Parameters to Predict the Outcome of Severe and Critical COVID-19 Patients when Admitted to the Hospital
Abstract
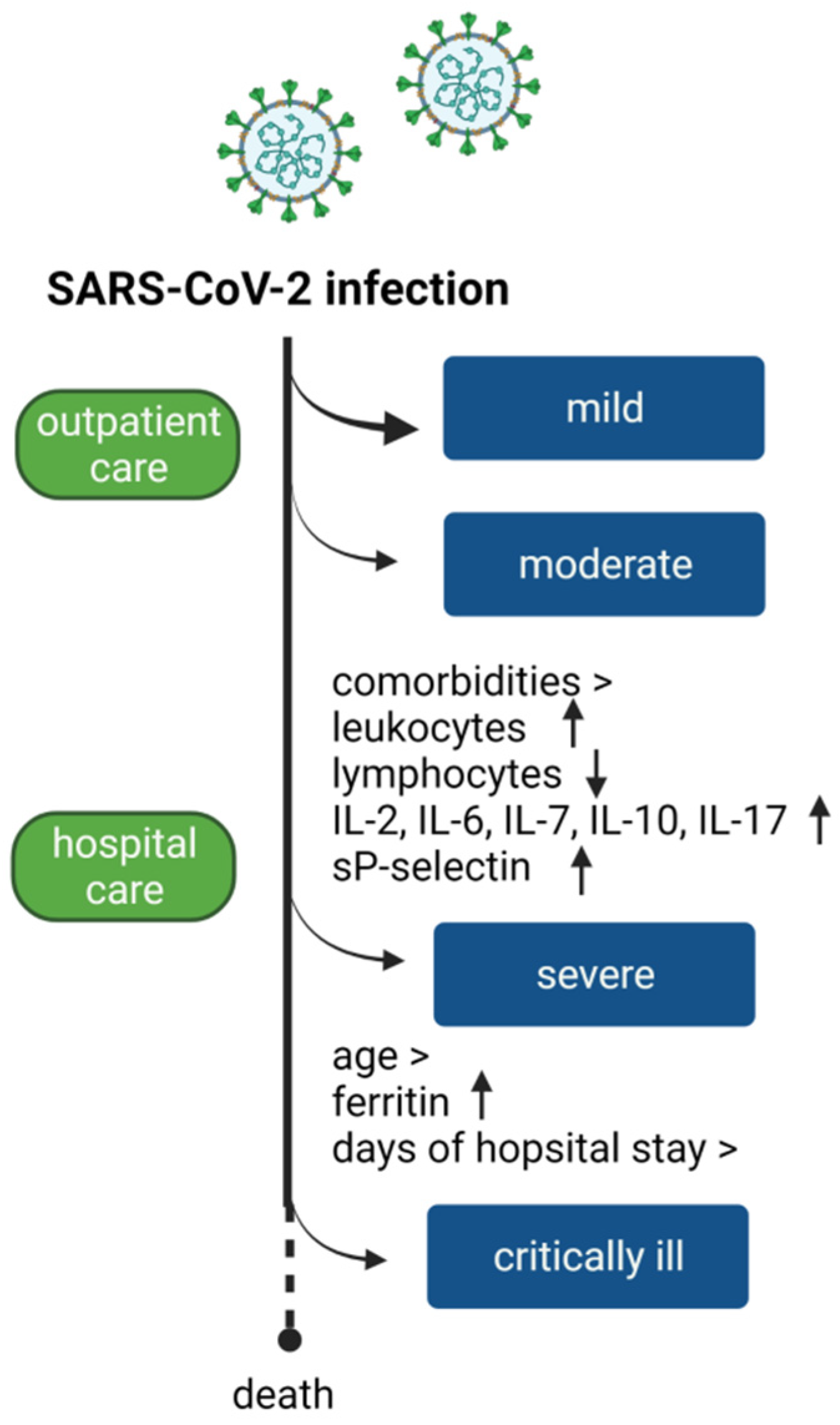
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Interleukins and sP-Selectin Measurement
2.3. Statistical Analysis
3. Results
3.1. Demographics and Laboratory Findings of Patients Infected
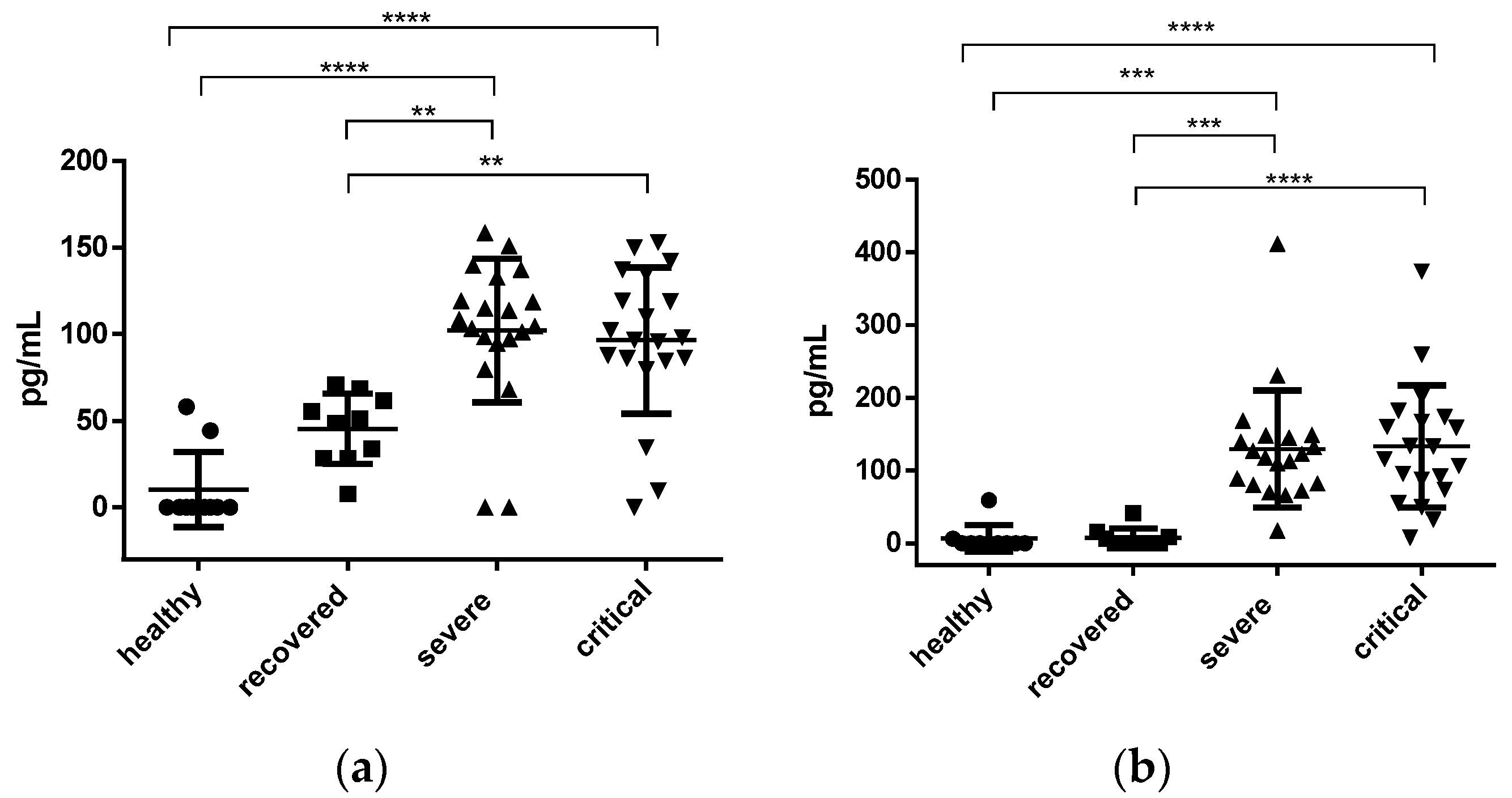
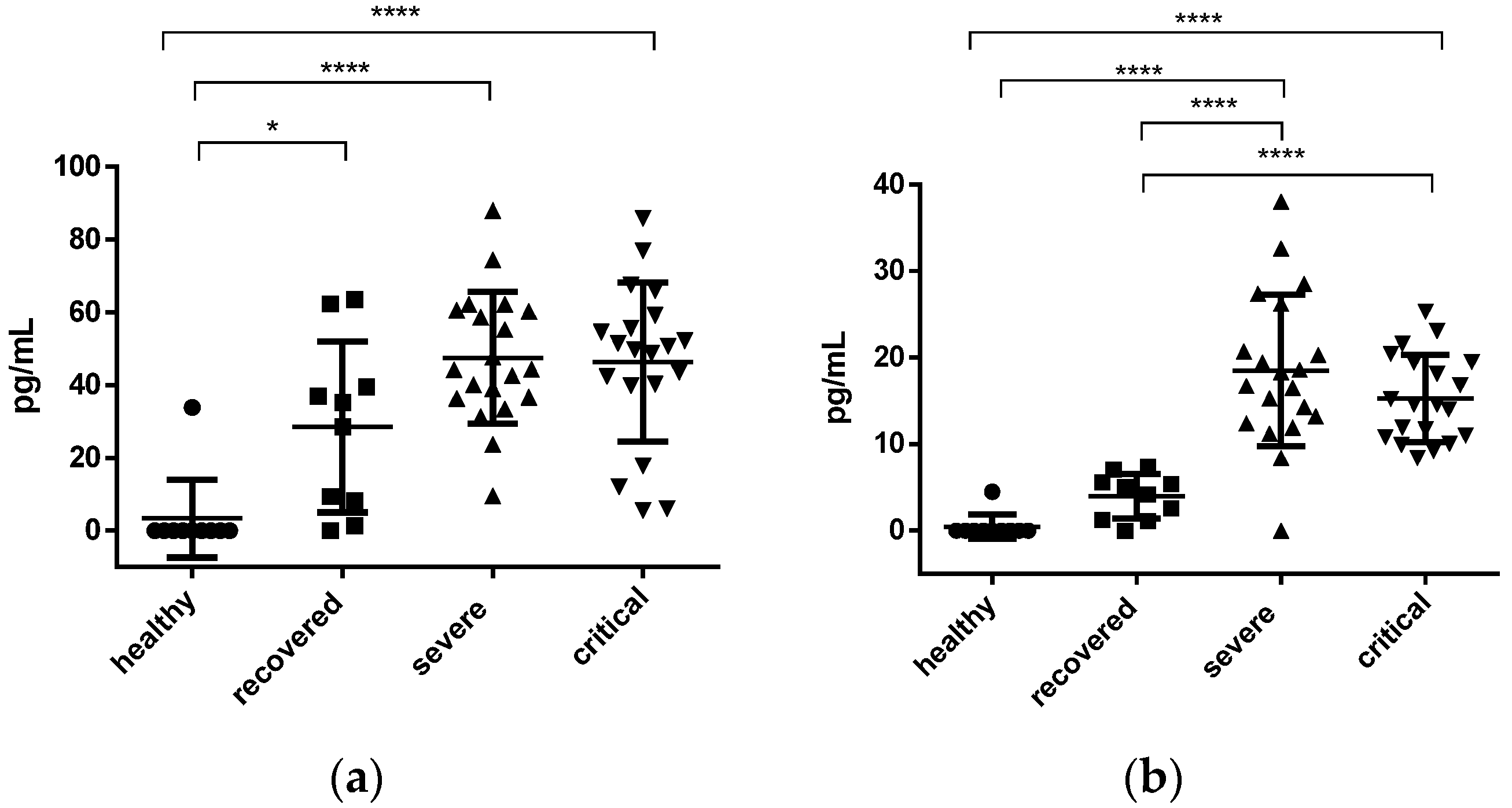
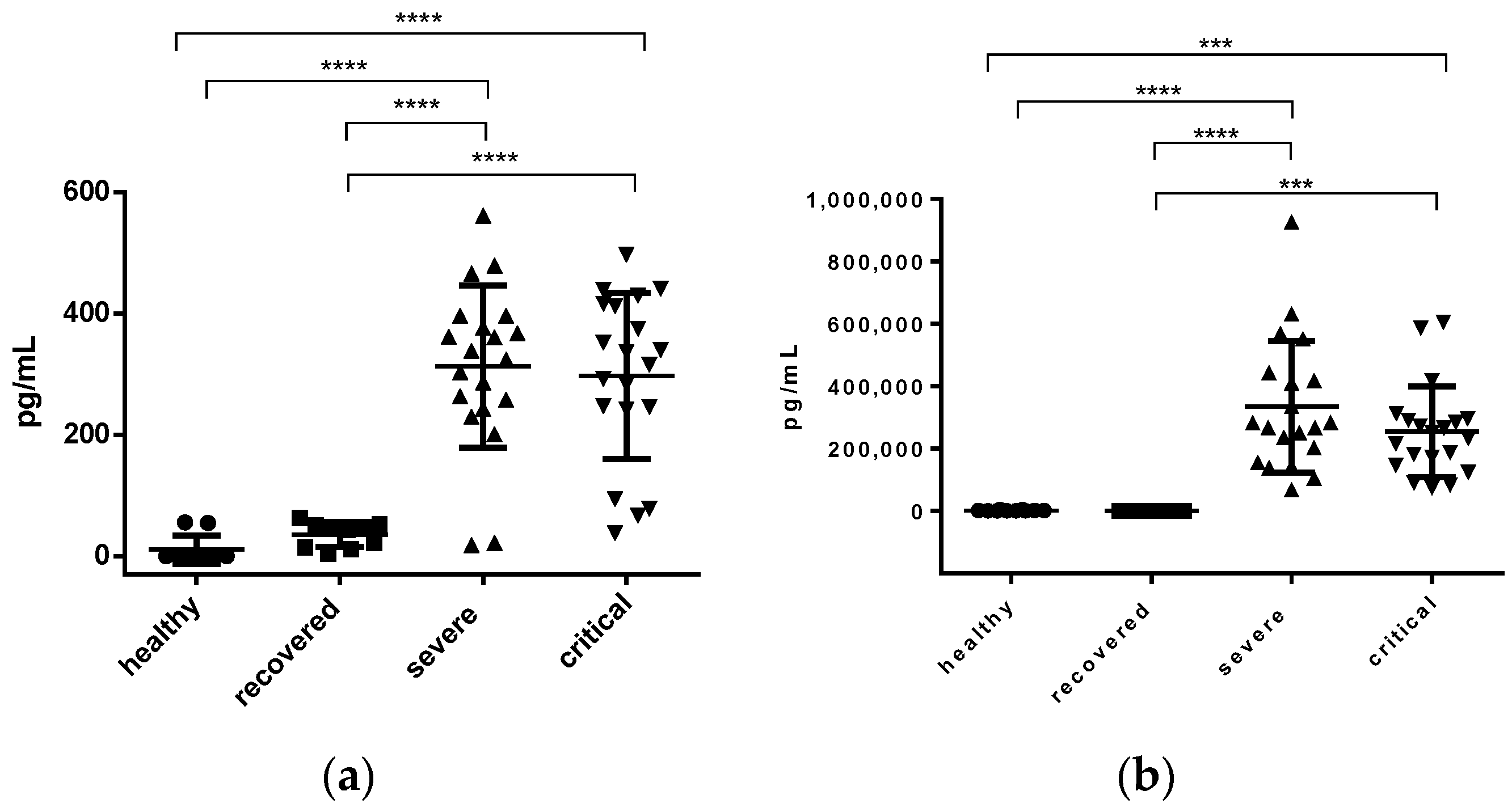
3.2. Interleukins and sP-Selectin
3.2.1. IL-2 and IL-6 Concentration
3.2.2. IL-7 and IL-10 Concentration
3.2.3. IL-17 and sP-Selectin Concentration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Channappanavar, R.; Perlman, S. Pathogenic human coronavirus infections: Causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017, 39, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Berube, J.; McNamara, M.; Saksena, S.; Hartman, M.; Arshad, T.; Bornheimer, S.J.; O’Gorman, M. Lymphocyte Subset Counts in COVID-19 Patients: A Meta-Analysis. Cytom. A 2020, 97, 772–776. [Google Scholar] [CrossRef] [PubMed]
- Nicolai, L.; Leunig, A.; Brambs, S.; Kaiser, R.; Weinberger, T.; Weigand, M.; Muenchhoff, M.; Hellmuth, J.C.; Ledderose, S.; Schulz, H.; et al. Immunothrombotic Dysregulation in COVID-19 Pneumonia Is Associated with Respiratory Failure and Coagulopathy. Circulation 2020, 142, 1176–1189. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.T.; Nakayama, T.; Wu, C.T.; Goltsev, Y.; Jiang, S.; Gall, P.A.; Liao, C.K.; Shih, L.C.; Schürch, C.M.; McIlwain, D.R.; et al. ACE2 localizes to the respiratory cilia and is not increased by ACE inhibitors or ARBs. Nat. Commun. 2020, 11, 5453. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Setiadi, H.; Yago, T.; Liu, Z.; McEver, R.P. Endothelial signaling by neutrophil-released oncostatin M enhances P-selectin-dependent inflammation and thrombosis. Blood Adv. 2019, 3, 168–183. [Google Scholar] [CrossRef]
