Efficacy of Guided Tissue Regeneration Using Frozen Radiation-Sterilized Allogenic Bone Graft as Bone Replacement Graft Compared with Deproteinized Bovine Bone Mineral in the Treatment of Periodontal Intra-Bony Defects: Randomized Controlled Trial
Abstract
:1. Introduction
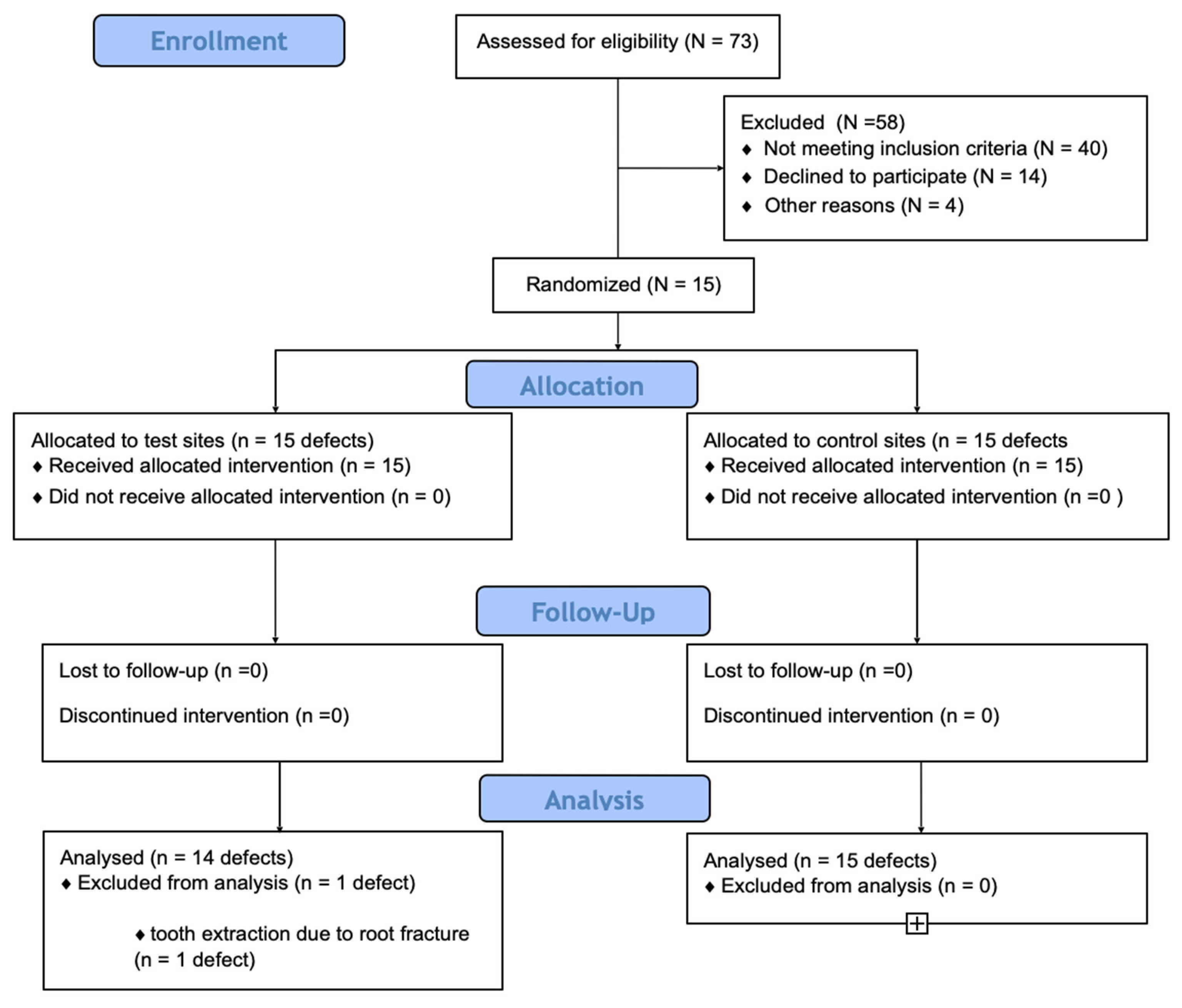
2. Materials and Methods
2.1. Study Design
2.2. Study Sample
2.3. Surgical Intervention
2.4. Clinical Outcomes
- Defect depth as the distance between the bottom of the defect and the most coronal point of the bony walls surrounding the defect;
- Defect width as the distance from the most coronal point of the bony walls surrounding the defect to the root surface;
- Defects were classified as one-wall, two-wall, and three-wall defects depending on the number of remaining walls.
