C-Reactive Protein and White Blood Cell Count in Cardiogenic Shock
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Patients, Design and Data Collection
2.2. Inclusion and Exclusion Criteria, Study Endpoints
2.3. Measurement of WBC Count and C-Reactive Protein
2.4. Statistical Methods
3. Results
3.1. Study Population
3.2. Correlation of Baseline CRP and WBC with Clinical and Laboratory Data
3.3. Prognostic Performance of CRP and WBC
3.4. Multivariate Cox Regression Models
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hochman, J.S.; Sleeper, L.A.; Webb, J.G.; Sanborn, T.A.; White, H.D.; Talley, J.D.; Buller, C.E.; Jacobs, A.K.; Slater, J.N.; Col, J.; et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N. Engl. J. Med. 1999, 341, 625–634. [Google Scholar] [CrossRef]
- Thiele, H.; Zeymer, U.; Neumann, F.J.; Ferenc, M.; Olbrich, H.G.; Hausleiter, J.; Richardt, G.; Hennersdorf, M.; Empen, K.; Fuernau, G.; et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N. Engl. J. Med. 2012, 367, 1287–1296. [Google Scholar] [CrossRef]
- Reynolds, H.R.; Hochman, J.S. Cardiogenic shock: Current concepts and improving outcomes. Circulation 2008, 117, 686–697. [Google Scholar] [CrossRef]
- Hochman, J.S. Cardiogenic shock complicating acute myocardial infarction: Expanding the paradigm. Circulation 2003, 107, 2998–3002. [Google Scholar] [CrossRef]
- Samsky, M.D.; Morrow, D.A.; Proudfoot, A.G.; Hochman, J.S.; Thiele, H.; Rao, S.V. Cardiogenic Shock After Acute Myocardial Infarction: A Review. JAMA 2021, 326, 1840–1850. [Google Scholar] [CrossRef]
- Lassus, J.; Tarvasmäki, T.; Tolppanen, H. Biomarkers in cardiogenic shock. Adv. Clin. Chem. 2022, 109, 31–73. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Fuernau, G.; Desch, S.; Freund, A.; Feistritzer, H.J.; Pöss, J.; Besler, C.; Lurz, P.; Büttner, P.; Thiele, H. Circulating Galectin-3 in Patients with Cardiogenic Shock Complicating Acute Myocardial Infarction Treated with Mild Hypothermia: A Biomarker Sub-Study of the SHOCK-COOL Trial. J. Clin. Med. 2022, 11, 7168. [Google Scholar] [CrossRef] [PubMed]
- Boras, E.; Slevin, M.; Alexander, M.Y.; Aljohi, A.; Gilmore, W.; Ashworth, J.; Krupinski, J.; Potempa, L.A.; Al Abdulkareem, I.; Elobeid, A.; et al. Monomeric C-reactive protein and Notch-3 co-operatively increase angiogenesis through PI3K signalling pathway. Cytokine 2014, 69, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef] [PubMed]
- Clyne, B.; Olshaker, J.S. The C-reactive protein. J. Emerg. Med. 1999, 17, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Mani, P.; Puri, R.; Schwartz, G.G.; Nissen, S.E.; Shao, M.; Kastelein, J.J.P.; Menon, V.; Lincoff, A.M.; Nicholls, S.J. Association of Initial and Serial C-Reactive Protein Levels with Adverse Cardiovascular Events and Death After Acute Coronary Syndrome: A Secondary Analysis of the VISTA-16 Trial. JAMA Cardiol. 2019, 4, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Kataja, A.; Tarvasmäki, T.; Lassus, J.; Sionis, A.; Mebazaa, A.; Pulkki, K.; Banaszewski, M.; Carubelli, V.; Hongisto, M.; Jankowska, E.; et al. Kinetics of procalcitonin, C-reactive protein and interleukin-6 in cardiogenic shock—Insights from the CardShock study. Int. J. Cardiol. 2021, 322, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Anand, I.S.; Latini, R.; Florea, V.G.; Kuskowski, M.A.; Rector, T.; Masson, S.; Signorini, S.; Mocarelli, P.; Hester, A.; Glazer, R.; et al. C-reactive protein in heart failure: Prognostic value and the effect of valsartan. Circulation 2005, 112, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Lamblin, N.; Mouquet, F.; Hennache, B.; Dagorn, J.; Susen, S.; Bauters, C.; de Groote, P. High-sensitivity C-reactive protein: Potential adjunct for risk stratification in patients with stable congestive heart failure. Eur. Heart J. 2005, 26, 2245–2250. [Google Scholar] [CrossRef]
- Welsh, C.; Welsh, P.; Mark, P.B.; Celis-Morales, C.A.; Lewsey, J.; Gray, S.R.; Lyall, D.M.; Iliodromiti, S.; Gill, J.M.R.; Pell, J.; et al. Association of Total and Differential Leukocyte Counts with Cardiovascular Disease and Mortality in the UK Biobank. Arter. Thromb. Vasc. Biol. 2018, 38, 1415–1423. [Google Scholar] [CrossRef]
- Brown, D.W.; Giles, W.H.; Croft, J.B. White blood cell count: An independent predictor of coronary heart disease mortality among a national cohort. J. Clin. Epidemiol. 2001, 54, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.; Wiberg, S.; Hassager, C.; Winther-Jensen, M.; Frikke-Schmidt, R.; Bang, L.E.; Lindholm, M.G.; Holmvang, L.; Moeller-Helgestad, O.; Ravn, H.B.; et al. Admission Leukocyte Count is Associated with Late Cardiogenic Shock Development and All-Cause 30-Day Mortality in Patients with St-Elevation Myocardial Infarction. Shock 2020, 53, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Desch, S.; Freund, A.; Akin, I.; Behnes, M.; Preusch, M.R.; Zelniker, T.A.; Skurk, C.; Landmesser, U.; Graf, T.; Eitel, I.; et al. Angiography after Out-of-Hospital Cardiac Arrest without ST-Segment Elevation. N. Engl. J. Med. 2021, 385, 2544–2553. [Google Scholar] [CrossRef]