- Wong, R.S.; Wu, A.; To, K.F.; Lee, N.; Lam, C.W.; Wong, C.K.; Chan, P.K.; Ng, M.H.; Yu, L.M.; Hui, D.S.; et al. Haematological manifestations in patients with severe acute respiratory syndrome: Retrospective analysis. BMJ 2003, 326, 1358–1362. [Google Scholar] [CrossRef]
- Prokop, M.; van Everdingen, W.; van Rees Vellinga, T.; Quarles van Ufford, H.; Stöger, L.; Beenen, L.; Geurts, B.; Gietema, H.; Krdzalic, J.; Schaefer-Prokop, C.; et al. CO-RADS: A Categorical CT Assessment Scheme for Patients Suspected of Having COVID-19-Definition and Evaluation. Radiology 2020, 296, E97–E104. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, G.; Cai, X.P.; Deng, J.W.; Zheng, L.; Zhu, H.H.; Zheng, M.; Yang, B.; Chen, Z. An overview of COVID-19. J. Zhejiang Univ. Sci. B 2020, 21, 343–360. [Google Scholar] [CrossRef]
- Chen, Y.; Klein, S.L.; Garibaldi, B.T.; Li, H.; Wu, C.; Osevala, N.M.; Li, T.; Margolick, J.B.; Pawelec, G.; Leng, S.X. Aging in COVID-19: Vulnerability, immunity and intervention. Ageing Res. Rev. 2021, 65, 101205. [Google Scholar] [CrossRef]
- Mertoglu, C.; Huyut, M.T.; Olmez, H.; Tosun, M.; Kantarci, M.; Coban, T.A. COVID-19 is more dangerous for older people and its severity is increasing: A case-control study. Med. Gas Res. 2022, 12, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Costa, V.O.; Nicolini, E.M.; da Costa, B.M.A.; Ferreira, V.H.P.; Tonisi, A.J.R.; Machado, N.M.; Moura, M.A.; Montessi, J.; de Castro Ferreira, L.; Campos, R.L.; et al. Sociodemographic, laboratory, image data and predictors of gravity risk in patients with COVID-19. PLoS ONE 2021, 16, e0256331. [Google Scholar] [CrossRef]
- Shamah-Levy, T.; Romero-Martínez, M.; Barrientos-Gutiérrez, T.; Cuevas-Nasu, L.; Bautista-Arredondo, S.; Colchero, M.A.; GaonaPineda, E.B.; Lazcano-Ponce, E.; Martínez-Barnetche, J.; Alpuche-Arana, C.; et al. Encuesta Nacional de Salud y Nutrición 2020 sobre COVID-19. Resultados Nacionales; Instituto Nacional de Salud Pública: Cuernavaca, Mexico, 2021; 192p. [Google Scholar]
