Cardiovascular Reasons for Access to a Tertiary Oncological Emergency Service: The CARILLON Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection and Study Outcomes
2.3. Statistical Analysis
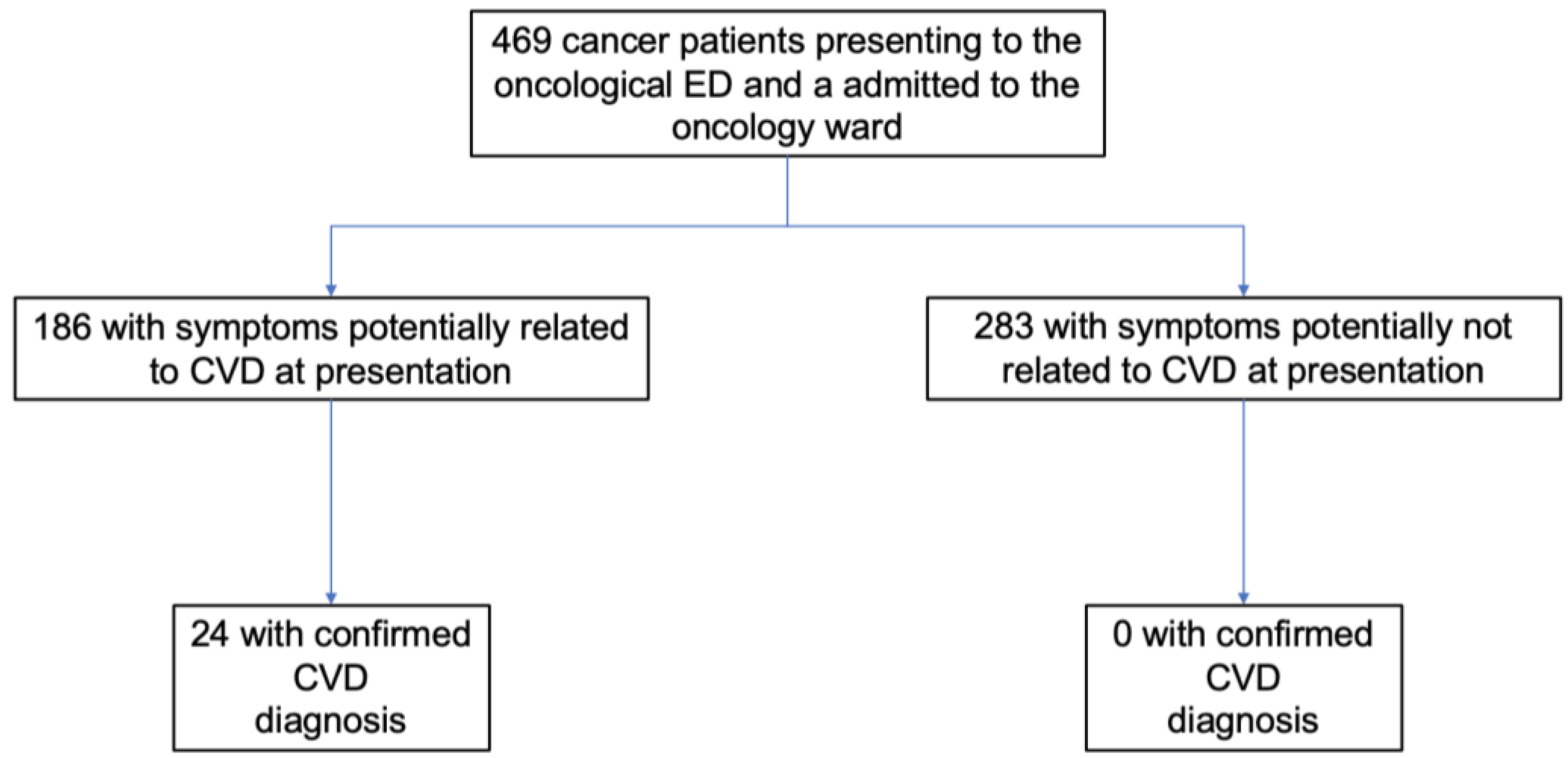
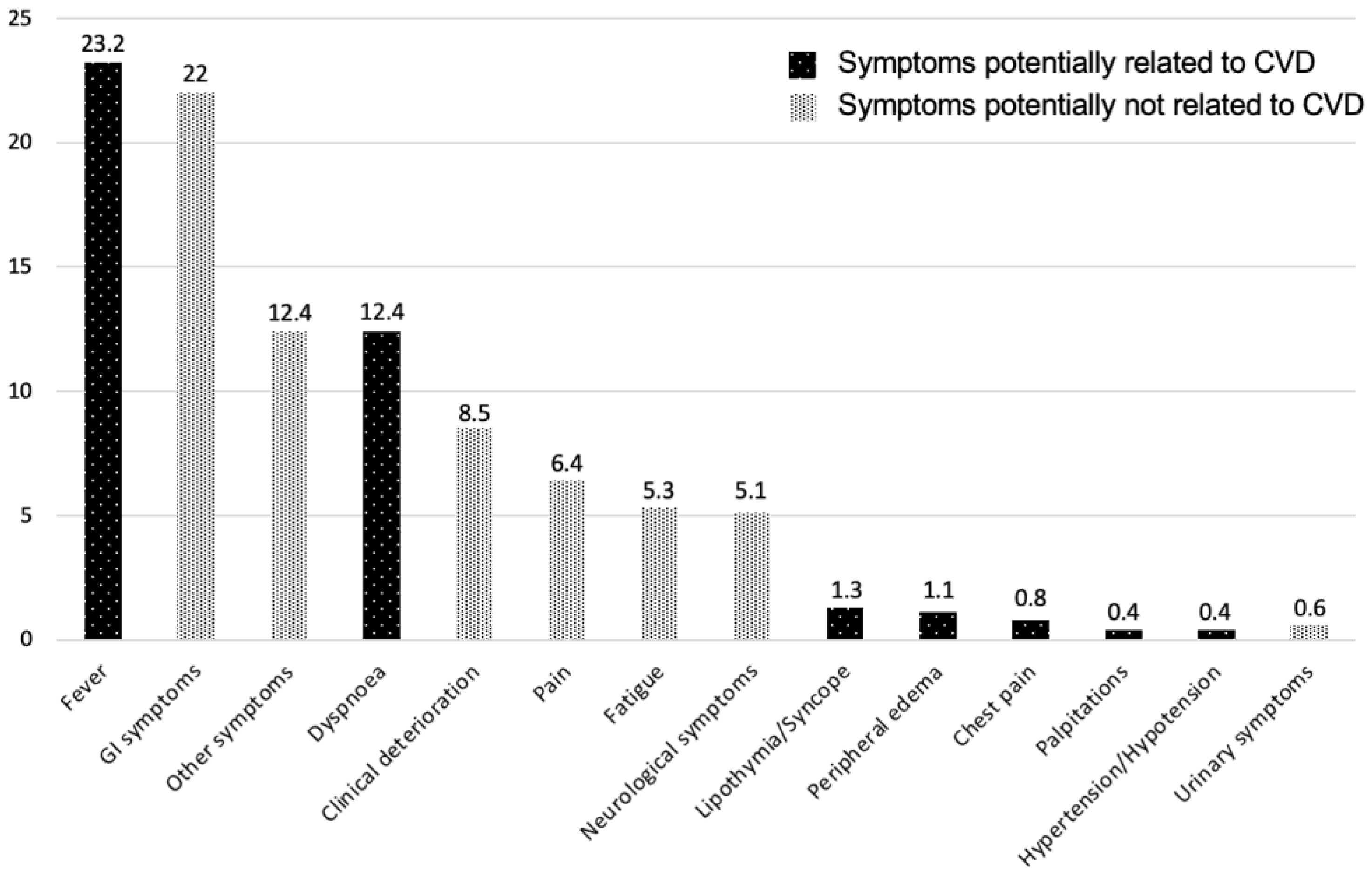
3. Results
3.1. Study and Population Characteristics
3.2. Study Outcomes
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mathers, C.D.; Loncar, D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006, 3, e442. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Abdel-Qadir, H.; Austin, P.C.; Lee, D.S.; Amir, E.; Tu, J.V.; Thavendiranathan, P.; Fung, K.; Anderson, G.M. A Population-Based Study of Cardiovascular Mortality Following Early-Stage Breast Cancer. JAMA Cardiol. 2017, 2, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Oeffinger, K.C.; Mertens, A.C.; Sklar, C.A.; Kawashima, T.; Hudson, M.M.; Meadows, A.T.; Friedman, D.L.; Marina, N.; Hobbie, W.; Kadan-Lottick, N.S.; et al. Chronic health conditions in adult survivors of childhood cancer. N. Engl. J. Med. 2006, 355, 1572–1582. [Google Scholar] [CrossRef]
- Gevaert, S.A.; Halvorsen, S.; Sinnaeve, P.R.; Sambola, A.; Gulati, G.; Lancellotti, P.; Van Der Meer, P.; Lyon, A.R.; Farmakis, D.; Lee, G.; et al. Evaluation and management of cancer patients presenting with acute cardiovascular disease: A Consensus Document of the Acute CardioVascular Care (ACVC) association and the ESC council of Cardio-Oncology-Part 1: Acute coronary syndromes and acute pericardial diseases. Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 947–959. [Google Scholar] [CrossRef] [PubMed]
- Malavasi, V.L.; Vitolo, M.; Proietti, M.; Diemberger, I.; Fauchier, L.; Marin, F.; Nabauer, M.; Potpara, T.S.; Dan, G.A.; Kalarus, Z.; et al. Impact of malignancy on outcomes in European patients with atrial fibrillation: A report from the ESC-EHRA EURObservational research programme in atrial fibrillation general long-term registry. Eur. J. Clin. Investig. 2022, 52, e13773. [Google Scholar] [CrossRef] [PubMed]
- Pennacchioni, A.; Nanni, G.; Sgura, F.A.; Imberti, J.F.; Monopoli, D.E.; Rossi, R.; Longo, G.; Arrotti, S.; Vitolo, M.; Boriani, G. Percutaneous pericardiocentesis for pericardial effusion: Predictors of mortality and outcomes. Intern. Emerg. Med. 2021, 16, 1771–1777. [Google Scholar] [CrossRef]