- Thiele, H.; Akin, I.; Sandri, M.; Fuernau, G.; de Waha, S.; Meyer-Saraei, R.; Nordbeck, P.; Geisler, T.; Landmesser, U.; Skurk, C.; et al. PCI Strategies in Patients with Acute Myocardial Infarction and Cardiogenic Shock. N. Engl. J. Med. 2017, 377, 2419–2432. [Google Scholar] [CrossRef]
- Thiele, H.; Akin, I.; Sandri, M.; de Waha-Thiele, S.; Meyer-Saraei, R.; Fuernau, G.; Eitel, I.; Nordbeck, P.; Geisler, T.; Landmesser, U.; et al. One-Year Outcomes after PCI Strategies in Cardiogenic Shock. N. Engl. J. Med. 2018, 379, 1699–1710. [Google Scholar] [CrossRef]
- Zeymer, U.; Bueno, H.; Granger, C.B.; Hochman, J.; Huber, K.; Lettino, M.; Price, S.; Schiele, F.; Tubaro, M.; Vranckx, P.; et al. Acute Cardiovascular Care Association position statement for the diagnosis and treatment of patients with acute myocardial infarction complicated by cardiogenic shock: A document of the Acute Cardiovascular Care Association of the European Society of Cardiology. Eur. Heart J. Acute Cardiovasc. Care 2020, 9, 183–197. [Google Scholar] [CrossRef]
- Forner, J.; Schupp, T.; Weidner, K.; Rusnak, J.; Jawhar, S.; Dulatahu, F.; Brück, L.M.; Behnes, M.; Hoffmann, U.; Bertsch, T.; et al. Cardiac Troponin I Reveals Diagnostic and Prognostic Superiority to Aminoterminal Pro-B-Type Natriuretic Peptide in Sepsis and Septic Shock. J. Clin. Med. 2022, 11, 6592. [Google Scholar] [CrossRef]
- Hanley, J.A.; McNeil, B.J. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 1983, 148, 839–843. [Google Scholar] [CrossRef]
- Johansson, P.I.; Stensballe, J.; Ostrowski, S.R. Shock induced endotheliopathy (SHINE) in acute critical illness—A unifying pathophysiologic mechanism. Crit. Care 2017, 21, 25. [Google Scholar] [CrossRef] [PubMed]
- Barron, H.V.; Cannon, C.P.; Murphy, S.A.; Braunwald, E.; Gibson, C.M. Association between white blood cell count, epicardial blood flow, myocardial perfusion, and clinical outcomes in the setting of acute myocardial infarction: A thrombolysis in myocardial infarction 10 substudy. Circulation 2000, 102, 2329–2334. [Google Scholar] [CrossRef]
- Ohlmann, P.; Jaquemin, L.; Morel, O.; El Behlgiti, R.; Faure, A.; Michotey, M.O.; Beranger, N.; Roul, G.; Schneider, F.; Bareiss, P.; et al. Prognostic value of C-reactive protein and cardiac troponin I in primary percutaneous interventions for ST-elevation myocardial infarction. Am. Heart J. 2006, 152, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Tomoda, H.; Aoki, N. Prognostic value of C-reactive protein levels within six hours after the onset of acute myocardial infarction. Am. Heart J. 2000, 140, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Ortolani, P.; Marzocchi, A.; Marrozzini, C.; Palmerini, T.; Saia, F.; Taglieri, N.; Baldazzi, F.; Silenzi, S.; Bacchi-Reggiani, M.L.; Guastaroba, P.; et al. Predictive value of high sensitivity C-reactive protein in patients with ST-elevation myocardial infarction treated with percutaneous coronary intervention. Eur. Heart J. 2008, 29, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Kasai, T.; Sato, A.; Ishiwata, S.; Yatsu, S.; Shitara, J.; Murata, A.; Kato, T.; Suda, S.; Matsue, Y.; et al. Association between C-reactive protein levels at hospital admission and long-term mortality in patients with acute decompensated heart failure. Heart Vessel. 2019, 34, 1961–1968. [Google Scholar] [CrossRef] [PubMed]
- Minami, Y.; Kajimoto, K.; Sato, N.; Hagiwara, N. Effect of Elevated C-Reactive Protein Level at Discharge on Long-Term Outcome in Patients Hospitalized for Acute Heart Failure. Am. J. Cardiol. 2018, 121, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, R.F. C-reactive protein, inflammation, and innate immunity. Immunol. Res. 2001, 24, 163–176. [Google Scholar] [CrossRef]
- Gabay, C.; Kushner, I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999, 340, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Du Clos, T.W.; Mold, C. C-reactive protein: An activator of innate immunity and a modulator of adaptive immunity. Immunol. Res. 2004, 30, 261–277. [Google Scholar] [CrossRef]
- Póvoa, P. C-reactive protein: A valuable marker of sepsis. Intensive Care Med. 2002, 28, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.J.Y.; Angus, M.I.; Nah, S.A.; Jacobsen, A.S.; Low, Y.; Choo, C.S.C.; Yap, T.L.; Chen, Y. Time course response of inflammatory markers in pediatric appendicitis. Pediatr. Surg. Int. 2020, 36, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Colley, C.M.; Fleck, A.; Goode, A.W.; Muller, B.R.; Myers, M.A. Early time course of the acute phase protein response in man. J. Clin. Pathol. 1983, 36, 203–207. [Google Scholar] [CrossRef]