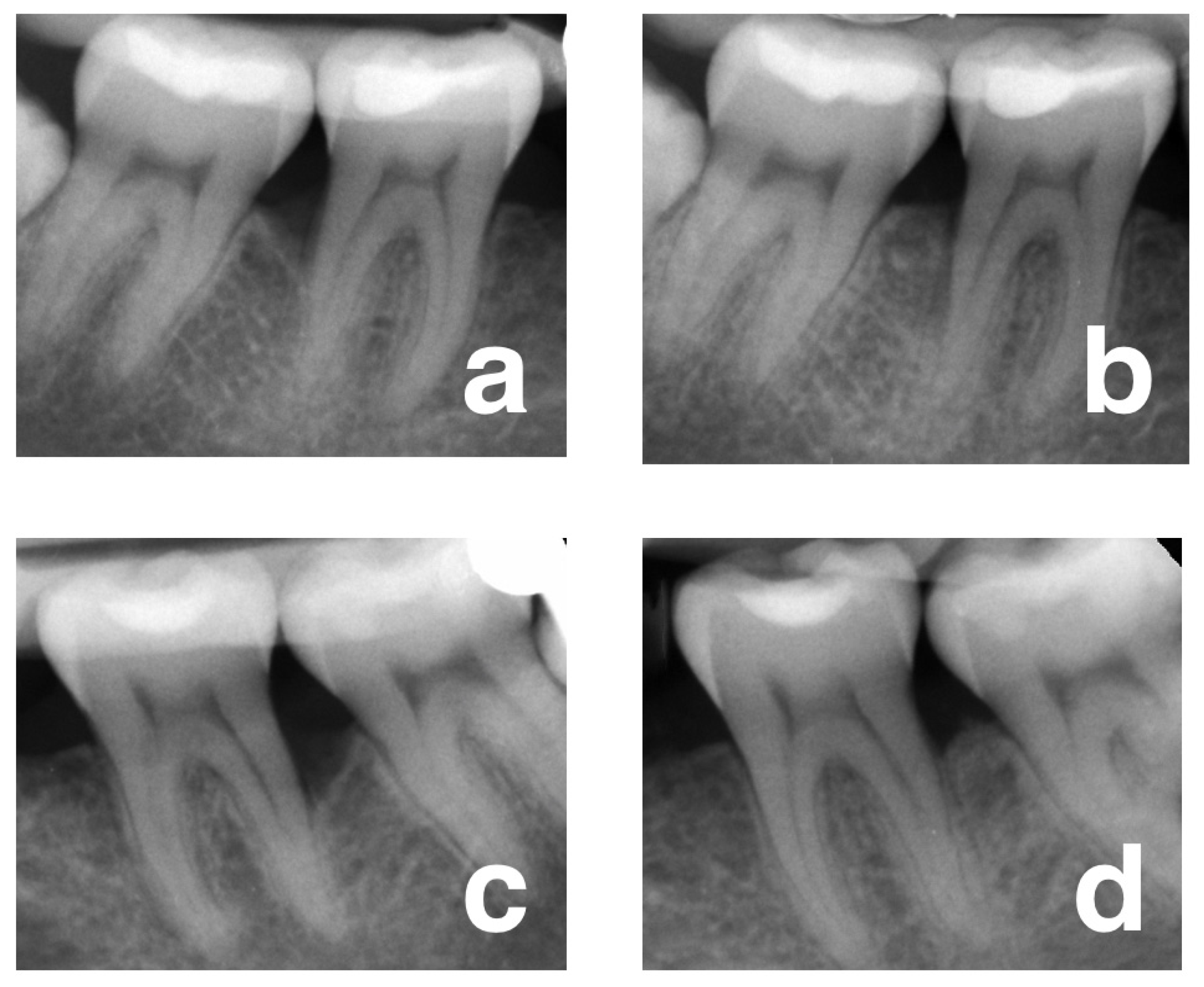
2.5. Radiological Outcomes
2.6. Patient-Reported Outcome Measures (PROMS)
2.7. Data Analysis
3. Results
3.1. Baseline Clinical Characteristics
3.2. Changes in Clinical and Radiological Parameters
3.3. Regression Analysis
3.4. Patient-Reported Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. S1), S173–S182. [Google Scholar] [CrossRef]
- Papapanou, P.N.; Wennströmm, J.L. The angular bony defect as indicator of further alveolar bone loss. J. Clin. Periodontol. 1991, 18, 317–322. [Google Scholar] [CrossRef]
- Rams, T.E.; Listgarten, M.A.; Slots, J. Radiographic alveolar bone morphology and progressive periodontitis. J. Periodontol. 2018, 89, 424–430. [Google Scholar] [CrossRef]
- Nibali, L.; Koidou, V.P.; Nieri, M.; Barbato, L.; Pagliaro, U.; Cairo, F. Regenerative surgery versus access flap for the treatment of intra-bony periodontal defects: A systematic review and meta-analysis. J. Clin. Periodontol. 2020, 47 (Suppl. S22), 320–351. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Herreram, D.; Kebschullm, M.; Chapple, I.; Jepsen, S.; Beglundh, T.; Sculean, A.; Tonetti, M.S. EFP Workshop Participants and Methodological Consultants. Treatment of stage I-III periodontitis-The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47 (Suppl. S22), 4–60. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Cortellini, P.; Lang, N.P.; Suvan, J.E.; Adriaens, P.; Dubravec, D.; Fonzar, A.; Fourmousis, I.; Rasperini, G.; Rossi, R.; et al. Clinical outcomes following treatment of human intrabony defects with GTR/bone replacement material or access flap alone. A multicenter randomized controlled clinical trial. J. Clin. Periodontol. 2004, 31, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact Mater. 2017, 2, 24–247. [Google Scholar] [CrossRef] [PubMed]
- Kao, R.T.; Nares, S.; Reynolds, M.A. Periodontal regeneration- intrabony defects: A systematic review from the AAP Regeneration Workshop. J. Periodontol. 2015, 86 (Suppl. S2), 77–104. [Google Scholar] [CrossRef]
- Bowers, G.M.; Chadroff, B.; Carnevale, R.; Mellonig, J.; Corio, R.; Emerson, J.; Stevens, M.; Romberg, E. Histologic evaluation of new attachment apparatus formation in humans. Part II. J. Periodontol. 1989, 60, 675–682. [Google Scholar] [CrossRef]
- Krasny, K.; Kamiński, A.; Krasny, M.; Czech, T.; Wojtowicz, A. Preparation of allogeneic bone for alveolar ridge augmentation. Cell Tissue Bank. 2017, 18, 313–321. [Google Scholar] [CrossRef]
- Dziedzic-Gocławska, A.; Kamiński, A.; Uhrynowska-Tyszkiewicz, I.; Stachowicz, W. Irradiation as a safety procedure in tissue banking. Cell Tissue Bank. 2005, 6, 201–219. [Google Scholar] [CrossRef]