- Kumar, A.; Arora, A.; Sharma, P.; Anikhindi, S.A.; Bansal, N.; Singla, V.; Khare, S.; Srivastava, A. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes Metab. Syndr. 2020, 14, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Lima-Martínez, M.M.; Carrera Boada, C.; Madera-Silva, M.D.; Marín, W.; Contreras, M. COVID-19 and diabetes: A bidirectional relationship. Clin. Investig. Arterioscler. 2021, 33, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Salazar, M.; Barochiner, J.; Espeche, W.; Ennis, I. COVID-19 and its relationship with hypertension and cardiovascular disease. Hipertens. Riesgo. Vasc. 2020, 37, 176–180. [Google Scholar] [CrossRef]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L., Jr.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T., Jr.; et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA 2003, 289, 2560–2572. [Google Scholar] [CrossRef]
- Corrigendum to: 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2019, 40, 475. [CrossRef]
- Díaz, E.; Rodríguez, A.; Martin-Loeches, I.; Lorente, L.; Del Mar Martín, M.; Pozo, J.C.; Montejo, J.C.; Estella, A.; Arenzana, Á.; Rello, J. Impact of obesity in patients infected with 2009 influenza A(H1N1). Chest 2011, 139, 382–386. [Google Scholar] [CrossRef]
- Louie, J.K.; Acosta, M.; Samuel, M.C.; Schechter, R.; Vugia, D.J.; Harriman, K.; Matyas, B.T. A novel risk factor for a novel virus: Obesity and 2009 pandemic influenza A (H1N1). Clin. Infect. Dis. 2011, 52, 301–312. [Google Scholar] [CrossRef]
- Zhou, Y.; Chi, J.; Lv, W.; Wang, Y. Obesity and diabetes as high-risk factors for severe coronavirus disease 2019 (COVID-19). Diabetes Metab. Res. Rev. 2021, 37, e3377. [Google Scholar] [CrossRef] [PubMed]
- Lighter, J.; Phillips, M.; Hochman, S.; Sterling, S.; Johnson, D.; Francois, F.; Stachel, A. Obesity in Patients Younger Than 60 Years Is a Risk Factor for COVID-19 Hospital Admission. Clin. Infect. Dis. 2020, 71, 896–897. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Zheng, K.I.; Wang, X.B.; Sun, Q.F.; Pan, K.H.; Wang, T.Y.; Chen, Y.P.; Targher, G.; Byrne, C.D.; George, J.; et al. Obesity Is a Risk Factor for Greater COVID-19 Severity. Diabetes. Care 2020, 43, e72–e74. [Google Scholar] [CrossRef] [PubMed]