- Chang, H.M.; Moudgil, R.; Scarabelli, T.; Okwuosa, T.M.; Yeh, E.T.H. Cardiovascular Complications of Cancer Therapy: Best Practices in Diagnosis, Prevention, and Management: Part 1. J. Am. Coll. Cardiol. 2017, 70, 2536–2551. [Google Scholar] [CrossRef]
- Chang, H.M.; Okwuosa, T.M.; Scarabelli, T.; Moudgil, R.; Yeh, E.T.H. Cardiovascular Complications of Cancer Therapy: Best Practices in Diagnosis, Prevention, and Management: Part 2. J. Am. Coll. Cardiol. 2017, 70, 2552–2565. [Google Scholar] [CrossRef]
- Tajiri, K.; Sekine, I.; Naito, H.; Murata, M.; Li, S.; Inoue, K.; Sasamura, R.; Nakajima, H.; Iida, N.; Nagashio, K.; et al. Cardiology consultation in oncology practice: A 5-year survey. Jpn. J. Clin. Oncol. 2020, 50, 1419–1425. [Google Scholar] [CrossRef]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef] [PubMed]
- Boriani, G.; Lee, G.; Parrini, I.; Lopez-Fernandez, T.; Lyon, A.R.; Suter, T.; Van der Meer, P.; Cardinale, D.; Lancellotti, P.; Zamorano, J.L.; et al. Anticoagulation in patients with atrial fibrillation and active cancer: An international survey on patient management. Eur. J. Prev. Cardiol. 2021, 28, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Kobo, O.; Moledina, S.M.; Raisi-Estabragh, Z.; Shanmuganathan, J.W.D.; Chieffo, A.; Al Ayoubi, F.; Alraies, M.C.; Biondi-Zoccai, G.; Elgendy, I.Y.; Mohamed, M.O.; et al. Emergency department cardiovascular disease encounters and associated mortality in patients with cancer: A study of 20.6 million records from the USA. Int. J. Cardiol. 2022, 363, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A.; Noble, S.; Lee, A.Y.Y.; Soff, G.; Meyer, G.; O’Connell, C.; Carrier, M. Role of direct oral anticoagulants in the treatment of cancer-associated venous thromboembolism: Guidance from the SSC of the ISTH. J. Thromb. Haemost. 2018, 16, 1891–1894. [Google Scholar] [CrossRef]
- Paterson, D.I.; Wiebe, N.; Cheung, W.Y.; Mackey, J.R.; Pituskin, E.; Reiman, A.; Tonelli, M. Incident Cardiovascular Disease Among Adults With Cancer: A Population-Based Cohort Study. JACC CardioOncol. 2022, 4, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Howard, E.; Steingart, R.M.; Armstrong, G.T.; Lyon, A.R.; Armenian, S.H.; Teresa Voso, M.; Cicconi, L.; Coco, F.L.; Minotti, G. Cardiovascular events in cancer survivors†. Semin. Oncol. 