Radiation Recall Pneumonitis Anticipates Bilateral Immune-Induced Pneumonitis in Non-Small Cell Lung Cancer
Abstract
:1. Introduction
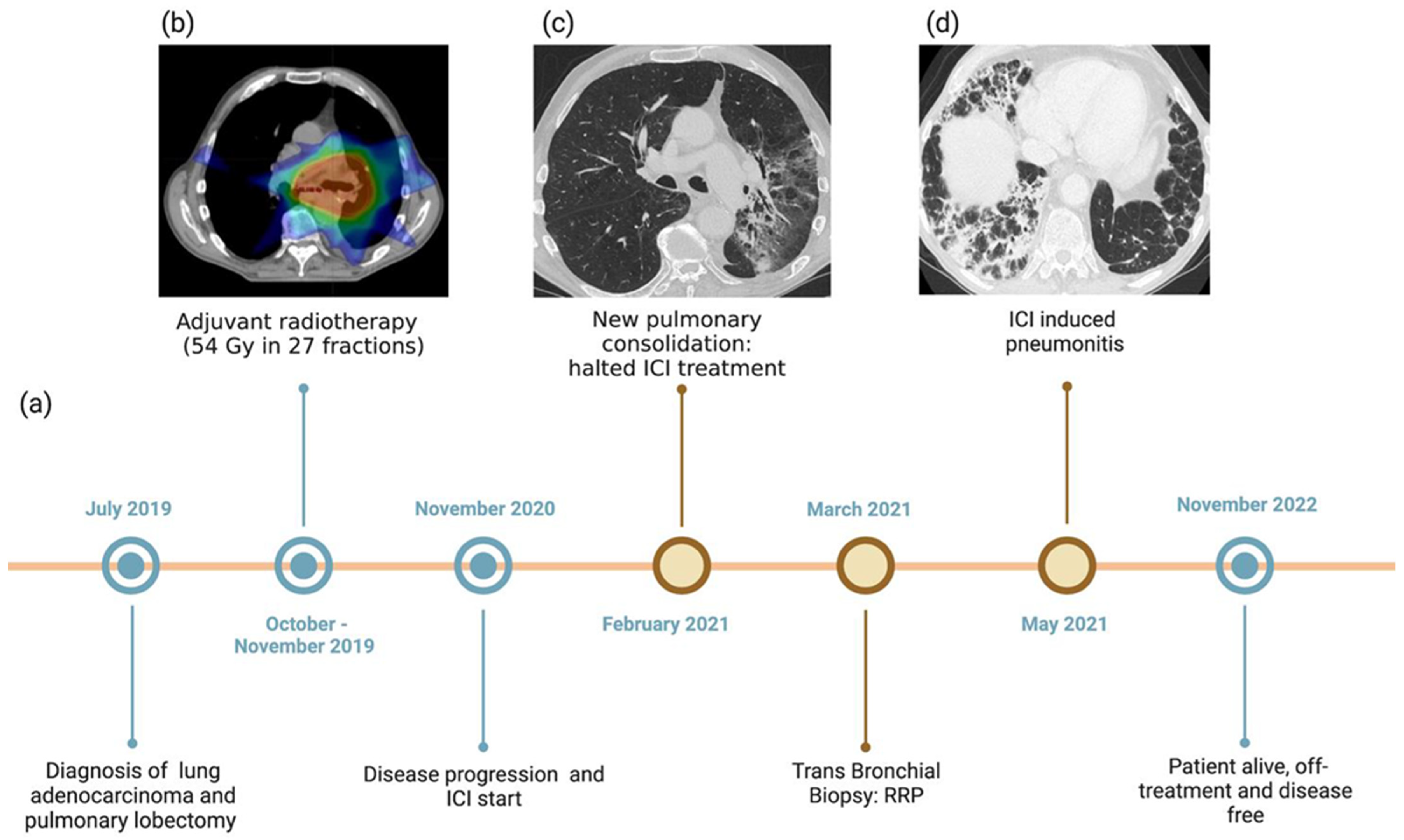
2. Case Presentation
3. Discussion
| Chest CT Findings | Clinical Findings and Onset Time | Other Exams/Tests | |
|---|---|---|---|
| Tumor progression/recurrence | Sequential enlargement of the mass/consolidation at the primary site; Enlargement occurring >12 months after RT completion; Disappearance of linear margins; development of bulging margins; Loss of air bronchogram; Increased soft tissue components. | Most local recurrences of malignancy occur within the first 3 years. | 18F-FDG-PET/CT demonstrating increased uptake Close follow-up with CT TBB (especially in high-risk patients) |
| Infection | Consolidation and/or ground-glass opacity with air bronchogram, Usually occurring outside the prior radiation field and/or not respecting the boundaries. | In the non-neutropenic patient, infection presentation, whether viral or bacterial, is similar to that in patients with no cancer. | Laboratory findings, e.g., blood culture, sputum culture, urinary antigen test, and sensitivity tests. Response to appropriate therapy |
| ICI-related pneumonitis | Several distinct radiologic patterns: OP is the most commonly seen, followed by NSIP and HP patterns. Usually bilateral, with involvement of >1 lobe, particularly lower lobes. Less likely to have well-demarcated borders. | Extremely variable onset time (fimmu). More frequent at around 3 months after ICI starts. Variable clinical presentation, ranging from asymptomatic to life-threatening respiratory compromise, possibly leading to death. | BAL: increased lymphocyte count, with CD4+ T-cells predominance TBB: inflammation and lymphocyte infiltration |
| Radiation recall pneumonitis | Consolidative or ground-glass opacities closely corresponding to the prior radiation field, with a well-defined linear demarcation from the adjacent normal lung. | Patient presentation ranged from asymptomatic to severely symptomatic, with non-productive cough and dyspnea. Median time interval between RT completion and IT-induced RRP onset: 450 days (range, 231–1859 days). Median time interval between IT initiation and RRP onset: 61 days (range, 4–520 days). | No well-established consensus on RRP diagnosis and treatment. Worth considering: treatment timeline, clinical symptoms, physical examination, radiological images revision, and TBB |
| Radiation-induced pneumonitis | Usually occurring in the radiated lung. Acute phase radiation pneumonitis: focal, diffuse, or non-uniform GGO and/or consolidative opacities in the high-dose region of the RT field, usually with sharp demarcation. Possible evolution into OP and CEP pattern: Consolidations can coalesce, with relatively sharp borders conforming to the radiation field rather than to anatomic boundaries. Chronic phase radiation fibrosis, in case of severe injury: Conventional pattern: irregular consolidation + volume loss + architectural distortion + traction bronchiectasis in the radiation field. Mass-like pattern: focal consolidation confined to the site of the original tumor. | The acute phase starts 4–12 weeks after RT completion The chronic phase commonly shows slow progression in the 6–12 months after RT completion, with stabilization within 2 years. | 18F-FDG-PET/CT demonstrating increased uptake Close follow-up with CT TBB (especially in high-risk patients) |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jan, P.-R.; Chang, J.W.-C. Radiation Recall Pneumonitis: A Rare Syndrome That Should Be Recognized. Cancers 2022, 14, 4642. [Google Scholar] [CrossRef]