- Dziedzic-Gocławska, A.; Ostrowski, K.; Stachowicz, W.; Michalik, J.; Grzesik, W. Effect of radiation sterilization on the osteoinductive properties and the rate of remodelling of bone implants preserved by lyophilization and deep-freezing. Clin. Orthop. 1991, 272, 30–37. [Google Scholar] [CrossRef]
- Stopa, Z.; Siewert-Gutowska, M.; Abed, K.; Szubińska-Lelonkiewicz, D.; Kamiński, A.; Fiedor, P. Evaluation of the safety and clinical efficacy of allogeneic bone grafts in the reconstruction of the maxilla and mandible. Trans. Proceed. 2018, 50, 2199–2201. [Google Scholar] [CrossRef]
- Cortellini, P.; Pini-Prato, G.; Tonetti, M.S. The modified papilla preservation technique. A new surgical approach for interproximal regenerative procedures. J. Periodontol. 1995, 66, 261–266. [Google Scholar] [CrossRef]
- Cortellini, P.; Pini-Prato, G.; Tonetti, M.S. The simplified papilla preservation flap. A novel surgical approach for the management of soft tissues in regenerative procedures. Int. J. Periodontics Restor. Dent. 1999, 19, 589–599. [Google Scholar]
- Cortellini, P.; Tonetti, M.S. Clinical concepts for regenerative therapy in intrabony defects. Periodontology 2015, 68, 282–307. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, T.J.; Drake, R.B.; Naylor, J.E. The plaque control record. J. Periodontol. 1972, 43, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, P.; Pini-Prato, G.; Tonetti, M.S. Periodontal regeneration of human infrabony defects I. Clinical measures. J. Periodontol. 1995, 64, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Schei, O.; Waerhaug, J.; Lovdal, A.; Arro, A. Alveolar bone loss as related to oral hygiene and age. J. Periodontol. 1959, 30, 7–16. [Google Scholar] [CrossRef]
- Jayakumar, A.; Rajababu, P.; Rohini, S.; Butchibabu, K.; Naveen, A.; Reddy, P.K.; Vidyasagar, S.; Satyanarayana, D.; Pavan Kumar, S. Multi-center, randomized clinical trial on efficacy and safety of recombinant human platelet-derived growth factor with ßtricalcium phosphate in human intra-osseous periodontal defects. J. Clin. Periodontol. 2011, 38, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Eickholz, P.; Hörr, T.; Klein, F.; Hassfeld, S.; Kim, T.S. Radiographic parameters for prognosis of periodontal healing of intrabony defects: Two different definitions of defect depth. J. Periodontol. 2004, 75, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Revill, S.I.; Robinson, J.O.; Rosen, M.; Hogg, M.I. The reliability of a linear analogue for evaluating pain. Anaesthesia 1976, 31, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Kiyak, H.A.; Hohl, T.; West, R.A.; McNeill, R.W. Psychologic changes in orthognatic surgery patients: A 24-month follow up. J. Oral Maxillofac. Surg. 1984, 42, 506–512. [Google Scholar] [CrossRef]