- Halpern, B.; Louzada, M.; Aschner, P.; Gerchman, F.; Brajkovich, I.; Faria-Neto, J.R.; Polanco, F.E.; Montero, J.; Juliá, S.M.M.; Lotufo, P.A.; et al. Obesity and COVID-19 in Latin America: A tragedy of two pandemics-Official document of the Latin American Federation of Obesity Societies. Obes. Rev. 2021, 22, e13165. [Google Scholar] [CrossRef] [PubMed]
- Ramadori, G. Albumin Infusion in Critically Ill COVID-19 Patients: Hemodilution and Anticoagulation. Int. J. Mol. Sci. 2021, 22, 7126. [Google Scholar] [CrossRef]
- Violi, F.; Cangemi, R.; Romiti, G.F.; Ceccarelli, G.; Oliva, A.; Alessandri, F.; Pirro, M.; Pignatelli, P.; Lichtner, M.; Carraro, A.; et al. Is Albumin Predictor of Mortality in COVID-19? Antioxid. Redox Signal. 2021, 35, 139–142. [Google Scholar] [CrossRef]
- Bannaga, A.S.; Tabuso, M.; Farrugia, A.; Chandrapalan, S.; Somal, K.; Lim, V.K.; Mohamed, S.; Nia, G.J.; Mannath, J.; Wong, J.L.; et al. C-reactive protein and albumin association with mortality of hospitalised SARS-CoV-2 patients: A tertiary hospital experience. Clin. Med. 2020, 20, 463–467. [Google Scholar] [CrossRef]
- Hariyanto, T.I.; Japar, K.V.; Kwenandar, F.; Damay, V.; Siregar, J.I.; Lugito, N.P.H.; Tjiang, M.M.; Kurniawan, A. Inflammatory and hematologic markers as predictors of severe outcomes in COVID-19 infection: A systematic review and meta-analysis. Am. J. Emerg. Med. 2021, 41, 110–119. [Google Scholar] [CrossRef]
- Li, Q.; Cao, Y.; Chen, L.; Wu, D.; Yu, J.; Wang, H.; He, W.; Chen, L.; Dong, F.; Chen, W.; et al. Hematological features of persons with COVID-19. Leukemia 2020, 34, 2163–2172. [Google Scholar] [CrossRef]
- Jiang, S.Q.; Huang, Q.F.; Xie, W.M.; Lv, C.; Quan, X.Q. The association between severe COVID-19 and low platelet count: Evidence from 31 observational studies involving 7613 participants. Br. J. Haematol. 2020, 190, e29–e33. [Google Scholar] [CrossRef]