- McGovern, K.; Ghaly, M. Radiation Recall Pneumonitis in the Setting of Immunotherapy and Radiation: A Focused Review. Future Sci. OA 2019, 5, FSO378. [Google Scholar] [CrossRef]
- Socinski, M.A.; Jotte, R.M. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef] [PubMed]
- Borst, J.; Busselar, J. Mechanism of action of PD-1 receptor/ligand targeted cancer immunotherapy. Eur. J. Immunol. 2021, 51, 1911–1920. [Google Scholar] [CrossRef] [PubMed]
- Anichini, A.; Perotti, V.E. Immune Escape Mechanisms in Non Small Cell Lung Cancer. Cancers 2020, 12, 3605. [Google Scholar] [CrossRef] [PubMed]
- Planchard, D.; Popat, S. Metastatic Non-Small Cell Lung Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2018, 29 (Suppl. 4), iv192–iv237. [Google Scholar] [CrossRef]
- Cousin, S.; Seneschal, J. Toxicity profiles of immunotherapy. Pharmacol. Ther. 2018, 181, 91–100. [Google Scholar] [CrossRef]
- Su, Q.; Zu, E.C. Risk of Pneumonitis and Pneumonia Associated with Immune Checkpoint Inhibitors for Solid Tumors: A Systematic Review and Meta-Analysis. Front. Immunol. 2019, 10, 108. [Google Scholar] [CrossRef]
- Schoenfeld, J.D.; Nishino, M. Pneumonitis Resulting from Radiation and Immune Checkpoint Blockade Illustrates Characteristic Clinical, Radiologic and Circulating Biomarker Features. J. Immunother. Cancer 2019, 7, 112. [Google Scholar] [CrossRef]
- Bradley, J.D. Long-Term Results of NRG Oncology RTOG 0617: Standard- Versus High-Dose Chemoradiotherapy with or without Cetuximab for Unresectable Stage III Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2020, 38, 706–714. [Google Scholar] [CrossRef]
- Kuang, Y.; Pierce, C.M. Chemoradiation-Induced Pneumonitis in Patients with Unresectable Stage III Non-Small Cell Lung Cancer: A Systematic Literature Review and Meta-Analysis. Lung Cancer 2022, 174, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Ryan, K.J.; Nero, D. Real-World Incidence and Cost of Pneumonitis Post-Chemoradiotherapy for Stage III Non-Small-Cell Lung Cancer. Future Oncol. 2020, 16, 4303–4313. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Hernández, M.; Maldonado, F. Radiation-Induced Lung Injury: Current Evidence. BMC Pulm. Med. 2021, 21, 9. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.W.; Day, C.N. Dosimetric Predictors of Pneumonitis in Locally Advanced Non-Small Cell Lung Cancer Patients Treated with Chemoradiation Followed by Durvalumab. Lung Cancer 2022, 170, 58–64. [Google Scholar] [CrossRef]
- Bledsoe, T.J.; Nath, S.K. Radiation Pneumonitis. Clin. Chest Med. 2017, 38, 201–208. [Google Scholar] [CrossRef]
- Zhang, X.J.; Sun, J.G. Prediction of Radiation Pneumonitis in Lung Cancer Patients: A Systematic Review. J. Cancer Res. Clin. Oncol. 2012, 138, 2103–2116. [Google Scholar] [CrossRef]