- Majzoub, J.; Barootchi, S.; Tavelli, L.; Wang, C.-W.; Chan, H.-L.; Wang, H.-L. Guided tissue regeneration combined with bone allograft in intrabony defects: Clinical outcomes and assessment of prognostic factors. J. Periodontol. 2020, 91, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Kher, V.K.; Bhongade, M.L.; Shori, T.; Kolte, A.P.; Dharamthok, S.B.; Shiraro, T.S. A comparative evaluation of the effectiveness of guided tissue regeneration by using a collagen membrane with or without decalcified freeze-dried bone allograft in the treatment of intrabony defects: A clinical and radiographic study. J. Indian Soc. Periodontol. 2013, 17, 484–489. [Google Scholar] [CrossRef]
- Jain, D.; Deepa, D. A comparative evaluation of freeze-dried bone allograft with and without bioresorbable guided tissue regeneration membrane Healiguide® in the treatment of Grade II furcation defects: A clinical study. J. Indian Soc. Periodontol. 2015, 19, 645–650. [Google Scholar]
- Academy Report. Position paper. Periodontal regeneration. J. Periodontol. 2005, 76, 1601–1622. [Google Scholar] [CrossRef]
- Atchuta, A.; Gooty, J.R.; Guntakandla, V.R.; Palakuru, S.K.; Durvasula, S.; Palaparthy, R. Clinical and radiographic evaluation of platelet-rich fibrin as an adjunct to bone grafting demineralized freeze-dried bone allograft in intrabony defects. J. Indian Soc. Periodontol. 2020, 24, 60–66. [Google Scholar] [CrossRef]
- Kamiński, A.; Grazka, E.; Jastrzębska, A.; Marowska, J.; Gut, G.; Wojciechowski, A.; Uhrynowska-Tyszkiewicz, I. Effect of accelerated electron beam on mechanical properties of human cortical bone: Influence of different processing methods. Cell Tissue Bank. 2012, 13, 375–386. [Google Scholar] [CrossRef]
- Sculean, A.; Berakdar, M.; Chiantella, G.C.; Donos, N.; Arweiler, N.B.; Auschill, T.M. Healing of intrabony defects following treatment with a bovine-derived xenograft and collagen membrane. J. Clin. Periodontol. 2003, 30, 73–80. [Google Scholar] [CrossRef]
- Stavropoulos, A.; Bertl, K.; Spineli, L.M.; Sculean, A.; Cortellini, P.; Tonetti, M. Medium- and long-term clinical benefits of periodontal regenerative/reconstructive procedures in intrabony defects: Systematic review and network meta-analysis of randomized controlled clinical trials. J. Clin. Periodontol. 2019, 48, 410–430. [Google Scholar] [CrossRef]
- Rakmanee, T.; Griffiths, G.S.; Auplish, G.; Darbar, U.; Petrie, A.; Olsen, I.; Donos, N. Radiographic outcomes following treatment of intrabony defect with guided tissue regeneration in aggressive periodontitis. Clin. Oral Investig. 2016, 20, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Linares, A.; Cortellini, P.; Lang, N.P.; Suvan, J.; Tonetti, M.S. European Reserch Group on Periodontology. Guided tissue regeneration/deproteinized bovine bone minera lor papilla preservation flaps alone for treatment of intrabony defects. II: Radiographic predictors and outcomes. J. Clin. Periodontol. 