- Amgalan, A.; Othman, M. Hemostatic laboratory derangements in COVID-19 with a focus on platelet count. Platelets 2020, 31, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Henry, B.M. COVID-19, ECMO, and lymphopenia: A word of caution. Lancet Respir. Med. 2020, 8, e24. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Xing, H.; Wei, Y.; Tang, Z.; Lu, X.; Wang, Z.; Liu, Y.; Xu, L.; Hu, L.; Wang, L.; et al. Monocyte volumetric parameters and lymph index are increased in SARS-CoV-2 infection. Int. J. Lab. Hematol. 2020, 42, e266–e269. [Google Scholar] [CrossRef] [PubMed]
- Demeester, S.; Demuyser, T.; Fauconnier, C.; Heestermans, R.; Orlando, C.; Depreter, B.; Jochmans, K. Routine haematology parameters in COVID-19 patients and clinical outcome: A Belgian single-centre study. Int. J. Lab. Hematol. 2020, 42, e252–e255. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Guo, Y.; Luo, Q.; Huang, Z.; Zhao, R.; Liu, S.; Le, A.; Li, J.; Wan, L. T-Cell Subset Counts in Peripheral Blood Can Be Used as Discriminatory Biomarkers for Diagnosis and Severity Prediction of Coronavirus Disease 2019. J. Infect. Dis. 2020, 222, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, S.; Liu, J.; Liang, B.; Wang, X.; Wang, H.; Li, W.; Tong, Q.; Yi, J.; Zhao, L.; et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine 2020, 55, 102763. [Google Scholar] [CrossRef]
- Gao, Y.D.; Ding, M.; Dong, X.; Zhang, J.J.; Kursat Azkur, A.; Azkur, D.; Gan, H.; Sun, Y.L.; Fu, W.; Li, W.; et al. Risk factors for severe and critically ill COVID-19 patients: A review. Allergy 2021, 76, 428–455. [Google Scholar] [CrossRef]
- Cheng, L.; Li, H.; Li, L.; Liu, C.; Yan, S.; Chen, H.; Li, Y. Ferritin in the coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. J. Clin. Lab. Anal. 2020, 34, e23618. [Google Scholar] [CrossRef]
- Grasselli, G.; Greco, M.; Zanella, A.; Albano, G.; Antonelli, M.; Bellani, G.; Bonanomi, E.; Cabrini, L.; Carlesso, E.; Castelli, G.; et al. Risk Factors Associated With Mortality Among Patients With COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Intern. Med. 2020, 180, 1345–1355. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Wei, L.; Ji, J.S.; Liu, Y.; Liu, R.; Zha, Y.; Chang, X.; Zhang, L.; Liu, Q.; et al. What are the risk factors of hospital length of stay in the novel coronavirus pneumonia (COVID-19) patients? A survival analysis in southwest China. PLoS ONE 2022, 17, e0261216. [Google Scholar] [CrossRef] [PubMed]