2019, 46, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Gevaert, S.A.; Halvorsen, S.; Sinnaeve, P.R.; Sambola, A.; Gulati, G.; Lancellotti, P.; Van Der Meer, P.; Lyon, A.R.; Farmakis, D.; Lee, G.; et al. Evaluation and management of cancer patients presenting with acute cardiovascular disease: A Clinical Consensus Statement of the Acute CardioVascular Care Association (ACVC) and the ESC council of Cardio-Oncology-part 2: Acute heart failure, acute myocardial diseases, acute venous thromboembolic diseases, and acute arrhythmias. Eur. Heart J. Acute Cardiovasc. Care 2022, 11, 865–874. [Google Scholar] [CrossRef]
- Vandyk, A.D.; Harrison, M.B.; Macartney, G.; Ross-White, A.; Stacey, D. Emergency department visits for symptoms experienced by oncology patients: A systematic review. Support. Care Cancer 2012, 20, 1589–1599. [Google Scholar] [CrossRef]
- Wang, Y.; Han, X.; Sun, J.; Li, C.; Adhikari, B.K.; Zhang, J.; Miao, X.; Chen, Z. Cardio-Oncology: A Myriad of Relationships Between Cardiovascular Disease and Cancer. Front. Cardiovasc. Med. 2022, 9, 727487. [Google Scholar] [CrossRef]
- Fogarassy, G.; Vathy-Fogarassy, Á.; Kenessey, I.; Veress, G.; Polgár, C.; Forster, T. Preventing cancer therapy-related heart failure: The need for novel studies. J. Cardiovasc. Med. 2021, 22, 459–468. [Google Scholar] [CrossRef]
- Gurizzan, C.; Roca, E.; Faggiano, A.; Paoli, D.; Dinatolo, E.; Masini, G.; Tomasi, C.; De Palma, G.; Metra, M.; Berruti, A.; et al. Rate of venous thromboembolism and atrial fibrillation in a real-world case series of advanced cancer patients: The CaTEV Study. J. Cardiovasc. Med. 2021, 22, 444–452. [Google Scholar] [CrossRef]
- Leiva, O.; AbdelHameid, D.; Connors, J.M.; Cannon, C.P.; Bhatt, D.L. Common Pathophysiology in Cancer, Atrial Fibrillation, Atherosclerosis, and Thrombosis: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol. 2021, 3, 619–634. [Google Scholar] [CrossRef] [PubMed]
- Schoormans, D.; Pedersen, S.S.; Dalton, S.; Rottmann, N.; van de Poll-Franse, L. Cardiovascular co-morbidity in cancer patients: The role of psychological distress. Cardiooncology 2016, 2, 9. [Google Scholar] [CrossRef] [PubMed]