2006, 33, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Inglehart, M.R. Enhancing periodontal health through regenerative approaches: A commentary on the need for patient-reported outcomes. J. Periodontol. 2015, 86, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Górski, B.; Jalowski, S.; Górska, R.; Zaremba, M. Treatment of intrabony defects with modified perforated membranes in aggressive periodontitis: Subtraction radiography outcomes, prognostic variables, and patient morbidity. Clin. Oral Investig. 2019, 23, 3005–3020. [Google Scholar] [CrossRef]
- Needleman, I.; Worthington, H.V.; Giedrys-Leeper, E.; Tucker, R. Guided tissue regeneration for periodontal infra-bony defects (review). Cochrane Database Syst. Rev. 2006, 19, CD001724. [Google Scholar]

| Variables | Test Sites (n = 15) | Control Sites (n = 15) | p |
|---|---|---|---|
| Tooth type (n) | |||
| Molars | 6 | 6 | |
| Premolars | 5 | 5 | |
| Upper incisors, canines | 4 | 4 | |
| Tooth position (n) | |||
| Maxillary teeth | 7 | 8 | |
| Mandibular teeth | 8 | 7 | |
| Radiographic angle (degrees) | 23.39 (20.39–26.40) ± 5.42 | 26.49 (22.04–30.45) ± 7.59 | 0.2467 |
| Intra-surgical measurements (mm) | 0.8029 | ||
| Defect depth | 6.00 (4.99–7.00) ± 1.81 | 5.80 (4.42–7.18) ± 2.48 | |
| Defect width | 3.73 (2.97–4.50) ± 1.39 | 3.06 (2.53–3.60) ± 0.96 | 0.1372 |
| Defect morphology (n) | |||
| One-wall | 5 | 4 | |
| Two-wall | 5 | 6 | |
| Three-wall | 5 | 5 |
| Baseline | 12 Months | p (Baseline-1-Year) | ∆ Change (Baseline-1-Year) | |
|---|---|---|---|---|
| PPD test (mm) | 7.67 (6.98–8.35) ± 1.23 | 3.23 (2.69–3.65) ± 0.83 | <0.0001 * <0.0001 * | 4.66 (3.78–5.51) ± 1.24 |
| PPD control | 7.40 (6.82–9.66) ± 1.06 | 3.63 (3.21–4.06) ± 0.77 | 3.57 (3.18–5.22) ± 1.11 | |
| p (test vs. control) | 0.3427 | 0.0429 * | 0.0190 * | |
| CAL test (mm) | 8.93 (8.13–9.73) ± 1.44 | 3.23 (2.69–3.65) ± 0.83 | <0.0001 * <0.0001 * | 5.54 (4.40–6.55) ± 1.18 |
| CAL control | 8.73 (7.81–9.69) ± 1.67 | 4.13 (3.25–5.02) ± 1.59 | 4.54 (3.95–5.22) ± 1.11 | |
| p (test vs. control) | 0.2219 | 0.1182 | 0.0891 | |
| GR test (mm) | 1.21 (5.21–6.57) ± 1.23 | 0.66 (0.42–0.91) ± 0.45 | 0.4981 0.2610 | 0.22 (−0.28–0.75) 0.91 |
| GR control | 1.47 (0.84–2.08) ± 1.12 | 1.03 (0.52–1.55) ± 0.93 | 0.45 (0.11–0.78) ± 0.66 | |
| p (test vs. control) | 0.7918 | 0.1651 | 0.3281 | |
| DD test (mm) | 5.89 (5.21–6.57) ± 1.23 | 0.66 (0.42–0.91) ± 0.45 | <0.0001 * <0.0001 * | - |
| DD control | 5.32 (4.31–6.35) ± 1.84 | 0.92 (0.58–1.27) ± 0.61 | ||
| p (test vs. control) | 0.2811 | 0.0334 * | ||