- Scott, J.A.; Villegas, A. Durvalumab after Chemoradiotherapy in Stage III Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef]
- Saito, G.; Oya, Y. Real-World Survey of Pneumonitis and Its Impact on Durvalumab Consolidation Therapy in Patients with Non-Small Cell Lung Cancer Who Received Chemoradiotherapy after Durvalumab Approval (HOPE-005/CRIMSON). Lung Cancer 2021, 161, 86–93. [Google Scholar] [CrossRef]
- Borghaei, H.; Gettinger, S. Five-Year Outcomes From the Randomized, Phase III Trials CheckMate 017 and 057: Nivolumab Versus Docetaxel in Previously Treated Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2021, 39, 723–733. [Google Scholar] [CrossRef]
- Reck, M.; Rodriguez-Abreu, D. Five-Year Outcomes with Pembrolizumab versus Chemotherapy for Metastatic Non-Small-Cell Lung Cancer with PD-L1 Tumor Proportion Score ≥ 50. J. Clin. Oncol. 2021, 39, 2339–2349. [Google Scholar] [CrossRef]
- Cui, P.; Liu, Z. Risk Factors for Pneumonitis in Patients Treated with Anti-programmed Death-1 Therapy: A Case-control Study. Cancer Med. 2018, 7, 4115–4120. [Google Scholar] [CrossRef] [PubMed]
- Haanen, J.; Obeid, M. Management of Toxicities from Immunotherapy: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2022, 33, 1217–1238. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, J.; Cottrell, T.L. Chronic Immune Checkpoint Inhibitor Pneumonitis. J. Immunother. Cancer 2020, 8, e000840. [Google Scholar] [CrossRef]
- Couey, M.A.; Bell, R.B. Delayed immune-related events (DIRE) after discontinuation of immunotherapy: Diagnostic hazard of autoimmunity at a distance. J. Immunother. Cancer 2019, 7, 165. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.J.; Ma, B.T. Delayed toxicities with anti-PD-1 and anti-PDL-1 immune checkpoint inhibitors (ICIs). J. Clin. Oncol. 2018, 36 (Suppl. 15), e15074. [Google Scholar] [CrossRef]
- Cousin, F.; Desir, C. Incidence, risk factors, and CT characteristics of radiation recall pneumonitis induced by immune checkpoint inhibitor in lung cancer. Radiother. Oncol. 2021, 157, 47–55. [Google Scholar] [CrossRef]
- McKay, M.J.; Foster, R. Radiation Recall Reactions: An Oncologic Enigma. Crit. Rev. Oncol. Hematol. 2021, 168, 103527. [Google Scholar] [CrossRef]
- Sharabi, A.B.; Lim, M. Radiation and Checkpoint Blockade Immunotherapy: Radiosensitisation and Potential Mechanisms of Synergy. Lancet Oncol. 2015, 16, e498–e509. [Google Scholar] [CrossRef]
- Riviere, P.; Sumner, W. Radiation Recall Pneumonitis After Treatment with Checkpoint Blockade Immunotherapy: A Case Series and Review of Literature. Front. Oncol. 2021, 11, 662954. Available online: https://www.frontiersin.org/articles/10.3389/fonc.2021.662954 (accessed on 6 December 2022). [CrossRef]
- Teng, F.; Li, M. Radiation Recall Pneumonitis Induced by PD-1/PD-L1 Blockades: Mechanisms and Therapeutic Implications. BMC Med. 2020, 18, 275. [Google Scholar] [CrossRef]
- Deutsch, E.; Besse, B. Can radiation-recall predict long lasting response to immune checkpoint inhibitors? Radiother. Oncol. 2020, 154, 125–127. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, Z. Radiation Recall Pneumonitis Induced by Anti-PD-1 Blockade: A Case Report and Review of the Literature. Front. Oncol. 2020, 10, 561. Available online: https://www.frontiersin.org/articles/10.3389/fonc.2020.00561 (accessed on 6 December 2022). [CrossRef] [PubMed]