- Castelli, G.P.; Pognani, C.; Meisner, M.; Stuani, A.; Bellomi, D.; Sgarbi, L. Procalcitonin and C-reactive protein during systemic inflammatory response syndrome, sepsis and organ dysfunction. Crit. Care 2004, 8, R234–R242. [Google Scholar] [CrossRef] [PubMed]
- Bahloul, M.; Bradii, S.; Turki, M.; Bouchaala, K.; Ben Hamida, C.; Chelly, H.; Ayedi, F.; Bouaziz, M. The value of sepsis biomarkers and their kinetics in the prognosis of septic shock due to bacterial infections. Anaesthesiol. Intensive Ther. 2021, 53, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Miki, T.; Iba, T. Kinetics of Circulating Damage-Associated Molecular Patterns in Sepsis. J. Immunol. Res. 2015, 2015, 424575. [Google Scholar] [CrossRef]
- Field, C.J.; Gougeon, R.; Marliss, E.B. Circulating mononuclear cell numbers and function during intense exercise and recovery. J. Appl. Physiol. 1991, 71, 1089–1097. [Google Scholar] [CrossRef]
- Gray, A.B.; Telford, R.D.; Collins, M.; Weidemann, M.J. The response of leukocyte subsets and plasma hormones to interval exercise. Med. Sci. Sport. Exerc. 1993, 25, 1252–1258. [Google Scholar] [CrossRef]
- McCarthy, D.A.; Macdonald, I.; Grant, M.; Marbut, M.; Watling, M.; Nicholson, S.; Deeks, J.J.; Wade, A.J.; Perry, J.D. Studies on the immediate and delayed leucocytosis elicited by brief (30-min) strenuous exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1992, 64, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Brenner, I.; Shek, P.N.; Zamecnik, J.; Shephard, R.J. Stress hormones and the immunological responses to heat and exercise. Int. J. Sport. Med. 1998, 19, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Soppi, E.; Lindroos, M.; Nikoskelainen, J.; Kalliomäki, J.L. Effect of cardiopulmonary resuscitation-induced stress on cell-mediated immunity. Intensive Care Med. 1984, 10, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, S.; Lange, T.; Born, J. Selective mobilization of cytotoxic leukocytes by epinephrine. J. Immunol. 2010, 184, 503–511. [Google Scholar] [CrossRef]
- Scanzano, A.; Cosentino, M. Adrenergic regulation of innate immunity: A review. Front. Pharm. 2015, 6, 171. [Google Scholar] [CrossRef] [PubMed]
- Benschop, R.J.; Rodriguez-Feuerhahn, M.; Schedlowski, M. Catecholamine-induced leukocytosis: Early observations, current research, and future directions. Brain Behav. Immun. 1996, 10, 77–91. [Google Scholar] [CrossRef]
- Sasmita, B.R.; Zhu, Y.; Gan, H.; Hu, X.; Xue, Y.; Xiang, Z.; Liu, G.; Luo, S.; Huang, B. Leukocyte and its Subtypes as Predictors of Short-Term Outcome in Cardiogenic Shock Complicating Acute Myocardial Infarction: A Cohort Study. Shock 2022, 57, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Akkus, M.N.; Polat, G.; Yurtdas, M.; Akcay, B.; Ercetin, N.; Cicek, D.; Doven, O.; Sucu, N. Admission levels of C-reactive protein and plasminogen activator inhibitor-1 in patients with acute myocardial infarction with and without cardiogenic shock or heart failure on admission. Int. Heart J. 2009, 50, 33–45. [Google Scholar] [CrossRef]
- Schrage, B.; Becher, P.M.; Bernhardt, A.; Bezerra, H.; Blankenberg, S.; Brunner, S.; Colson, P.; Cudemus Deseda, G.; Dabboura, S.; Eckner, D.; et al. Left Ventricular Unloading Is Associated with Lower Mortality in Patients with Cardiogenic Shock Treated with Venoarterial Extracorporeal Membrane Oxygenation: Results from an International, Multicenter Cohort Study. Circulation 2020, 142, 2095–2106. [Google Scholar] [CrossRef]
- Shah, T.; Newcombe, P.; Smeeth, L.; Addo, J.; Casas, J.P.; Whittaker, J.; Miller, M.A.; Tinworth, L.; Jeffery, S.; Strazzullo, P.; et al. Ancestry as a determinant of mean population C-reactive protein values: Implications for cardiovascular risk prediction. Circ. Cardiovasc. Genet. 2010, 3, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Best, L.G.; Zhang, Y.; Lee, E.T.; Yeh, J.L.; Cowan, L.; Palmieri, V.; Roman, M.; Devereux, R.B.; Fabsitz, R.R.; Tracy, R.P.; et al. C-reactive protein as a predictor of cardiovascular risk in a population with a high prevalence of diabetes: The Strong Heart Study. Circulation 2005, 112, 1289–1295. [Google Scholar] [CrossRef] [PubMed]
- Siemens Healthcare Diagnostics Inc. Assays der Atellica CH und IM Systeme sowie Dimension Vista im Vergleich. In CH-89 CH und 1 IM-28 IM: Assayspezifische Methodenvergleichsgrafiken—Regressions-und Differenzgrafiken; Übereinstimmungstabellen, 17.08.2018; Siemens Healthcare Diagnostics Inc.: Deerfield, IL, USA, 2018; p. 84. Available online: https://www.siemens-healthineers.com/de/laboratory-diagnostics (accessed on 13 December 2022).