- Chow, E.J.; Mueller, B.A.; Baker, K.S.; Cushing-Haugen, K.L.; Flowers, M.E.; Martin, P.J.; Friedman, D.L.; Lee, S.J. Cardiovascular hospitalizations and mortality among recipients of hematopoietic stem cell transplantation. Ann. Intern. Med. 2011, 155, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Rivera, D.R.; Gallicchio, L.; Brown, J.; Liu, B.; Kyriacou, D.N.; Shelburne, N. Trends in Adult Cancer-Related Emergency Department Utilization: An Analysis of Data From the Nationwide Emergency Department Sample. JAMA Oncol. 2017, 3, e172450. [Google Scholar] [CrossRef] [PubMed]
- Caterino, J.M.; Adler, D.; Durham, D.D.; Yeung, S.J.; Hudson, M.F.; Bastani, A.; Bernstein, S.L.; Baugh, C.W.; Coyne, C.J.; Grudzen, C.R.; et al. Analysis of Diagnoses, Symptoms, Medications, and Admissions Among Patients With Cancer Presenting to Emergency Departments. JAMA Netw. Open 2019, 2, e190979. [Google Scholar] [CrossRef]
- Galderisi, M.; Santoro, C.; Bossone, E.; Mancusi, C. Rationale and proposal for cardio-oncology services in Italy. J. Cardiovasc. Med. 2022, 23, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Boriani, G.; Imberti, J.F.; Valenti, A.C.; Malavasi, V.L.; Vitolo, M. Managing atrial fibrillation: The need for an individualized approach even in the emergency department. Intern. Emerg. Med. 2020, 15, 9–12. [Google Scholar] [CrossRef]
- Malavasi, V.L.; Vitolo, M.; Colella, J.; Montagnolo, F.; Mantovani, M.; Proietti, M.; Potpara, T.S.; Lip, G.Y.H.; Boriani, G. Rhythm- or rate-control strategies according to 4S-AF characterization scheme and long-term outcomes in atrial fibrillation patients: The FAMo (Fibrillazione Atriale in Modena) cohort. Intern. Emerg. Med. 2021, 17, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Imberti, J.F.; Kotalczyk, A.; Wang, Y.; Lip, G.Y.H.; Investigators, C.R. 4S-AF scheme and ABC pathway guided management improves outcomes in atrial fibrillation patients. Eur. J. Clin. Investig. 2022, 52, e13751. [Google Scholar] [CrossRef] [PubMed]
- Vitolo, M.; Proietti, M.; Malavasi, V.L.; Bonini, N.; Romiti, G.F.; Imberti, J.F.; Fauchier, L.; Marin, F.; Nabauer, M.; Potpara, T.S.; et al. Adherence to the “Atrial fibrillation Better Care” (ABC) pathway in patients with atrial fibrillation and cancer: A report from the ESC-EHRA EURObservational Research Programme in atrial fibrillation (EORP-AF) General Long-Term Registry. Eur. J. Intern. Med. 2022, 105, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Imberti, J.F.; Mei, D.A.; Vitolo, M.; Bonini, N.; Proietti, M.; Potpara, T.; Lip, G.Y.H.; Boriani, G. Comparing atrial fibrillation guidelines: Focus on stroke prevention, bleeding risk assessment and oral anticoagulant recommendations. Eur. J. Intern. Med. 2022, 101, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Laufer-Perl, M.; Gilon, D.; Kapusta, L.; Iakobishvili, Z. The Role of Speckle Strain Echocardiography in the Diagnosis of Early Subclinical Cardiac Injury in Cancer Patients—Is There More Than Just Left Ventricle Global Longitudinal Strain? J. Clin. Med. 2021, 10, 154. [Google Scholar] [CrossRef]
- Boriani, G.; Guerra, F.; De Ponti, R.; D’Onofrio, A.; Accogli, M.; Bertini, M.; Bisignani, G.; Forleo, G.B.; Landolina, M.; Lavalle, C.; et al. Five waves of COVID-19 pandemic in Italy: Results of a national survey evaluating the impact on activities related to arrhythmias, pacing, and electrophysiology promoted by AIAC (Italian Association of Arrhythmology and Cardiac Pacing). Intern. Emerg. Med. 2022; in press. [Google Scholar] [CrossRef] [PubMed]
- Ticinesi, A.; Nouvenne, A.; Cerundolo, N.; Parise, A.; Prati, B.; Guerra, A.; Meschi, T. Trends of COVID-19 Admissions in an Italian Hub during the Pandemic Peak: Large Retrospective Study Focused on Older Subjects. J. Clin. Med. 2021, 10, 1115. [Google Scholar] [CrossRef] [PubMed]
- Bonfioli, G.; Tomasoni, D.; Metra, M.; Adamo, M. Coronavirus disease 2019 and cardiovascular disease: What we have learnt during the last 2 years. J. Cardiovasc. Med. 2022, 23, 710–714. [Google Scholar] [CrossRef]
- España, P.P.; Bilbao, A.; García-Gutiérrez, S.; Lafuente, I.; Anton-Ladislao, A.; Villanueva, A.; Uranga, A.; Legarreta, M.J.; Aguirre, U.; Quintana, J.M.; et al. Predictors of mortality of COVID-19 in the general population and nursing homes. Intern. Emerg. Med. 2021, 16, 1487–1496. [Google Scholar] [CrossRef]
- Griewing, S.; Gremke, N.; Kreutz, J.; Schieffer, B.; Timmermann, L.; Markus, B. Chronological Development of Cardiovascular Disease in Times of COVID-19: A Retrospective Analysis of Hospitalized Diseases of the Circulatory System and COVID-19 Patients of a German University Hospital. J. Cardiovasc. Dev. Dis. 2022, 9, 325. [Google Scholar] [CrossRef]