| LDF test (mm) | - | 5.22 (4.56–5.54) ± 1.11 | - | - |
| LDF control | 4.33 (3.40–5.18) ± 1.74 | |||
| p (test vs. control) | 0.0478 * | |||
| %DF test (mm) | - | 85.89 (81.29–95.11) ± 8.90 | - | - |
| %DF control | 83.27 (77.61–90.37) ± 11.41 | |||
| p (test vs. control) | 0.1091 |
| Parameter | Regression Coefficient | Standard Error | Confidence Interval | p | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| CAL gain from baseline to 1 year as dependent variable. R2 = 0.5649 | |||||
| Intercept | 5.6065 | 3.5427 | 7.0054 | 4.2077 | 0.1319 |
| Gender | 0.3713 | 0.6305 | 0.6202 | 0.1223 | 0.5636 |
| Age | −0.0308 | 0.0570 | −0.008 | −0.0534 | 0.5955 |
| Surgical procedure (test vs. control) | −0.1939 | 0.5223 | 0.0123 | −0.4001 | 0.7150 |
| Tooth type (incisors, canines, premolars vs. molars) | −0.3964 | 0.3969 | −0.239 | −0.5532 | 0.3319 |
| Tooth position (upper vs. lower) | 1.0250 | 0.5503 | 1.2423 | 0.8077 | 0.0798 |
| FMPS | −0.0826 | 0.0581 | −0.059 | −0.1056 | 0.1735 |
| FMBS | 0.0778 | 0.0456 | 0.0959 | 0.0598 | 0.1062 |
| PPD | −0.1706 | 0.3491 | −0.0328 | −0.3085 | 0.6312 |
| DD | −0.0306 | 0.2670 | 0.0748 | −0.1360 | 0.9100 |
| RVG angle | −0.1669 | 0.0474 | −0.1482 | −0.1856 | 0.0026 * |
| PPD reduction from baseline to 1 year as dependent variable. R2 = 0.6335 | |||||
| Intercept | 4.2998 | 2.4676 | 5.2742 | 3.3255 | 0.0984 |
| Gender | −0.3789 | 0.5490 | −0.162 | −0.5957 | 0.4988 |
| Age | −0.0848 | 0.0486 | −0.065 | −0.1041 | 0.0982 |
| Surgical procedure (test vs. control) | 0.1001 | 0.4756 | 0.2879 | −0.0877 | 0.8356 |
| Tooth type (incisors, canines, premolars vs. molars) | −0.3512 | 0.3612 | −0.2085 | −0.4939 | 0.3437 |
| Tooth position (upper vs. lower) | 0.4019 | 0.5002 | 0.5994 | 0.2044 | 0.4321 |
| FMPS | 0.0029 | 0.0527 | 0.0237 | −0.0179 | 0.9566 |
| FMBS | 0.0662 | 0.0414 | 0.0826 | 0.0498 | 0.1274 |
| CAL | 0.4270 | 0.1965 | 0.5046 | 0.3494 | 0.0434 * |
| DD | 0.1468 | 0.2418 | 0.2423 | 0.0513 | 0.5514 |
| RVG angle | −0.0771 | 0.0407 | −0.061 | −0.0932 | 0.074 |
| LDF from baseline to 1 year as the dependent variable. R2 = 0.7145 | |||||
| Intercept | 6.3908 | 3.0015 | 7.5760 | 5.2057 | 0.0473 |
| Gender | −0.1927 | 0.5437 | 0.0219 | −0.4074 | 0.7270 |
| Age | −0.0066 | 0.0504 | 0.0133 | −0.0265 | 0.8969 |
| Surgical procedure (test vs. control) | 0.5582 | 0.4464 | 0.7345 | 0.3820 | 0.2270 |
| Tooth type (incisors, canines, premolars vs. molars) | −0.6148 | 0.3317 | −0.4838 | −0.7459 | 0.0803 |
| Tooth position (upper vs. lower) | −0.0079 | 0.4854 | 0.1837 | −0.1996 | 0.9871 |
| FMPS | 0.0125 | 0.0504 | 0.0324 | −0.0073 | 0.8066 |
| FMBS | 0.0000 | 0.0392 | 0.0155 | −0.0154 | 0.9992 |
| PPD | −0.1305 | 0.3070 | −0.0093 | −0.2518 | 0.6756 |
| CAL | 0.3715 | 0.2162 | 0.4569 | 0.2861 | 0.1029 |