- Zacho, J.; Tybjaerg-Hansen, A.; Jensen, J.S.; Grande, P.; Sillesen, H.; Nordestgaard, B.G. Genetically elevated C-reactive protein and ischemic vascular disease. N. Engl. J. Med. 2008, 359, 1897–1908. [Google Scholar] [CrossRef] [PubMed]

| All Patients (n = 240) | Survivor (n = 108) | Non-Survivor (n = 132) | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Age , median; (IQR) | 74 | (63–81) | 72 | (62–80) | 74 | (64–81) | 0.284 |
| Male sex, n (%) | 149 | (62.1) | 70 | (64.8) | 79 | (59.8) | 0.430 |
| Body mass index (kg/m2), median; (IQR) | 26.60 | (24.20–30.25) | 26.20 | (24.20–29.40) | 26.80 | (24.50–30.50) | 0.228 |
| Vital signs, median; (IQR) | |||||||
| Body temperature (°C) | 36.0 | (34.9–36.5) | 36.1 | (35.3–36.6) | 35.8 | (34.6–36.5) | 0.026 |
| Heart rate (bpm) | 88 | (71–110) | 85 | (69–107) | 95 | (72–112) | 0.088 |
| Systolic blood pressure (mmHg) | 109 | (93–130) | 109 | (93–130) | 107 | (91–128) | 0.355 |
| Respiratory rate (breaths/min) | 20 | (17–24) | 19 | (16–23) | 20 | (18–25) | 0.144 |
| Cardiovascular risk factors,n (%) | |||||||
| Arterial hypertension | 175 | (72.9) | 82 | (75.9) | 93 | (70.5) | 0.343 |
| Diabetes mellitus | 98 | (41.0) | 39 | (36.4) | 59 | (44.7) | 0.197 |
| Hyperlipidemia | 130 | (54.2) | 60 | (55.6) | 70 | (53.0) | 0.696 |
| Smoking | 86 | (36.0) | 39 | (36.4) | 47 | (35.6) | 0.893 |
| Prior medical history,n (%) | |||||||
| Coronary artery disease | 89 | (37.1) | 41 | (38.0) | 48 | (36.4) | 0.508 |
| Congestive heart failure | 85 | (35.4) | 40 | (37.0) | 45 | (34.1) | 0.635 |
| Atrial fibrillation | 80 | (33.3) | 37 | (34.3) | 43 | (32.6) | 0.783 |
| Chronic kidney disease | 85 | (35.4) | 39 | (36.1) | 46 | (34.8) | 0.839 |
| Stroke | 32 | (13.3) | 18 | (16.7) | 14 | (10.6) | 0.169 |
| COPD | 46 | (19.2) | 18 | (16.7) | 28 | (21.2) | 0.373 |
| Liver cirrhosis | 8 | (3.3) | 5 | (4.6) | 3 | (2.3) | 0.317 |
| Malignancy | 38 | (15.8) | 17 | (15.7) | 21 | (15.9) | 0.972 |
| Immunosuppression | 19 | (7.9) | 6 | (5.6) | 13 | (9.8) | 0.220 |
| Medication on admission, n (%) | |||||||
| ACE-inhibitor | 82 | (37.1) | 39 | (36.8) | 43 | (37.4) | 0.927 |
| ARB | 40 | (18.0) | 20 | (18.7) | 20 | (17.4) | 0.801 |
| Beta-blocker | 122 | (55.2) | 59 | (55.7) | 63 | (54.8) | 0.896 |
| ARNI | 8 | (3.6) | 5 | (4.7) | 3 | (2.6) | 0.395 |