- Boriani, G.; Palmisano, P.; Guerra, F.; Bertini, M.; Zanotto, G.; Lavalle, C.; Notarstefano, P.; Accogli, M.; Bisignani, G.; Forleo, G.B.; et al. Impact of COVID-19 pandemic on the clinical activities related to arrhythmias and electrophysiology in Italy: Results of a survey promoted by AIAC (Italian Association of Arrhythmology and Cardiac Pacing). Intern. Emerg. Med. 2020, 15, 1445–1456. [Google Scholar] [CrossRef]
- Dyba, T.; Randi, G.; Bray, F.; Martos, C.; Giusti, F.; Nicholson, N.; Gavin, A.; Flego, M.; Neamtiu, L.; Dimitrova, N. The European cancer burden in 2020: Incidence and mortality estimates for 40 countries and 25 major cancers. Eur. J. Cancer 2021, 157, 308–347. [Google Scholar] [CrossRef]
- Brignole, M.; Moya, A.; de Lange, F.J.; Deharo, J.C.; Elliott, P.M.; Fanciulli, A. 2018 ESC Guidelines for the diagnosis and management of syncope. Eur. Heart J. 2018, 39, 1883–1948. [Google Scholar] [CrossRef] [PubMed]
- O’Grady, N.P.; Barie, P.S.; Bartlett, J.G.; Bleck, T.; Carroll, K.; Kalil, A.C.; Linden, P.; Maki, D.G.; Nierman, D.; Pasculle, W.; et al. Guidelines for evaluation of new fever in critically ill adult patients: 2008 update from the American College of Critical Care Medicine and the Infectious Diseases Society of America. Crit. Care Med. 2008, 36, 1330–1349. [Google Scholar] [CrossRef]
- Erhardt, L.; Herlitz, J.; Bossaert, L.; Halinen, M.; Keltai, M.; Koster, R.; Marcassa, C.; Quinn, T.; van Weert, H. Task force on the management of chest pain. Eur. Heart J. 2002, 23, 1153–1176. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Charton, E.; Cuer, B.; Cottone, F.; Efficace, F.; Touraine, C.; Hamidou, Z.; Fiteni, F.; Bonnetain, F.; Woronoff-Lemsi, M.-C.; Bascoul-Mollevi, C.; et al. Time to deterioration in cancer randomized clinical trials for patient-reported outcomes data: A systematic review. Qual. Life Res. 2020, 29, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; de Haes, J.C.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Adler, Y.; Charron, P.; Imazio, M.; Badano, L.; Barón-Esquivias, G.; Bogaert, J.; Brucato, A.; Gueret, P.; Klingel, K.; Lionis, C.; et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC)Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2015, 36, 2921–2964. [Google Scholar] [PubMed]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [PubMed]