- Kadapatti, K.; Shendge, J.; Kulkarni, A.; Mane, J.; Patil, R.; Gandhi, S.; Sonone, A.; Deshmukh, U.; Rahulgade, P. Analysis of mortality in COVID-19 patients admitted to an intensive care unit. Indian J. Crit. Care Med. 2021, 25, S67–S68. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; Cohen, S.L.; et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Anka, A.U.; Tahir, M.I.; Abubakar, S.D.; Alsabbagh, M.; Zian, Z.; Hamedifar, H.; Sabzevari, A.; Azizi, G. Coronavirus disease 2019 (COVID-19): An overview of the immunopathology, serological diagnosis and management. Scand. J. Immunol. 2021, 93, e12998. [Google Scholar] [CrossRef]
- Lee, W.W.; Teo, T.H.; Lum, F.M.; Andiappan, A.K.; Amrun, S.N.; Rénia, L.; Rötzschke, O.; Ng, L.F. Virus infection drives IL-2 antibody complexes into pro-inflammatory agonists in mice. Sci. Rep. 2016, 6, 37603. [Google Scholar] [CrossRef]
- Luo, X.H.; Zhu, Y.; Mao, J.; Du, R.C. T cell immunobiology and cytokine storm of COVID-19. Scand. J. Immunol. 2021, 93, e12989. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, S.; Subbian, S. Critical Determinants of Cytokine Storm and Type I Interferon Response in COVID-19 Pathogenesis. Clin. Microbiol. Rev. 2021, 34, e00299-20. [Google Scholar] [CrossRef]
- Jimenez-Gastelum, G.R.; Espinoza-Ortega, A.M.; Ramos-Payan, R.; Aguilar-Medina, M.; Lopez-Gutierrez, J.; Villegas-Mercado, C.; Ochoa-Ramirez, L.A.; Rendon-Aguilar, H.; Osuna-Ramos, J.F.; Rios-Tostado, J.J.; et al. More Evidence of the Link of Interleukin-6 and Interleukin-10 with Critical COVID-19: A Report in Mexican Patients. Iran J. Immunol. 2021, 18, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Zhang, J.W.; Zhang, W.; Lin, Y.L.; Wang, Q. Circulating levels of IL-2, IL-4, TNF-alpha, IFN-gamma, and C-reactive protein are not associated with severity of COVID-19 symptoms. J. Med. Virol. 