| Aldosterone antagonist | 39 | (17.7) | 18 | (17.1) | 21 | (18.3) | 0.828 |
| Diuretics | 105 | (47.3) | 46 | (43.4) | 59 | (50.9) | 0.266 |
| ASA | 66 | (27.5) | 31 | (28.7) | 35 | (26.5) | 0.706 |
| P2Y12-inhibitor | 18 | (7.5) | 8 | (7.4) | 10 | (7.6) | 0.961 |
| Statin | 107 | (48.2) | 55 | (51.9) | 52 | (44.8) | 0.293 |
| All Patients (n = 240) | Survivor (n = 108) | Non-Survivor (n = 132) | p Value | ||||
|---|---|---|---|---|---|---|---|
| Cause of CS, n (%) | |||||||
| Acute myocardial infarction | 115 | (47.9) | 41 | (38.0) | 74 | (56.1) | |
| Arrhythmic | 28 | (11.7) | 21 | (19.4) | 7 | (5.3) | |
| ADHF | 63 | (26.3) | 27 | (25.0) | 36 | (27.3) | |
| Pulmonary embolism | 13 | (5.4) | 4 | (3.7) | 9 | (6.8) | 0.001 |
| Vitium | 11 | (4.6) | 8 | (7.4) | 3 | (2.3) | |
| Cardiomyopathy | 7 | (2.9) | 4 | (3.7) | 3 | (2.3) | |
| Pericardial tamponade | 3 | (1.3) | 3 | (2.8) | 0 | (0.0) | |
| Classification of CS, n (%) | |||||||
| Stage A | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | |
| Stage B | 6 | (2.5) | 6 | (5.6) | 0 | (0.0) | |
| Stage C | 87 | (36.6) | 51 | (47.2) | 36 | (27.3) | 0.001 |
| Stage D | 19 | (7.9) | 9 | (8.3) | 10 | (7.6) | |
| Stage E | 128 | (53.3) | 42 | (38.9) | 86 | (65.2) | |
| Transthoracic echocardiography | |||||||
| LVEF >55%, n, (%) | 25 | (10.4) | 14 | (13.0) | 11 | (8.3) | |
| LVEF 54–41%, n, (%) | 28 | (11.7) | 18 | (16.7) | 10 | (7.6) | |
| LVEF 40–30%, n, (%) | 54 | (22.5) | 31 | (28.7) | 23 | (17.4) | 0.004 |
| LVEF <30%, n, (%) | 117 | (48.8) | 40 | (37.0) | 77 | (58.3) | |
| LVEF not documented, n, (%) | 16 | (6.7) | 5 | (4.6) | 11 | (8.3) | |
| VCI (cm), median; (IQR) | 1.8 | (1.5–2.2) | 1.8 | (1.5–2.2) | 1.9 | (1.6–2.2) | 0.345 |
| TAPSE (mm), median; (IQR) | 15 | (11–18) | 17 | (11–20) | 14 | (11–17) | 0.066 |
| Cardiopulmonary resuscitation | |||||||
| OHCA, n (%) | 93 | (38.8) | 34 | (31.5) | 59 | (44.7) | 0.001 |
| IHCA, n (%) | 35 | (14.6) | 8 | (7.4) | 27 | (20.5) | |
| Shockable rhythm, n (%) | 171 | (71.8) | 78 | (72.9) | 93 | (71.0) | 0.745 |
| Non-shockable rhythm, n (%) | 67 | (28.2) | 29 | (27.1) | 38 | (29.0) | |
| ROSC (min), median; (IQR) | 15 | (10–29) | 12 | (5–20) | 17 | (11–30) | 0.004 |
| Respiratory status | |||||||
| Mechanical ventilation, n (%) | 141 | (59.5) | 54 | (50.0) | 87 | (67.4) | 0.006 |