| Total Cohort (n = 469) | Potential CVD Symptoms (n = 186) | Potential Non-CVD Symptoms (n = 283) | p-Value | |
|---|---|---|---|---|
| Female sex, n (%) | 243/469 (51.8) | 89/186 (47.8) | 154/283 (54.4) | 0.16 |
| Age (years), median (IQR) | 68.0 (59.1–76.3) | 69.3 (59.9–77.6) | 69.0 (59.4–75.9) | 0.49 |
| Caucasian race, n (%) | 457/469 (97.4) | 181/186 (97.3) | 276/283 (97.5) | 1.00 |
| BMI (Kg/m2), median (IQR) | 25.0 (22.0–28.8) | 25.1 (22.0–28.9) | 25.0 (22.0–28.8) | 0.97 |
| BSA (m2), median (IQR) | 1.8 (1.6–1.9) | 1.8 (1.6–2.0) | 1.8 (1.7–1.9) | 0.26 |
| Hematologic cancer, n (%) | 133/469 (28.4) | 78/186 (41.9) | 55/283 (19.4) | <0.01 |
| Active cancer, n (%) | 469/469 (100) | 186/186 (100) | 283/283 (100) | - |
| CV risk factors | ||||
| Hypertension, n (%) | 259/467 (55.5) | 107/184 (58.2) | 152/283 (53.7) | 0.35 |
| DM, n (%) | 98/466 (21.0) | 42/184 (22.8) | 56/282 (19.9) | 0.44 |
| Dyslipidemia, n (%) | 133/466 (28.5) | 60/184 (32.6) | 73/282 (25.9) | 0.17 |
| Smoking | ||||
| Never, n (%) | 293/443 (66.1) | 119/175 (68.0) | 174/268 (64.9) | 0.75 |
| Former, n (%) | 99/443 (22.3) | 38/175 (21.7) | 61/268 (22.8) | |
| Active, n (%) | 51/443 (11.5) | 18/175 (10.3) | 33/268 (12.3) | |
| Comorbidities | ||||
| IHD, n (%) | 45/464 (9.7) | 18/184 (9.8) | 27/281 (9.6) | 0.95 |
| CABG, n (%) | 8/465 (1.7) | 4/184 (2.2) | 4/281 (1.4) | 0.72 |
| PCI, n (%) | 32/465 (6.9) | 11/184 (6.0) | 21/281 (7.5) | 0.53 |
| COPD, n (%) | 38/457 (8.3) | 16/182 (8.8) | 22/275 (8.0) | 0.76 |
| PAD, n (%) | 54/464 (11.6) | 21/183 (11.5) | 33/281 (11.7) | 0.93 |
| AF/AFL, n (%) | 54/466 (11.6) | 31/184 (16.8) | 23/282 (8.2) | <0.01 |
| CIED | ||||
| No CIED, n (%) | 452/465 (97.2) | 178/184 (96.7) | 274/281 (97.5) | 0.15 |
| PM, n (%) | 9/465 (1.9) | 6/184 (3.3) | 3/281 (1.1) | |
| ICD, n (%) | 2/465 (0.4) | 0/184 (0.0) | 2/281 (0.7) | |
| CRTD, n (%) | 2/465 (0.4) | 0/184 (0.0) | 2/281 (0.7) | |
| LVEF, median (IQR) | 58 (55–60) | 58 (55–60) | 60 (55–61) | 0.32 |
| Heart rhythm | ||||
| SR, n (%) | 387/423 (91.5) | 146/170 (85.9) | 241/253 (95.3) | <0.01 |
| AF, n (%) | 29/423 (6.9) | 19/170 (11.2) | 10/253 (4.0) | |
| CIED-induced, n (%) | 7/423 (1.6) | 5/170 (2.9) | 2/253 (0.8) | |
| Main ECG features | ||||
| 1st-degree AVB, n (%) | 15/425 (3.5) | 7/170 (4.1) | 8/255 (3.1) | 0.60 |
| LBBB, n (%) | 6/425 (1.4) | 1/170 (0.6) | 5/255 (2.0) | 0.41 |
