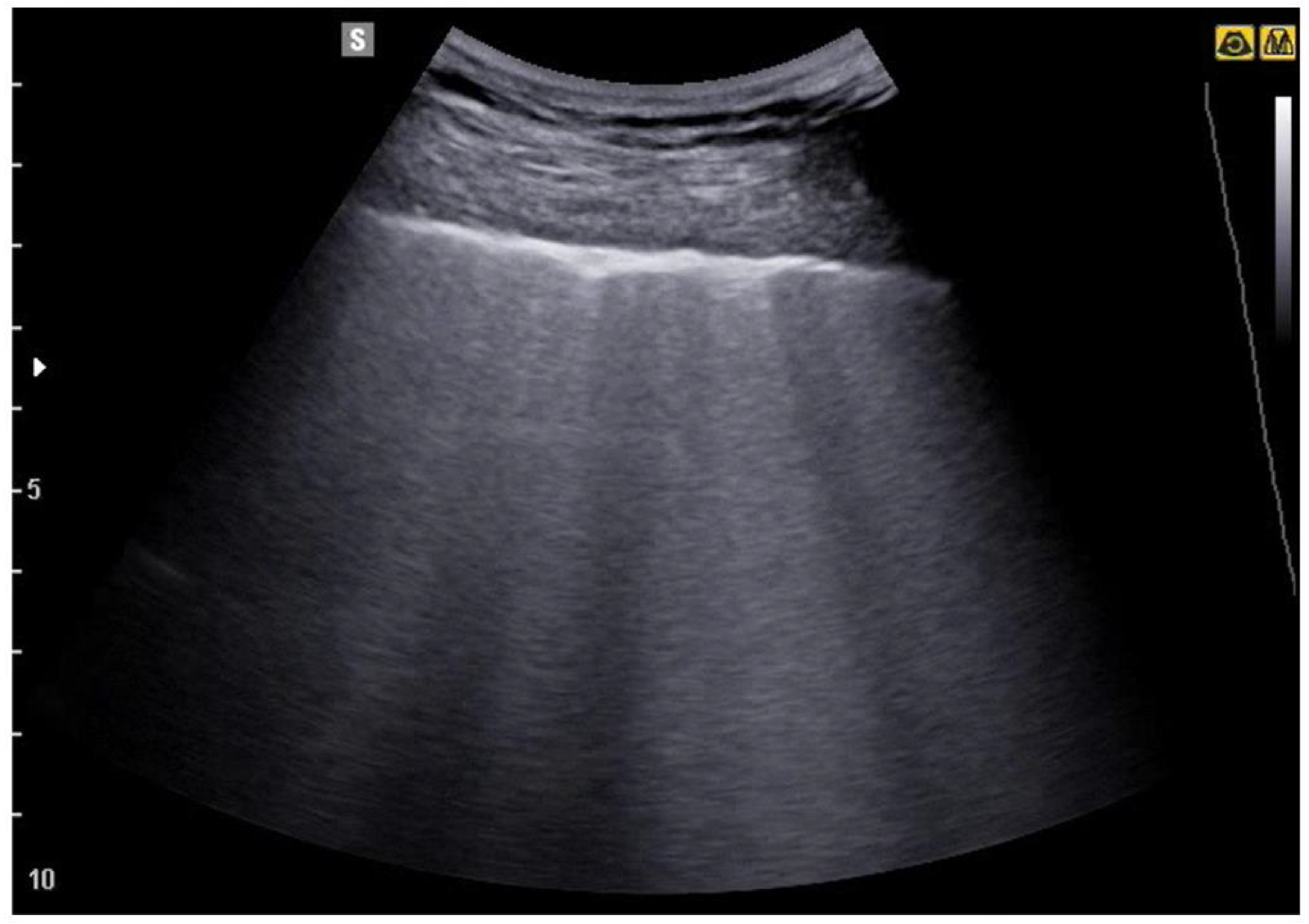

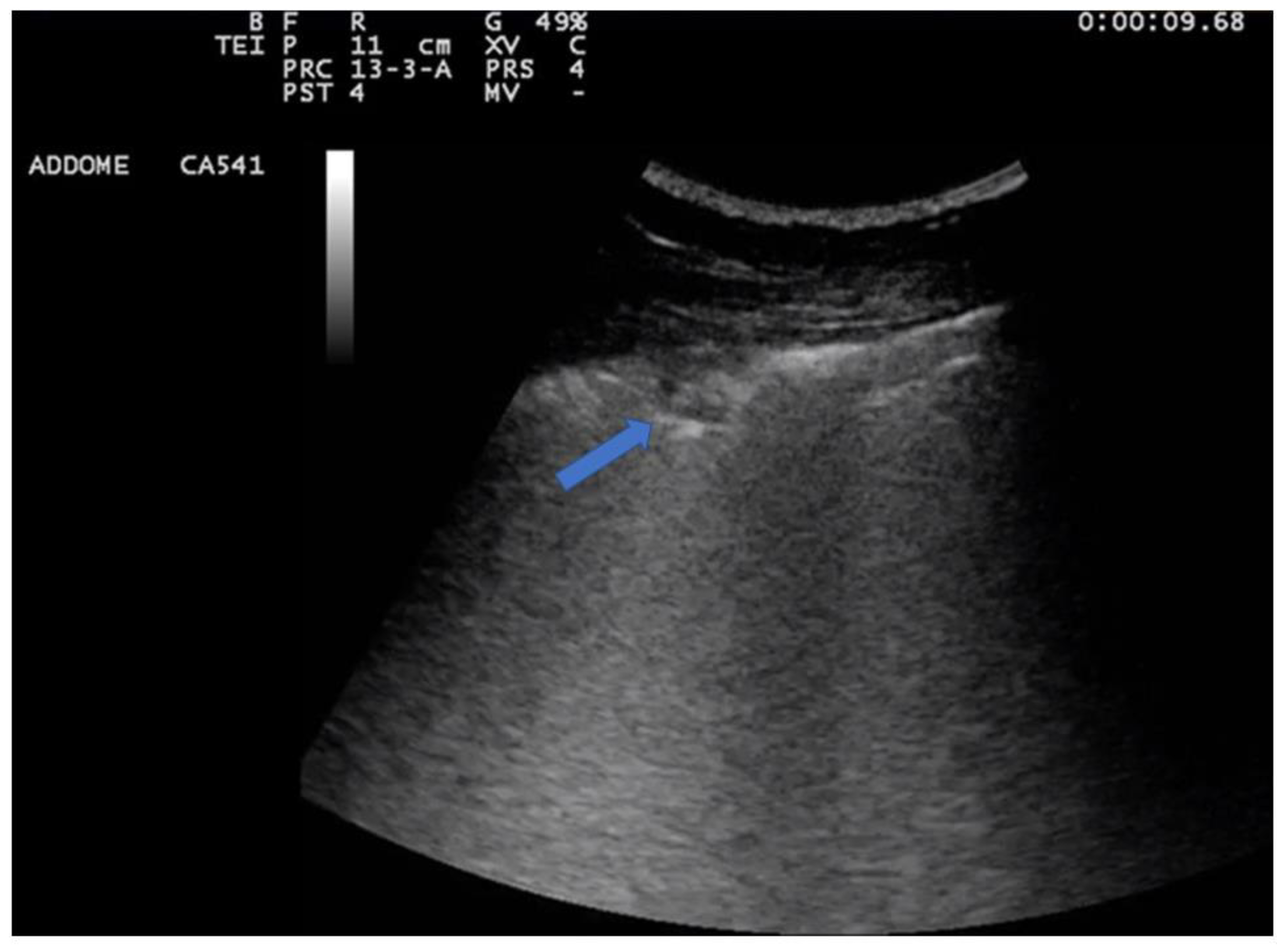
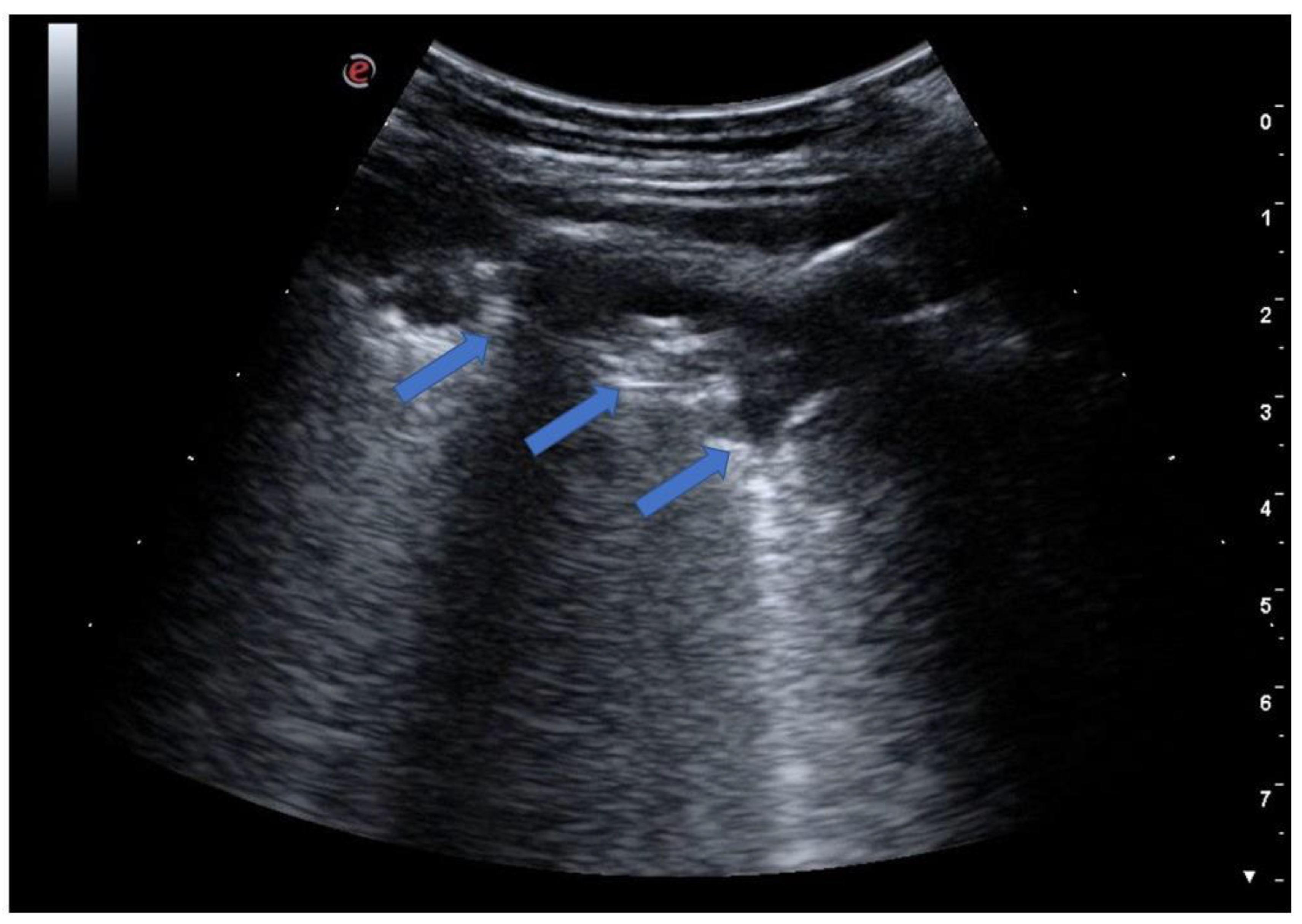
| Features | Complications | |
|---|---|---|
| Streptococcus pneumoniae | Lobar consolidation | Pleural effusion |
| Staphilococcus aureus | Multifocal consolidations | Abscess-empyema |
| Klebsiella pneumoniae | Upper lobes consolidations | Abscess |
| Pseudomonas aeruginosa | Multifocal consolidations | Abscess |
| Haemophilus influenzae, legionella pneumophila, moraxella catarralis, chlamidia pneumoniae, mycoplasma pneumoniae | Multifocal B-lines | Consolidation, pleural effusion |