2021, 93, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Guzik, T.J.; Mohiddin, S.A.; Dimarco, A.; Patel, V.; Savvatis, K.; Marelli-Berg, F.M.; Madhur, M.S.; Tomaszewski, M.; Maffia, P.; D’Acquisto, F.; et al. COVID-19 and the cardiovascular system: Implications for risk assessment, diagnosis, and treatment options. Cardiovasc. Res. 2020, 116, 1666–1687. [Google Scholar] [CrossRef] [PubMed]
- Grobler, C.; Maphumulo, S.C.; Grobbelaar, L.M.; Bredenkamp, J.C.; Laubscher, G.J.; Lourens, P.J.; Steenkamp, J.; Kell, D.B.; Pretorius, E. COVID-19: The Rollercoaster of Fibrin(Ogen), D-Dimer, Von Willebrand Factor, P-Selectin and Their Interactions with Endothelial Cells, Platelets and Erythrocytes. Int. J. Mol. Sci. 2020, 21, 5168. [Google Scholar] [CrossRef] [PubMed]
- Woollard, K.J.; Suhartoyo, A.; Harris, E.E.; Eisenhardt, S.U.; Jackson, S.P.; Peter, K.; Dart, A.M.; Hickey, M.J.; Chin-Dusting, J.P. Pathophysiological levels of soluble P-selectin mediate adhesion of leukocytes to the endothelium through Mac-1 activation. Circ. Res. 2008, 103, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- Kanagala, P.; Arnold, J.R.; Khan, J.N.; Singh, A.; Gulsin, G.S.; Squire, I.B.; McCann, G.P.; Ng, L.L. Plasma P-selectin is a predictor of mortality in heart failure with preserved ejection fraction. ESC Heart Fail. 2021, 8, 2328–2333. [Google Scholar] [CrossRef] [PubMed]
- Watany, M.M.; Abdou, S.; Elkolaly, R.; Elgharbawy, N.; Hodeib, H. Evaluation of admission levels of P, E and L selectins as predictors for thrombosis in hospitalized COVID-19 patients. Clin. Exp. Med. 2022, 22, 567–575. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | All Patients (n = 40) | Severe (n = 20) | Critical (n = 20) | p Value |
|---|---|---|---|---|
| Age, years | 55.25 a ± 2.399 b | 49.50 ± 3.276 | 61.00 ± 3.067 | 0.0145 * |
| Sex | Men 22 c (55%) d | 8 (40%) | 14 (70%) | 0.1110 |
| Women 18 (45%) | 12 (60%) | 6 (30%) | ||
| Any comorbidity | Diabetes 19 (47.5%) | 11 (55%) | 8 (40%) | 0.5273 |
| Hypertension 10 (25%) | 5 (25%) | 5 (25%) | >0.999 | |
| Obesity I 1 (2.5%) | 1 (5%) | 0 | >0.999 | |