| Duration of mechanical ventilation (days), mean; (IQR) | 2 | (1–6) | 2 | (0–7) | 2 | (1–5) | 0.097 |
| PaO2/FiO2 ratio, median; (IQR) | 206 | (133–338) | 200 | (143–356) | 213 | (121–337) | 0.737 |
| PaO2, mmHg, median; (IQR) | 102 | (78–160) | 101 | (78–145) | 103 | (77–165) | 0.661 |
| Multiple organ support during ICU | |||||||
| Dosis norepinephrine on admission (µg/kg/min), median; (IQR) | 0.1 | (0.0–0.3) | 0.1 | (0.0–0.2) | 0.2 | (0.1–0.6) | 0.001 |
| Mechanical circulatory assist device, n (%) | 21 | (8.8) | 3 | (2.8) | 18 | (13.6) | 0.003 |
| Baseline laboratory values, (median, (IQR)) | |||||||
| pH | 7.29 | (7.22–7.37) | 7.31 | (7.24–7.37) | 7.28 | (7.19–7.37) | 0.049 |
| Lactate (mmol/L) | 3.4 | (1.7–6.9) | 2.7 | (1.6–4.3) | 4.5 | (2.2–9.7) | 0.001 |
| Sodium (mmol/L) | 138 | (136–141) | 138 | (136–140) | 138 | (136–141) | 0.460 |
| Potassium (mmol/L) | 4.3 | (3.8–4.9) | 4.2 | (3.7–4.9) | 4.4 | (3.9–5.0) | 0.449 |
| Creatinine (mg/dL) | 1.49 | (1.16–2.19) | 1.36 | (1.07–2.00) | 1.60 | (1.23–2.31) | 0.014 |
| Hemoglobin (g/dL) | 12.4 | (10.3–14.0) | 12.5 | (10.0–14.2) | 12.4 | (10.7–13.8) | 0.701 |
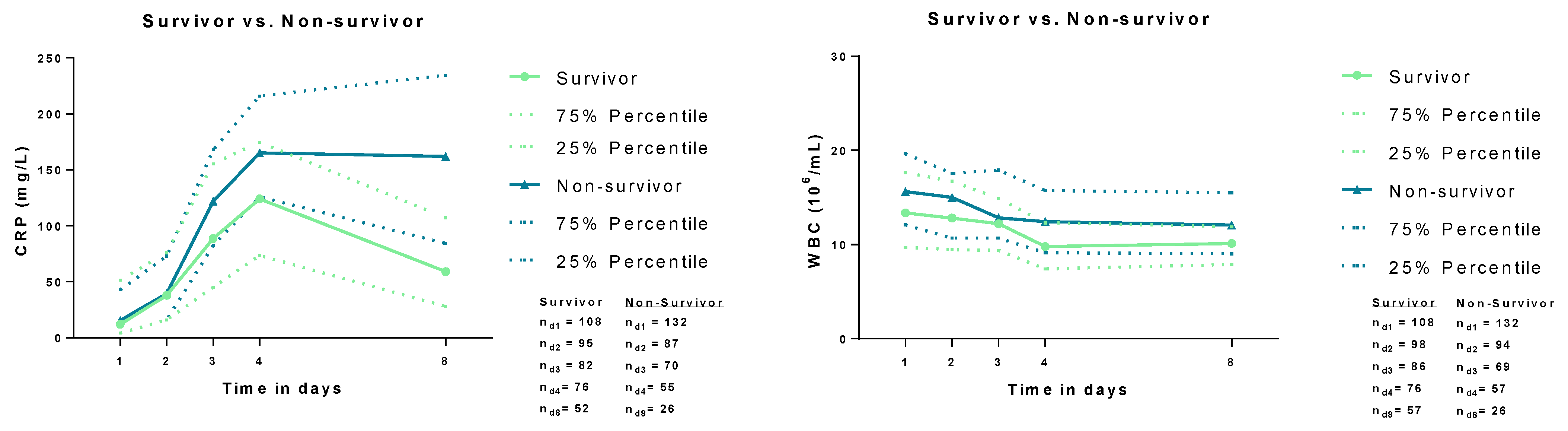
| WBC count (106/mL) | 14.73 | (10.45–18.69) | 13.36 | (9.70–17.65) | 15.61 | (12.10–19.65) | 0.005 |
| Platelets (106/mL) | 222 | (167–272) | 218 | (159–286) | 224 | (176–263) | 0.964 |
| INR | 1.18 | (1.08–1.39) | 1.13 | (1.05–1.32) | 1.20 | (1.11–1.46) | 0.001 |
| D-dimer (mg/L) | 9.86 | (2.46–32.00) | 5.49 | (1.99–15.74) | 18.19 | (3.46–32.00) | 0.005 |
| AST (U/L) | 129 | (45–312) | 104 | (37–205) | 167 | (61–488) | 0.016 |
| ALT (U/L) | 77 | (32–178) | 55 | (29–113) | 96 | (35–254) | 0.019 |
| Bilirubin (mg/dL) | 0.62 | (0.43–1.00) | 0.60 | (0.41–0.96) | 0.65 | (0.46–1.00) | 0.346 |
| Troponin I (µg/L) | 0.764 | (0.169–6.158) | 0.335 | (0.092–2.511) | 1.929 | (0.349–12.430) | 0.001 |
| NT-pro BNP (pg/mL) | 4866 | (971–13618) | 4480 | (479–12842) | 5281 | (1245–14104) | 0.213 |
| Procalcitonin (ng/mL) | 0.30 | (0.11–0.94) | 0.31 | (0.07–0.67) | 0.28 | (0.17–1.38) | 0.529 |
| CRP (mg/L) | 13 | (4–46) | 12 | (4–51) | 16 | (4–43) | 0.698 |
| Primary endpoint | |||||||
| All-cause mortality at 30 days, n (%) | 132 | (55.0) | 0 | (0.0) | 132 | (100.0) | - |
| Follow up data, n (%) | |||||||
| ICU time (days), median; (IQR) | 4 | (2–8) | 4 | (3–10) | 3 | (2–6) | 0.001 |