| Anaerobes | Upper lobes consolidations | Abscess |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boccatonda, A.; Cocco, G.; D’Ardes, D.; Delli Pizzi, A.; Vidili, G.; De Molo, C.; Vicari, S.; Serra, C.; Cipollone, F.; Schiavone, C.; et al. Infectious Pneumonia and Lung Ultrasound: A Review. J. Clin. Med. 2023, 12, 1402. https://doi.org/10.3390/jcm12041402
Boccatonda A, Cocco G, D’Ardes D, Delli Pizzi A, Vidili G, De Molo C, Vicari S, Serra C, Cipollone F, Schiavone C, et al. Infectious Pneumonia and Lung Ultrasound: A Review. Journal of Clinical Medicine. 2023; 12(4):1402. https://doi.org/10.3390/jcm12041402
Chicago/Turabian StyleBoccatonda, Andrea, Giulio Cocco, Damiano D’Ardes, Andrea Delli Pizzi, Gianpaolo Vidili, Chiara De Molo, Susanna Vicari, Carla Serra, Francesco Cipollone, Cosima Schiavone, and et al. 2023. "Infectious Pneumonia and Lung Ultrasound: A Review" Journal of Clinical Medicine 12, no. 4: 1402. https://doi.org/10.3390/jcm12041402
APA StyleBoccatonda, A., Cocco, G., D’Ardes, D., Delli Pizzi, A., Vidili, G., De Molo, C., Vicari, S., Serra, C., Cipollone, F., Schiavone, C., & Guagnano, M. T. (2023). Infectious Pneumonia and Lung Ultrasound: A Review. Journal of Clinical Medicine, 12(4), 1402. https://doi.org/10.3390/jcm12041402