- Kalisz, K.R.; Ramaiya, N.H. Immune Checkpoint Inhibitor Therapy–Related Pneumonitis: Patterns and Management. Radiographics 2019, 39, 1923–1937. [Google Scholar] [CrossRef] [PubMed]
- Nobashi, T.W.; Nishimoto, Y. Clinical and Radiological Features of Immune Checkpoint Inhibitor-Related Pneumonitis in Lung Cancer and Non-Lung Cancers. Br. J. Radiol. 2020, 93, 1115. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Pan, Y. Differentiation between Immune Checkpoint Inhibitor-related and Radiation Pneumonitis in Lung Cancer by CT Radiomics and Machine Learning. Med. Phys. 2022, 49, 1547–1558. [Google Scholar] [CrossRef]
- Qiu, Q.; Xing, L. Development and Validation of a Radiomics Nomogram Using Computed Tomography for Differentiating Immune Checkpoint Inhibitor-Related Pneumonitis From Radiation Pneumonitis for Patients with Non-Small Cell Lung Cancer. Front. Immunol. 2022, 13, 870842. Available online: https://www.frontiersin.org/articles/10.3389/fimmu.2022.870842 (accessed on 6 December 2022). [CrossRef]
- Strange, C.D.; Shroff, G.S. Imaging of the post-radiation chest in lung cancer. Clin. Radiol. 2022, 77, 19–30. [Google Scholar] [CrossRef]
- Yin, J.; Wu, Y. Checkpoint Inhibitor Pneumonitis Induced by Anti-PD-1/PD-L1 Therapy in Non-Small-Cell Lung Cancer: Occurrence and Mechanism. Front. Immunol. 2022, 7, 830631. [Google Scholar] [CrossRef]
- Cho, J.Y.; Kim, J. Characteristics, incidence, and risk factors of immune checkpoint inhibitor-related pneumonitis in patients with non-small cell lung cancer. Lung Cancer 2018, 125, 150–156. [Google Scholar] [CrossRef]
- Colen, R.R.; Fujii, T. Radiomics to predict immunotherapy-induced pneumonitis: Proof of concept. Investig. New Drugs 2018, 36, 601–607. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torresan, S.; Zussino, G.; Cortiula, F.; Follador, A.; Fasola, G.; Girometti, R.; Cereser, L. Radiation Recall Pneumonitis Anticipates Bilateral Immune-Induced Pneumonitis in Non-Small Cell Lung Cancer. J. Clin. Med. 2023, 12, 1266. https://doi.org/10.3390/jcm12041266
Torresan S, Zussino G, Cortiula F, Follador A, Fasola G, Girometti R, Cereser L. Radiation Recall Pneumonitis Anticipates Bilateral Immune-Induced Pneumonitis in Non-Small Cell Lung Cancer. Journal of Clinical Medicine. 2023; 12(4):1266. https://doi.org/10.3390/jcm12041266
Chicago/Turabian StyleTorresan, Sara, Gaia Zussino, Francesco Cortiula, Alessandro Follador, Gianpiero Fasola, Rossano Girometti, and Lorenzo Cereser. 2023. "Radiation Recall Pneumonitis Anticipates Bilateral Immune-Induced Pneumonitis in Non-Small Cell Lung Cancer" Journal of Clinical Medicine 12, no. 4: 1266. https://doi.org/10.3390/jcm12041266
APA StyleTorresan, S., Zussino, G., Cortiula, F., Follador, A., Fasola, G., Girometti, R., & Cereser, L. (2023). Radiation Recall Pneumonitis Anticipates Bilateral Immune-Induced Pneumonitis in Non-Small Cell Lung Cancer. Journal of Clinical Medicine, 12(4), 1266. https://doi.org/10.3390/jcm12041266