| Death ICU, n (%) | 131 | (54.6) | 4 | (3.7) | 127 | (96.2) | 0.001 |
| CRP | WBC | |||
|---|---|---|---|---|
| r | p-Value | r | p-Value | |
| Day 1 | ||||
| Age | 0.085 | 0.191 | −0.248 | 0.001 |
| WBC count (106/mL) | −0.089 | 0.171 | − | − |
| Platelet count (106/mL) | −0.026 | 0.694 | 0.377 | 0.001 |
| Albumin (g/L) | −0.430 | 0.001 | −0.025 | 0.717 |
| Bilirubin (mg/dL) | 0.301 | 0.001 | −0.077 | 0.342 |
| CRP (mg/L) | − | − | −0.089 | 0.171 |
| Procalcitonin (ng/mL) | 0.511 | 0.001 | 0.024 | 0.834 |
| cTNI (µg/L) | 0.002 | 0.980 | 0.289 | 0.001 |
| NT-pro BNP (pg/mL) | 0.548 | 0.001 | −0.114 | 0.260 |
| Mechanical ventilation days | −0.194 | 0.003 | 0.147 | 0.023 |
| Creatinine (mg/dL) | 0.341 | 0.001 | 0.099 | 0.127 |
| Intensive care days | −0.138 | 0.033 | 0.076 | 0.243 |
| Day 3 | ||||
| Age | −0.078 | 0.339 | 0.117 | 0.146 |
| WBC count (106/mL) | 0.255 | 0.002 | − | − |
| Platelet count (106/mL) | 0.004 | 0.958 | 0.355 | 0.001 |
| Albumin (g/L) | −0.359 | 0.001 | −0.102 | 0.223 |
| Bilirubin (mg/dL) | −0.006 | 0.950 | −0.166 | 0.058 |
| CRP (mg/L) | − | − | 0.255 | 0.002 |
| Procalcitonin (ng/mL) | 0.397 | 0.004 | 0.123 | 0.395 |
| cTNI (µg/L) | 0.281 | 0.010 | 0.317 | 0.003 |
| NT-pro BNP (pg/mL) | −0.011 | 0.943 | 0.158 | 0.324 |
| Mechanical ventilation days | 0.308 | 0.001 | 0.144 | 0.073 |
| Creatinine | 0.180 | 0.028 | 0.202 | 0.012 |
| Intensive care days | 0.300 | 0.001 | 0.074 | 0.363 |
| CRP | WBC | p Value * | |
|---|---|---|---|
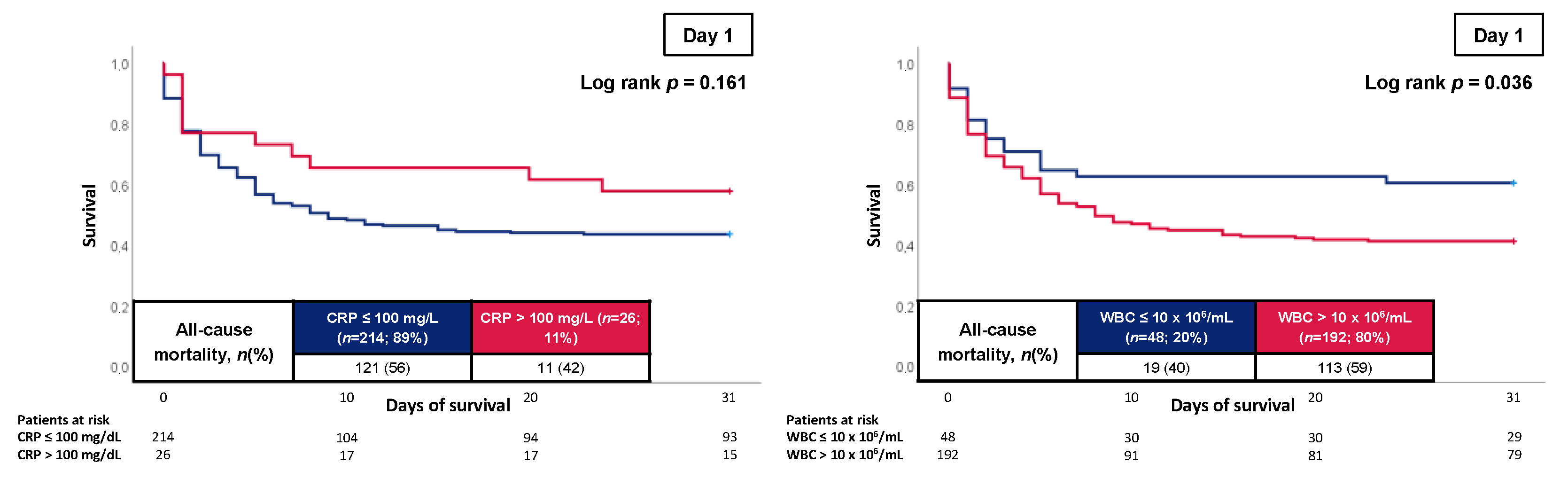
| Day 1 | 0.514 (0.440–0.589); p = 0.700 | 0.605 (0.533–0.677); p = 0.005 | 0.081 |
| Day 2 | 0.510 (0.426–0.595); p = 0.813 | 0.572 (0.491–0.653); p = 0.085 | 0.298 |
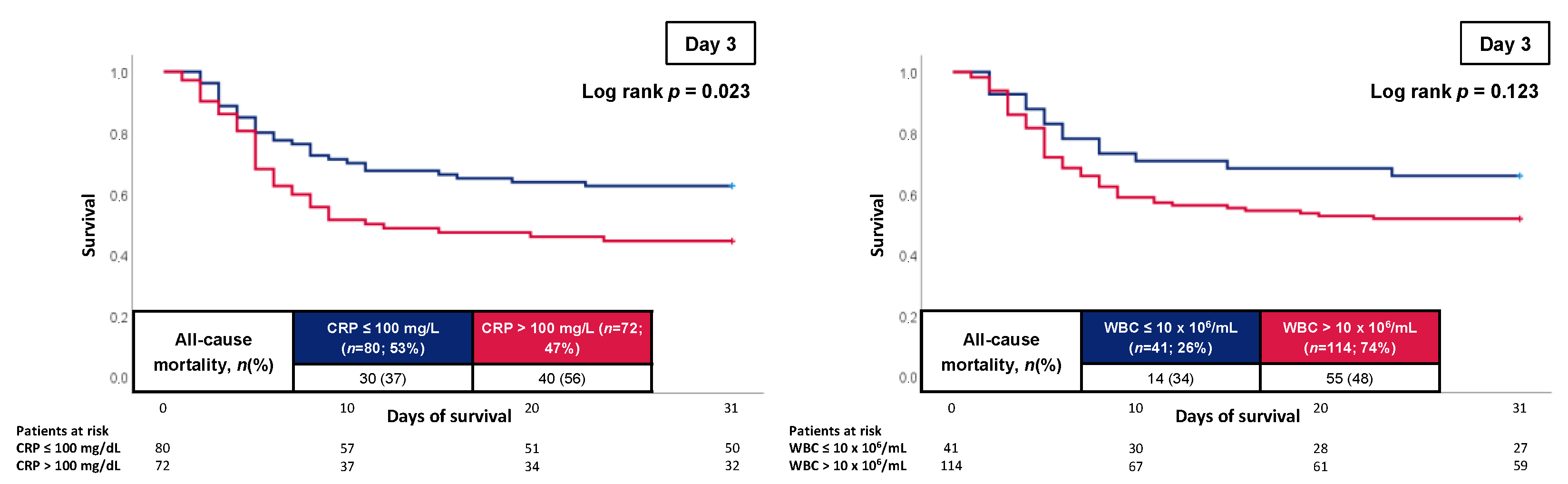
| Day 3 | 0.623 (0.534–0.712); p = 0.009 | 0.597 (0.507–0.687); p = 0.039 | 0.688 |
| Day 4 | 0.673 (0.580–0.766); p = 0.001 | 0.663 (0.589–0.758); p = 0.001 | 0.884 |
| Day 8 | 0.754 (0.633–0.875); p = 0.001 | 0.639 (0.499–0.778); p = 0.044 | 0.210 |
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Age | 1.008 | 0.994–1.021 | 0.254 | 1.013 | 0.997–1.029 | 0.103 |
| BMI (kg/m2) | 1.014 | 0.983–1.046 | 0.386 | 1.005 | 0.970–1.042 | 0.774 |
| Mechanical ventilation | 1.583 | 1.095–2.289 | 0.015 | 1.512 | 1.005–2.277 | 0.047 |