| RBBB, n (%) | 28/424 (6.6) | 7/169 (4.1) | 21/255 (8.2) | 0.10 |
| CV medications | ||||
| Antiplatelets, n (%) | 48/453 (10.6) | 20/181 (11.0) | 28/272 (10.3) | 0.80 |
| Anticoagulant, n (%) | 93/453 (20.5) | 52/181 (28.7) | 41/272 (15.1) | <0.01 |
| ACEi/ARB, n (%) | 87/453 (19.2) | 29/181 (16.0) | 58/272 (21.3) | 0.16 |
| BB, n (%) | 150/453 (33.1) | 68/181 (37.6) | 82/272 (30.1) | 0.10 |
| Potential CVD Symptoms | Potential Non-CVD Symptoms | HR (95% CI) | p | |||
|---|---|---|---|---|---|---|
| n/N (%) | Events/ 100 pts-Months | n/N (%) | Events/ 100 pts-Months | |||
| Mortality | 80/186 (43.0) | 8.9 | 124/283 (43.8) | 11.2 | 0.85 (0.64–1.12) | 0.24 |
| New in-hospital CV events | 15/186 (8.1) | 1.7 | 17/283 (6.0) | 1.5 | 1.03 (0.77–1.37) | 0.83 |
| Days, Median (IQR) | Days, Median (IQR) | |||||
| Length of stay | 12 (8–18) | 12 (7–20) | - | 0.57 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imberti, J.F.; Maisano, A.; Rampini, F.; Minnocci, M.; Bertuglia, F.; Mantovani, M.; Cherubini, B.; Mei, D.A.; Ferrara, L.; Bonini, N.; et al. Cardiovascular Reasons for Access to a Tertiary Oncological Emergency Service: The CARILLON Study. J. Clin. Med. 2023, 12, 962. https://doi.org/10.3390/jcm12030962
Imberti JF, Maisano A, Rampini F, Minnocci M, Bertuglia F, Mantovani M, Cherubini B, Mei DA, Ferrara L, Bonini N, et al. Cardiovascular Reasons for Access to a Tertiary Oncological Emergency Service: The CARILLON Study. Journal of Clinical Medicine. 2023; 12(3):962. https://doi.org/10.3390/jcm12030962
Chicago/Turabian StyleImberti, Jacopo F., Anna Maisano, Francesca Rampini, Melania Minnocci, Filippo Bertuglia, Marta Mantovani, Benedetta Cherubini, Davide A. Mei, Leonardo Ferrara, Niccolò Bonini, and et al. 2023. "Cardiovascular Reasons for Access to a Tertiary Oncological Emergency Service: The CARILLON Study" Journal of Clinical Medicine 12, no. 3: 962. https://doi.org/10.3390/jcm12030962
APA StyleImberti, J. F., Maisano, A., Rampini, F., Minnocci, M., Bertuglia, F., Mantovani, M., Cherubini, B., Mei, D. A., Ferrara, L., Bonini, N., Valenti, A. C., Vitolo, M., Longo, G., & Boriani, G. (2023). Cardiovascular Reasons for Access to a Tertiary Oncological Emergency Service: The CARILLON Study. Journal of Clinical Medicine, 12(3), 962. https://doi.org/10.3390/jcm12030962