| RVG angle | −0.1191 | 0.0386 | −0.1038 | −0.1343 | 0.0064 * |
| Test (n = 15) | Control (n = 15) | Test (n = 15) | Control (n = 15) | |||||
|---|---|---|---|---|---|---|---|---|
| Number of Subjects (%) | Number of Subjects (%) | p Value | Intensity (VAS) Mean ±SD | Minimum–Maximum | Intensity (VAS) Mean ±SD | Minimum–Maximum | p Value | |
| Discomfort | 11 (73.33) | 12 (80.00) | 1 | 27.27 ± 21.33 | 2–57 | 28.42 ± 18.64 | 3–55 | 0.8921 |
| Pain | 11 (73.33) | 12 (80.00) | 1 | 28.73 ± 15.95 | 2–45 | 27.08 ± 14.13 | 3–45 | 0.7957 |
| Edema | 11 (73.33) | 12 (80.00) | 1 | 23.40 ± 14.45 | 3–47 | 33.09 ± 18.16 | 3–57 | 0.1949 |
| Eating impairment | 14 (93.33) | 15 (100) | 1 | 30.73 ± 12.16 | 4–48 | 37.20 ± 24.05 | 1–76 | 0.4393 |
| Speaking impairment | 8 (53.33) | 9 (60.00) | 1 | 17.00 ± 11.56 | 3–35 | 14.56 ± 12.01 | 1–35 | 0.6760 |
| Interferences with daily activities | 7 (46.66) | 8 (53.33%) | 1 | 15.00 ± 8.54 | 3–26 | 14.38 ± 9.13 | 1–28 | 0.8937 |
| Interferences with work | 7 (46.66) | 8 (53.33%) | 1 | 13.00 ± 11.90 | 4–27 | 12.00 ± 11.87 | 2–32 | 0.8885 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brodzikowska, A.; Górski, B.; Szerszeń, M.; Sanz, M. Efficacy of Guided Tissue Regeneration Using Frozen Radiation-Sterilized Allogenic Bone Graft as Bone Replacement Graft Compared with Deproteinized Bovine Bone Mineral in the Treatment of Periodontal Intra-Bony Defects: Randomized Controlled Trial. J. Clin. Med. 2023, 12, 1396. https://doi.org/10.3390/jcm12041396
Brodzikowska A, Górski B, Szerszeń M, Sanz M. Efficacy of Guided Tissue Regeneration Using Frozen Radiation-Sterilized Allogenic Bone Graft as Bone Replacement Graft Compared with Deproteinized Bovine Bone Mineral in the Treatment of Periodontal Intra-Bony Defects: Randomized Controlled Trial. Journal of Clinical Medicine. 2023; 12(4):1396. https://doi.org/10.3390/jcm12041396
Chicago/Turabian StyleBrodzikowska, Aniela, Bartłomiej Górski, Marcin Szerszeń, and Mariano Sanz. 2023. "Efficacy of Guided Tissue Regeneration Using Frozen Radiation-Sterilized Allogenic Bone Graft as Bone Replacement Graft Compared with Deproteinized Bovine Bone Mineral in the Treatment of Periodontal Intra-Bony Defects: Randomized Controlled Trial" Journal of Clinical Medicine 12, no. 4: 1396. https://doi.org/10.3390/jcm12041396
APA StyleBrodzikowska, A., Górski, B., Szerszeń, M., & Sanz, M. (2023). Efficacy of Guided Tissue Regeneration Using Frozen Radiation-Sterilized Allogenic Bone Graft as Bone Replacement Graft Compared with Deproteinized Bovine Bone Mineral in the Treatment of Periodontal Intra-Bony Defects: Randomized Controlled Trial. Journal of Clinical Medicine, 12(4), 1396. https://doi.org/10.3390/jcm12041396