| Obesity II 4 (10%) | 2 (10%) | 2 (10%) | >0.999 | |
| Obesity III 3 (7.5%) | 0 | 3 (15%) | 0.2308 | |
| Obesity IV 1 (2.5%) | 0 | 1 (5%) | >0.999 | |
| Laboratory data | Albumin e 3.430 ± 0.1343 | 3.595 ± 0.2089 | 3.265 ± 0.1658 | 0.2236 |
| Leukocytes f 12,505 ± 886.4 | 11,695 ± 1073 | 13,315 ± 1416 | 0.3677 | |
| Lymphocytes g 913.6 ± 84.70 | 995.5 ± 130.3 | 831.8 ± 108.5 | 0.3404 | |
| Platelets h 279,517 ± 16,638 | 299,909 ± 26,825 | 259,125 ± 19,314 | 0.2248 | |
| Ferritin i 712.9 ± 69.26 | 574.5 ± 97.16 | 851.3 ± 90.76 | 0.0441 * | |
| Days of hospital stay | 14.95 ± 1.128 | 10.10 ± 0.8611 | 19.80 ± 1.417 | 0.0001 **** |
| Death | 21 (52.5%) | 3 (15%) | 18 (90%) | 0.0001 **** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chávez-Ocaña, S.d.C.; Bravata-Alcántara, J.C.; Cortés-Ortiz, I.A.; Reyes-Sandoval, A.; García-Machorro, J.; Herrera-Gonzalez, N.E. Parameters to Predict the Outcome of Severe and Critical COVID-19 Patients when Admitted to the Hospital. J. Clin. Med. 2023, 12, 1323. https://doi.org/10.3390/jcm12041323
Chávez-Ocaña SdC, Bravata-Alcántara JC, Cortés-Ortiz IA, Reyes-Sandoval A, García-Machorro J, Herrera-Gonzalez NE. Parameters to Predict the Outcome of Severe and Critical COVID-19 Patients when Admitted to the Hospital. Journal of Clinical Medicine. 2023; 12(4):1323. https://doi.org/10.3390/jcm12041323
Chicago/Turabian StyleChávez-Ocaña, Sonia del Carmen, Juan Carlos Bravata-Alcántara, Iliana Alejandra Cortés-Ortiz, Arturo Reyes-Sandoval, Jazmín García-Machorro, and Norma Estela Herrera-Gonzalez. 2023. "Parameters to Predict the Outcome of Severe and Critical COVID-19 Patients when Admitted to the Hospital" Journal of Clinical Medicine 12, no. 4: 1323. https://doi.org/10.3390/jcm12041323
APA StyleChávez-Ocaña, S. d. C., Bravata-Alcántara, J. C., Cortés-Ortiz, I. A., Reyes-Sandoval, A., García-Machorro, J., & Herrera-Gonzalez, N. E. (2023). Parameters to Predict the Outcome of Severe and Critical COVID-19 Patients when Admitted to the Hospital. Journal of Clinical Medicine, 12(4), 1323. https://doi.org/10.3390/jcm12041323