| Hb (mg/dL) | 0.987 | 0.924–1.055 | 0.698 | 0.983 | 0.914–1.057 | 0.644 |
| WBC > 10 × 106/mL | 1.643 | 1.010–2.672 | 0.045 | 1.785 | 1.021–3.121 | 0.042 |
| Platelets (106/mL) | 0.999 | 0.997–1.001 | 0.424 | 0.999 | 0.997–1.001 | 0.458 |
| INR | 1.371 | 1.068–1.759 | 0.013 | 1.339 | 1.033–1.735 | 0.027 |
| CRP > 100 mg/L | 0.656 | 0.354–1.216 | 0.181 | 0.670 | 0.334–1.346 | 0.260 |
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Age | 1.008 | 0.994–1.021 | 0.254 | 1.005 | 0.986–1.024 | 0.636 |
| BMI (kg/m2) | 1.014 | 0.983–1.046 | 0.386 | 0.983 | 0.935–1.034 | 0.506 |
| Mechanical ventilation | 1.583 | 1.095–2.289 | 0.015 | 1.309 | 0.746–2.300 | 0.348 |
| Hb (mg/dL) | 0.944 | 0.848–1.050 | 0.289 | 0.922 | 0.817–1.041 | 0.191 |
| WBC > 10 × 106/mL | 1.565 | 0.870–2.815 | 0.135 | 1.571 | 0.842–2.931 | 0.155 |
| Platelets (106/mL) | 0.998 | 0.995–1.001 | 0.171 | 0.998 | 0.995–1.001 | 0.284 |
| INR | 1.596 | 1.120–2.274 | 0.010 | 1.615 | 1.076–2.424 | 0.021 |
| CRP > 100 mg/L | 1.702 | 1.060–2.735 | 0.028 | 1.693 | 1.008–2.843 | 0.047 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dudda, J.; Schupp, T.; Rusnak, J.; Weidner, K.; Abumayyaleh, M.; Ruka, M.; Egner-Walter, S.; Forner, J.; Müller, J.; Bertsch, T.; et al. C-Reactive Protein and White Blood Cell Count in Cardiogenic Shock. J. Clin. Med. 2023, 12, 965. https://doi.org/10.3390/jcm12030965
Dudda J, Schupp T, Rusnak J, Weidner K, Abumayyaleh M, Ruka M, Egner-Walter S, Forner J, Müller J, Bertsch T, et al. C-Reactive Protein and White Blood Cell Count in Cardiogenic Shock. Journal of Clinical Medicine. 2023; 12(3):965. https://doi.org/10.3390/jcm12030965
Chicago/Turabian StyleDudda, Jonas, Tobias Schupp, Jonas Rusnak, Kathrin Weidner, Mohammad Abumayyaleh, Marinela Ruka, Sascha Egner-Walter, Jan Forner, Julian Müller, Thomas Bertsch, and et al. 2023. "C-Reactive Protein and White Blood Cell Count in Cardiogenic Shock" Journal of Clinical Medicine 12, no. 3: 965. https://doi.org/10.3390/jcm12030965
APA StyleDudda, J., Schupp, T., Rusnak, J., Weidner, K., Abumayyaleh, M., Ruka, M., Egner-Walter, S., Forner, J., Müller, J., Bertsch, T., Kittel, M., Akin, I., & Behnes, M. (2023). C-Reactive Protein and White Blood Cell Count in Cardiogenic Shock. Journal of Clinical Medicine, 12(3), 965. https://doi.org/10.3390/jcm12030965






